Abstract
Introduction
The use of trans-sacral implants to treat fractures of the sacrum is limited by the variable pelvic anatomy. We were interested in how many trans-sacral implants can be placed per pelvis? If a trans-sacral implant cannot be placed in S1, where is the cortex perforated, and is the use of sacroiliac screws safe in these pelves?
Materials and methods
3D pelvic models were created from CT scans of 156 individuals without fractures (92 European and 64 Japanese, 79 male and 77 female, mean age 66.7 ± 13.7 years). Trans-sacral implants with a diameter of 7.3 mm were positioned virtually with and without a surrounding safe zone of 12 mm diameter.
Results
Fifty-one percent of pelves accommodated trans-sacral implants in S1 with a safe zone. Twenty-two percent did not offer enough space in S1 for an implant even when ignoring the safe zone. Every pelvis had sufficient space for a trans-sacral implant in S2, in 78% including a safe zone as well. In S1, implant perforation was observed in the sacral ala and iliac fossa in 69%, isolated iliac fossa perforation in 23% and perforation of the sacral ala in 8%. Bilateral sacroiliac screw placement was always possible in S1.
Conclusions
The use of trans-sacral implants in S1 requires meticulous preoperative planning to avoid injury of neurovascular structures. S2 more consistently offers space for trans-sacral implants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Demographic changes lead to increasing numbers of elderly patients experiencing osteoporosis-associated fragility fractures of the pelvis (FFP) [1, 2]. These fractures are usually treated non-operatively. However, in cases of intractable pain during mobilization, or fracture displacement with consequent instability, operative stabilization is performed [3]. Sacral fractures are commonly treated with minimally-invasive sacroiliac (SI) screws [4]. However, 14–20% of elderly patients with osteoporosis experience backing out of conventional SI screws [5–7]. In high-energy fractures, up to 17% have demonstrated SI screw loosening, with vertical shear fractures as risk factor [8]. Trans-sacral implants can be used to lessen this complication [6, 9–11], as they do not depend on screw purchase in the sacral body’s decreased bone mass [12, 13]. They anchor in the cortical bone of the posterior ilium bilaterally and if using a trans-sacral bar, they can exerting some compression on the sacral fracture [9]. They further may prevent secondary contralateral sacral fractures [14] due to higher stress also experienced in the contralateral sacral ala when a pubic rami fracture is present [15]. Trans-sacral implants showed biomechanical benefits over SI screws [16, 17] without clinical disadvantages of anchorage in the non-injured SI joint [18]. Alternatively, SI screws can be augmented with cement to enhance screw purchase in the osteoporotic sacral body [19].
Trans-sacral implant positioning in S1 is restricted by the highly variable anatomy of the upper sacrum [20, 21]. The safe corridor for trans-sacral implants is smaller than that for unilateral SI screws, because the implants must pass an isthmus bilaterally in the sacrum [22]. Measurements of three-dimensional (3D) models or computed tomography (CT) scans have indicated that 14–41% of pelves do not offer enough space to safely accommodate a trans-sacral implant in S1 [23–29]. When respecting a safe zone of 12 mm diameter, trans-sacral corridor S1 is not available in 52% of pelves [29]. In 77–100% of pelves where implant positioning was impossible or critical in S1, there was enough space for an implant in S2 [24–26, 28–30]. Anatomically adequate trans-sacral corridors in S3 were reported in 15–24% [28, 29, 31]. The use of multiple implants in the posterior pelvic ring lead to less screw loosening [6] and higher biomechanical stability [32, 33]. However, information regarding the maximal number of implants possible, the location of cortical bone perforation, and the possibility of using SI screws is limited. Further, the pre- and intraoperative imaging of the corridor’s borders is difficult and may not help to identify pelves suitable for trans-sacral implants.
We used virtual trans-sacral implant positioning to answer how many implants can be placed per pelvis, with or without surrounding safe zones? In pelves not offering sufficient space for a trans-sacral implant in S1, we were interested in the location of cortical perforation, and whether conventional SI screws are safe.
Methods
CT data
Clinical CT scans of 92 European (EU; 48 female, 44 male) and 64 Japanese (JP; 29 female, 35 male) individuals were included. Mean age was 66.7 years (± 13.7, range 26–91). Individuals with bony pathology other than osteoporosis (13) or fractures (6), as well as those with sacralization of L5 (11) or lumbarization of S1 (1) and spina bifida (1) were excluded. The data of the European individuals was obtained from the CT database of the AO Research Institute Davos, Switzerland. CT scanners Siemens Sensation 64 and Siemens Definition (Siemens AG, Erlangen, Germany) were used with 120 kpV voltage and B45f Kernel. The mean voxel size was 0.75 × 0.75 × 1.02 mm. Postmortem scans with the GE LightSpeed VCT (GE Healthcare, Waukesha, USA) were used for the Japanese group, with application of 120 kpV tube voltage and “Standard Kernel”. The mean voxel size was 0.68 × 0.68 × 1.11 mm.
Virtual implant positioning
A 3D model was created for each pelvis using Amira software (Amira 5.4.3, Visage Imaging, Inc., Berlin, Germany) [20]. The trans-sacral corridors were visualized in the lateral semitransparent view. A screw with a diameter of 7.3 mm and variable length was used for virtual implant positioning. A safe zone with a diameter of 12 mm was optionally displayed around the screw. After manual orientation of the pelves in the lateral view to obtain the maximal possible trans-sacral diameter, trans-sacral implants were virtually placed in S1, S2, and S3, with or without safe zones.
If the S1 trans-sacral corridor did not offer sufficient space for an implant, the cortical perforation of the virtual implant was located and classified as: isolated perforation in the iliac fossa, isolated extraosseous implant in the sacral ala, and extraosseous location in the iliac fossa and the sacrum.
When trans-sacral implant placement in S1 was not possible, conventional SI screws were positioned from the outer table of the ilium to the S1 vertebral body.
Statistics
The Chi-square test was used to compare nominal groups. A value of p ≤ 0.05 was considered statistically significant. Statistical analysis was performed using GraphPad QuickCalcs (https://www.graphpad.com/quickcalcs/contingency1/; accessed April 2017).
Results
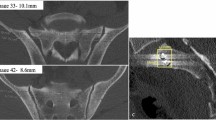
Trans-sacral implants were virtually placed in S1, S2, and S3 whenever possible. Pelves were clustered according to the maximum number of implants accommodated by S1 (Table 1). Male pelves were more often spacious enough to fit two trans-sacral implants including a safe zone in S1 (p 0.01, Fig. 1). In 15 pelves (10%), the trans-sacral corridor was not “high” (cranio-caudally) enough to accommodate a screw with a surrounding safety zone, but it was “wide” (antero-posteriorly) enough for two trans-sacral implants without safe zones. Fifty-one percent of the pelves (80 individuals; 53 EU and 27 JP, p 0.058) offered enough space in S1 for a trans-sacral implant including a surrounding safe zone. All pelves provided enough space in S2 to place a trans-sacral implant with 7.3 mm diameter. Implant positioning in S2 including a safe zone was possible in 78% (75% EU and 83% JP, p 0.25). In S3, 18% of the pelves accommodated trans-sacral implants (10% EU and 31% JP, p 0.002; Fig. 2). However, none of these had space for a safe zone in S3. Pelves without space for a safe zone in S1 more often accommodated implants with safe zones in S2 (p 0.031) and implants without safe zones in S3 (p < 0.001). In addition, pelves unable to accommodate any implant in S1 more often had space for a trans-sacral implant in S3 (p < 0.001), however they offered only non-significantly more often space for an implant with safe zone in S2 (p 0.09). Considering only implants surrounded by a safe zone, 24% pelves had space for three implants, 25% accommodated two implants, 44% had space for one implant and only 7% for none.
Of the 35 individuals lacking space in S1 for a trans-sacral implant, 69% of perforations occurred in the sacral ala as well as the iliac fossa (12 EU and 12 JP; Fig. 2). Isolated perforation of the iliac fossa was present in 8 pelves (23%; 4 EU and 4 JP; Fig. 3). Isolated osseous perforation of the sacral ala was observed in 3 pelves (8%; 2 EU and 1 JP).
There is not enough space in S1 for a trans-sacral implant, the perforation occurs in the iliac fossa. Although there is no critical anatomic structure in the iliac fossa, there is the risk of slinging along the cortex of the sacral ala. Note that the iliocortical density is the corridor’s cranial limit
Bilateral placement of an SI screw obliquely from postero-inferior to anterio-superior was possible in all pelves, irrespective of the space available for a trans-sacral implant (Fig. 2).
Discussion
Using virtual implant positioning in 3D pelvic models, this study confirmed space for trans-sacral implants in S1 including safe zones in half of pelves. There was always sufficient space in S2 to accommodate trans-sacral implants. In cases where there was no space for a trans-sacral implant in S1, virtual placement of SI screws was always possible.
The use of multiple implants in the posterior pelvic ring is encouraged by higher biomechanical stability [32, 33] as well as less screw loosening in FFP [6]. A recent study showed good clinical results with only 1% of guide wire cortical breaching using navigation and an average of 2.3 posterior implants (mostly placed trans-sacral) [11]. Half of the pelves in our study accommodated two or more trans-sacral implants surrounding a safe zone in S1 and S2 together.
Implants are commonly positioned in S1. There, one fifth of the pelves did not offer sufficient space to place a trans-sacral implant; however, half of the pelves had corridors large enough to accommodate an implant with a surrounding safe zone in S1. The remaining 27% had space for implants without a safe zone. This corresponds to manual measurement of the corridor dimensions [29]. Previous publications stated that trans-sacral implant placement in S1 is impossible for 14–41% of pelves [23–29, 34]. The highly variable anatomy of the upper sacrum results in anatomical conditions which make implant positioning critical [20, 21]. Larger trans-sacral corridors in S1 were observed with higher pelvic incidence, larger sacral curvature and more cranially positioned SI joints [21]. Morphological features were defined using radiographs and CT scans in these sacra, called “dysmorphic” [35, 36]. However, their assessment is subjective, and no single feature was found to predict a small corridor [26]. More objective criteria have been discussed; however, reliability has not yet been tested. These include the “sacral dysmorphism score,” a ratio of two angles measured in reformatted CT planes [26], and evaluation of the “lateral sacral triangle” on a lateral radiograph [37]. Radiographically, the corridor’s limits are difficult to assess, especially the upper border [38]. This is due to the inconsistent location of the limiting structure (sacral ala, upper limit of the SI joint, or the iliac fossa) [29] and the individual angulation of outlet and inlet views [39].
Alternatively, if space for trans-sacral implants in S1 is limited, implants can be positioned in S2 [40]. In this study, implant positioning was always possible in S2, with 22% not accommodating a surrounding safe zone. Previous studies have found that 52–100% of pelves offer space for trans-sacral implant positioning in S2 [24, 25, 27–29, 34]. The less space there is in S1, the larger the space in S2 [21, 24, 27, 29, 34]. In “dysmorphic” sacra or pelves not accommodating a trans-sacral implant in S1, 77–100% accommodate implants in S2 [24–26, 28–30, 34, 40, 41]. The biomechanical benefits of S2 fixation, however, are not yet clear. The sacrum rotates ventrally and caudally around a rotation center located at the S2 level [42]. Trans-sacral fixation in S2 is less stable than unilateral S1 fixation using an open book injury model [32]. Rotational stability is higher with S1 compared to S2 fixation using a spinopelvic dissociation model [16]. In cases of a transverse fracture line between S1 and S2, which occurs in two thirds of fragility fractures of the sacrum [43], there is no stabilizing benefit of using an S2 implant. Although trans-sacral fixation in S3 is possible [28, 29, 31], the biomechanical benefit is debatable due to the caudal position within the SI joint. In addition, since the use of trans-sacral implants in S2 and S3 approaches the sciatic notch, there may be an increased risk of injuring the superior gluteal neurovascular bundle.
Cortical perforations by the implant in S1 were located in the sacral ala, the region of the SI joint, and the iliac fossa. This corresponds to the variable location of the cranial limit of the trans-sacral corridor in S1 [29]. Thus, not only the sacrum, but also the iliac fossa determines availability of the trans-sacral corridor S1. When perforating the iliac fossa, the risk of continued sliding along the sacral ala with the drill and injury to nerve root L5 may be substantial. Intraoperatively, the iliocortical density is often taken as the upper limit for implant positioning when using image intensifier [35]. However, this radiographic structure was found to represent the cortex of the iliac fossa [44]. Reliance on that structure may, therefore, be dangerous [45], especially in pelves where cortical perforation occurred in the sacral ala. In the light of the often critical corridor dimensions and the difficulty of intraoperative corridor visualization, a detailed preoperative planning is required. As the maximal diameter is in the anterior part of the oval-shaped corridor [29], we adapt a coronal plane in the pelvic CT to the sacral inclination of S1 and measure the corridor’s dimension in this coronal and the axial view. The addition of navigation techniques my help intraoperatively [11, 46], however, clinical studies are inconclusive about their superiority, favoring 3D compared to 2D navigation techniques [47–50].
When trans-sacral implantation was not possible in S1, there was always enough space for bilateral SI screws. The corridor for SI screws in sacra with limited space is oriented from postero-caudally to antero-cranially [22, 36, 51, 52]. As SI screws aim for the sacral body of S1, they pass only one isthmus. This leads to the “vestibule concept” [41], where the corridor has the form of an hourglass, with the narrow and limiting diameter formed by the sacra ala and the S1 foramen [22, 41, 53].
This study was limited by the exclusion of sacralized L5 or lumbarized S1 vertebrae; only sacra with five fused sacral vertebrae were studied. Sacra with a lumbosacral transitional vertebra (LSTV) are known to have larger trans-sacral corridors in the first sacral vertebrae when S1 is defined as the vertebra below the LSTV [25]. “Dysmorphic” sacral segments have attributes of LSTV and are reported to have small or absent trans-sacral corridors in S1, with large S2 corridors [30, 40]. Another limitation of this study may be that the outer cortical border was segmented; thus, in reality, the corridor may be slightly smaller due to cortical thickness. Our study collective was comprised only of individuals without pelvic fracture. Fracture displacement impacts trans-sacral corridor size and notably decreases the corridor’s diameter [54]. In contrast to our study, pelves are typically assessed intraoperatively via radiographs or navigation. Implant positioning is more difficult using these indirect means of anatomic visualization. One additional limitation is that a single observer carried out the virtual implant positioning.
Conclusion
Safe trans-sacral implantation in S1 was only possible in half of the individuals studied due to variable anatomy. In the others, S2 could be a useful and safe alternative. Multiple trans-sacral implants are mostly possible, in half of the pelves there was space for two or more trans-sacral implants with a safe zone. The iliocortical density can be used as an anatomic landmark for the upper limit of trans-sacral corridors, but the sacral ala may be lower and thus, at risk for implant perforation. Therefore, thorough individual preoperative planning using CT data is mandatory to assess pelves amenable to trans-sacral implants. Intraoperatively, 3D navigation may help in safe implant positioning. When using an oblique trajectory from postero-caudal to antero-cranial, SI screws are a safe alternative to trans-sacral implants.
References
Andrich S, Haastert B, Neuhaus E, Neidert K, Arend W, Ohmann C, Grebe J, Vogt A, Jungbluth P, Rösler G, Windolf J, Icks A (2015) Epidemiology of pelvic fractures in germany: considerably high incidence rates among older people. PLoS ONE 10:e0139078. https://doi.org/10.1371/journal.pone.0139078
Kannus P, Parkkari J, Niemi S, Sievänen H (2015) Low-trauma pelvic fractures in elderly finns in 1970–2013. Calcif Tissue Int 97:577–580. https://doi.org/10.1007/s00223-015-0056-8
Rommens PM, Wagner D, Hofmann A (2017) Fragility fractures of the pelvis. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.16.00057
Routt ML Jr, Simonian PT, Mills WJ (1997) Iliosacral screw fixation: early complications of the percutaneous technique. J Orthop Trauma 11:584–589
Reuther G, Röhner U, Will T, Dehne I, Petereit U (2014) CT-guided screw fixation of vertical sacral fractures in local anaesthesia using a standard CT. RöFo Fortschritte Auf Dem Geb Röntgenstrahlen Nukl 186:1134–1139. https://doi.org/10.1055/s-0034-1366605
Eckardt H, Egger A, Hasler RM, Zech CJ, Vach W, Suhm N, Morgenstern M, Saxer F (2017) Good functional outcome in patients suffering fragility fractures of the pelvis treated with percutaneous screw stabilisation: assessment of complications and factors influencing failure. Injury 48:2717–2723. https://doi.org/10.1016/j.injury.2017.11.002
Wagner D, Ossendorf C, Gruszka D, Hofmann A, Rommens PM (2015) Fragility fractures of the sacrum: how to identify and when to treat surgically? Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc 41:349–362. https://doi.org/10.1007/s00068-015-0530-z
Kim J-W, Oh C-W, Oh J-K, Kyung H-S, Park K-H, Yoon S-D, Yoon S-H (2016) The incidence of and factors affecting iliosacral screw loosening in pelvic ring injury. Arch Orthop Trauma Surg 136:921–927. https://doi.org/10.1007/s00402-016-2471-3
Mehling I, Hessmann MH, Rommens PM (2012) Stabilization of fatigue fractures of the dorsal pelvis with a trans-sacral bar. Operative technique and outcome. Injury 43:446–451. https://doi.org/10.1016/j.injury.2011.08.005
Rommens PM, Wagner D, Hofmann A (2012) Surgical management of osteoporotic pelvic fractures: a new challenge. Eur J Trauma Emerg Surg Off Publ Eur Trauma Soc 38:499–509. https://doi.org/10.1007/s00068-012-0224-8
Balling H (2019) Additional sacroplasty does not improve clinical outcome in minimally invasive navigation-assisted screw fixation procedures for nondisplaced insufficiency fractures of the sacrum. Spine (Phila Pa 1976) 44(8):534–542. https://doi.org/10.1097/BRS.0000000000002899
Wagner D, Hofmann A, Kamer L, Sawaguchi T, Richards RG, Noser H, Gruszka D, Rommens PM (2018) Fragility fractures of the sacrum occur in elderly patients with severe loss of sacral bone mass. Arch Orthop Trauma Surg 138:971–977. https://doi.org/10.1007/s00402-018-2938-5
Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Rommens PM (2016) Sacral bone mass distribution assessed by averaged three-dimensional CT models: implications for pathogenesis and treatment of fragility fractures of the sacrum. J Bone Jt Surg Am 98:584–590. https://doi.org/10.2106/JBJS.15.00726
Lattauschke A, Klauke F, Ullrich BW, Hofmann GO, Mendel T (2017) Course of operative treatment of a sacral insufficiency fracture: successful or serious treatment? Unfallchirurg 120:890–895. https://doi.org/10.1007/s00113-017-0403-5
Ricci P-L, Maas S, Kelm J, Gerich T (2018) Finite element analysis of the pelvis including gait muscle forces: an investigation into the effect of rami fractures on load transmission. J Exp Orthop. https://doi.org/10.1186/s40634-018-0151-7
Zhao Y, Li J, Wang D, Liu Y, Tan J, Zhang S (2012) Comparison of stability of two kinds of sacro-iliac screws in the fixation of bilateral sacral fractures in a finite element model. Injury 43:490–494. https://doi.org/10.1016/j.injury.2011.12.023
Tabaie SA, Bledsoe JG, Moed BR (2013) Biomechanical comparison of standard iliosacral screw fixation to transsacral locked screw fixation in a type C zone II pelvic fracture model. J Orthop Trauma 27:521–526. https://doi.org/10.1097/BOT.0b013e3182781102
Heydemann J, Hartline B, Gibson ME, Ambrose CG, Munz JW, Galpin M, Achor TS, Gary JL (2016) Do transsacral–transiliac screws across uninjured sacroiliac joints affect pain and functional outcomes in trauma patients? Clin Orthop Relat Res 474:1417–1421. https://doi.org/10.1007/s11999-015-4596-z
Höch A, Pieroh P, Henkelmann R, Josten C, Böhme J (2017) In-screw polymethylmethacrylate-augmented sacroiliac screw for the treatment of fragility fractures of the pelvis: a prospective, observational study with 1-year follow-up. BMC Surg 17:132. https://doi.org/10.1186/s12893-017-0330-y
Wagner D, Kamer L, Rommens PM, Sawaguchi T, Richards RG, Noser H (2014) 3D statistical modeling techniques to investigate the anatomy of the sacrum, its bone mass distribution, and the trans-sacral corridors. J Orthop Res Off Publ Orthop Res Soc 32:1543–1548. https://doi.org/10.1002/jor.22667
Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Hofmann A, Rommens PM (2017) Morphometry of the sacrum and its implication on trans-sacral corridors using a CT data-based 3D statistical model. Spine J Off J N Am Spine Soc 17:1141–1147. https://doi.org/10.1016/j.spinee.2017.03.023
Day CS, Prayson MJ, Shuler TE, Towers J, Gruen GS (2000) Transsacral versus modified pelvic landmarks for percutaneous iliosacral screw placement—a computed tomographic analysis and cadaveric study. Am J Orthop Belle Mead NJ 29:16–21
Hasenboehler EA, Stahel PF, Williams A, Smith WR, Newman JT, Symonds DL, Morgan SJ (2011) Prevalence of sacral dysmorphia in a prospective trauma population: Implications for a “safe” surgical corridor for sacro-iliac screw placement. Patient Saf Surg 5:8. https://doi.org/10.1186/1754-9493-5-8
Gras F, Hillmann S, Rausch S, Klos K, Hofmann GO, Marintschev I (2015) Biomorphometric analysis of ilio-sacro-iliacal corridors for an intra-osseous implant to fix posterior pelvic ring fractures. J Orthop Res Off Publ Orthop Res Soc 33:254–260. https://doi.org/10.1002/jor.22754
Lee JJ, Rosenbaum SL, Martusiewicz A, Holcombe SA, Wang SC, Goulet JA (2015) Transsacral screw safe zone size by sacral segmentation variations. J Orthop Res Off Publ Orthop Res Soc 33:277–282. https://doi.org/10.1002/jor.22739
Kaiser SP, Gardner MJ, Liu J, Routt MLC, Morshed S (2014) Anatomic determinants of sacral dysmorphism and implications for safe iliosacral screw placement. J Bone Jt Surg Am 96:e120. https://doi.org/10.2106/JBJS.M.00895
Mendel T, Noser H, Kuervers J, Goehre F, Hofmann GO, Radetzki F (2013) The influence of sacral morphology on the existence of secure S1 and S2 transverse bone corridors for iliosacroiliac screw fixation. Injury 44:1773–1779. https://doi.org/10.1016/j.injury.2013.08.006
Radetzki F, Wohlrab D, Goehre F, Noser H, Delank KS, Mendel T (2014) Anatomical conditions of the posterior pelvic ring regarding bisegmental transverse sacroiliac screw fixation: a 3D morphometric study of 125 pelvic CT datasets. Arch Orthop Trauma Surg 134:1115–1120. https://doi.org/10.1007/s00402-014-2022-8
Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Uesugi M, Ossendorf C, Rommens PM (2017) Critical dimensions of trans-sacral corridors assessed by 3D CT models. Relevance for implant positioning in fractures of the sacrum. J Orthop Res Off Publ Orthop Res Soc 35:2577–2584. https://doi.org/10.1002/jor.23554
Conflitti JM, Graves ML, Chip Routt ML Jr (2010) Radiographic quantification and analysis of dysmorphic upper sacral osseous anatomy and associated iliosacral screw insertions. J Orthop Trauma 24:630–636. https://doi.org/10.1097/BOT.0b013e3181dc50cd
Eastman JG, Adams MR, Frisoli K, Chip Routt ML (2018) Is S3 a viable osseous fixation pathway. J Orthop Trauma 32:93–99. https://doi.org/10.1097/BOT.0000000000001036
Zhang L, Peng Y, Du C, Tang P (2014) Biomechanical study of four kinds of percutaneous screw fixation in two types of unilateral sacroiliac joint dislocation: a finite element analysis. Injury 45:2055–2059. https://doi.org/10.1016/j.injury.2014.10.052
Jazini E, Klocke N, Tannous O, Johal HS, Hao J, Salloum K, Gelb DE, Nascone JW, Belin E, Hoshino CM, Hussain M, OʼToole RV, Bucklen B, Ludwig SC (2017) Does lumbopelvic fixation add stability? A cadaveric biomechanical analysis of an unstable pelvic fracture model. J Orthop Trauma 31:37–46. https://doi.org/10.1097/BOT.0000000000000703
Gras F, Gottschling H, Schröder M, Marintschev I, Hofmann GO, Burgkart R (2016) Transsacral osseous corridor anatomy is more amenable to screw insertion in males: a biomorphometric analysis of 280 pelves. Clin Orthop 474:2304–2311. https://doi.org/10.1007/s11999-016-4954-5
Routt ML Jr, Simonian PT, Agnew SG, Mann FA (1996) Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: a cadaveric and clinical study. J Orthop Trauma 10:171–177
Miller AN, Routt MLC Jr (2012) Variations in sacral morphology and implications for iliosacral screw fixation. J Am Acad Orthop Surg 20:8–16. https://doi.org/10.5435/JAAOS-20-01-008
Mendel T, Noser H, Wohlrab D, Stock K, Radetzki F (2011) The lateral sacral triangle—a decision support for secure transverse sacroiliac screw insertion. Injury 42:1164–1170
Kim JW, Quispe JC, Hao J, Herbert B, Hake M, Mauffrey C (2016) Fluoroscopic views for a more accurate placement of iliosacral screws: an experimental study. J Orthop Trauma 30:34–40. https://doi.org/10.1097/BOT.0000000000000426
Graves ML, Routt MLC Jr (2011) Iliosacral screw placement: are uniplanar changes realistic based on standard fluoroscopic imaging? J Trauma 71:204–208. https://doi.org/10.1097/TA.0b013e31821e842a (discussion 208)
Gardner MJ, Morshed S, Nork SE, Ricci WM, Chip Routt ML Jr (2010) Quantification of the upper and second sacral segment safe zones in normal and dysmorphic sacra. J Orthop Trauma 24:622–629. https://doi.org/10.1097/BOT.0b013e3181cf0404
Carlson DA, Scheid DK, Maar DC, Baele JR, Kaehr DM (2000) Safe placement of S1 and S2 iliosacral screws: the “vestibule” concept. J Orthop Trauma 14:264–269
Hammer N, Steinke H, Lingslebe U, Bechmann I, Josten C, Slowik V, Böhme J (2013) Ligamentous influence in pelvic load distribution. Spine J Off J N Am Spine Soc 13:1321–1330. https://doi.org/10.1016/j.spinee.2013.03.050
Linstrom NJ, Heiserman JE, Kortman KE, Crawford NR, Baek S, Anderson RL, Pitt AM, Karis JP, Ross JS, Lekovic GP, Dean BL (2009) Anatomical and biomechanical analyses of the unique and consistent locations of sacral insufficiency fractures. Spine 34:309–315. https://doi.org/10.1097/BRS.0b013e318191ea01
Wong-Chung J, Jamsheer N, Nabar U, Aradi AJ (1997) Two parallel linear densities on lateral radiographs of the lumbosacral spine: neither iliopectineal lines nor basis ossis sacri. Br J Radiol 70:58–61
Day AC, Stott PM, Boden RA (2007) The accuracy of computer-assisted percutaneous iliosacral screw placement. Clin Orthop 463:179–186
Takao M, Hamada H, Sakai T, Sugano N (2019) Factors influencing the accuracy of iliosacral screw insertion using 3D fluoroscopic navigation. Arch Orthop Trauma Surg 139(2):189–195. https://doi.org/10.1007/s00402-018-3055-1
Zwingmann J, Hauschild O, Bode G, Südkamp NP, Schmal H (2013) Malposition and revision rates of different imaging modalities for percutaneous iliosacral screw fixation following pelvic fractures: a systematic review and meta-analysis. Arch Orthop Trauma Surg 133:1257–1265. https://doi.org/10.1007/s00402-013-1788-4
Teo AQA, Yik JH, Jin Keat SN, Murphy DP, O’Neill GK (2018) Accuracy of sacroiliac screw placement with and without intraoperative navigation and clinical application of the sacral dysmorphism score. Injury. https://doi.org/10.1016/j.injury.2018.05.027
Richter PH, Gebhard F, Dehner C, Scola A (2016) Accuracy of computer-assisted iliosacral screw placement using a hybrid operating room. Injury 47:402–407. https://doi.org/10.1016/j.injury.2015.11.023
Matityahu A, Kahler D, Krettek C, Stöckle U, Grutzner PA (2014) Three-dimensional navigation is more accurate than two-dimensional navigation or conventional fluoroscopy for percutaneous sacroiliac screw fixation in the dysmorphic sacrum: a randomized multicenter study. J Orthop Trauma 28:4
Morshed S, Choo K, Kandemir U, Kaiser SP (2015) Internal fixation of posterior pelvic ring injuries using iliosacral screws in the dysmorphic upper sacrum. JBJS Essent Surg Tech 5:e3. https://doi.org/10.2106/JBJS.ST.N.00006
Bastian JD, Jost J, Cullmann JL, Aghayev E, Keel MJB, Benneker LM (2015) Percutaneous screw fixation of the iliosacral joint: optimal screw pathways are frequently not completely intraosseous. Injury 46:2003–2009. https://doi.org/10.1016/j.injury.2015.06.044
Mendel T, Appelt K, Kuhn P, Suhm N (2008) Bony sacroiliac corridor. A virtual volume model for the accurate insertion of transarticular screws. Unfallchirurg 111:19–26. https://doi.org/10.1007/s00113-007-1386-4
Reilly MC, Bono CM, Litkouhi B, Sirkin M, Behrens FF (2003) The effect of sacral fracture malreduction on the safe placement of iliosacral screws. J Orthop Trauma 17:88–94
Acknowledgements
We thank Thomas Heldstab for his technical assistance. Some data based on previous research, which has been co-funded by the TK System of the AO Foundation, Davos, Switzerland, and by Depuy Synthes company, Zuchwil, Switzerland. We would like to thank Depuy Synthes to provide us with a virtual screw template.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Wagner, Author Kamer, Author Sawaguchi, Author Noser, Author Uesugi, Author Baranowski, Author Gruszka, and Author Rommens declare that they have no conflict of interest.
Ethical approval
The database of the AO Research Institute Davos is registered at the “Eidgenössischer Öffentlichkeits- und Datenschutzbeauftragter” (EDÖP, Bern, Switzerland, No. 200700006). All patients agreed to anonymous use of their CT data for research purposes. For the Japanese data, ethical approval was given by the institutional review board of the Tsukuba Medical Center (No. 2012-013).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wagner, D., Kamer, L., Sawaguchi, T. et al. Space available for trans-sacral implants to treat fractures of the pelvis assessed by virtual implant positioning. Arch Orthop Trauma Surg 139, 1385–1391 (2019). https://doi.org/10.1007/s00402-019-03204-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-019-03204-9