Abstract
Introduction
Patients suffering from osteoporosis-associated fragility fractures of the sacrum (FFS; also termed sacral insufficiency fractures) are increasingly observed. They have typical fracture patterns with fracture lines located in the sacral ala. When treating these patients operatively, iliosacral screw loosening is not uncommon. We aimed to study the sacral bone mass in patients presenting with a FFS using 3D statistical models.
Materials and methods
3D models of averaged Hounsfield units (HU) were generated based on CT scans from 13 patients with a unilateral FFS (mean age 79.6 years; 11 females, 2 males). The control group without fractures consisted of 28 males and 32 females (mean age of 68.3 years). A virtual bone probe along the trans-sacral corridors S1 and S2 was taken.
Results
The bone mass distribution in the fractured sacra was similar to the control group, however, with overall lower HU. Large zones of negative HU were located in the sacral ala. In the fractured sacra, the HU in the sacral ala was significantly lower on the non-injured side when comparing to the fractured side (p < 0.001) as well as compared to the non-fractured group (p < 0.001). Low bone mass was observed in sacral body S1 (40 HU) and S2 (20 HU).
Conclusions
The extensive area of negative HU may explain the fracture location in the sacral ala. The low HU in the sacral bodies advocates the use of trans-sacral implants or augmented iliosacral screws to enhance the strength of fracture fixation. The increased HU in the fractured ala could be explained by fracture-asssociated hemorrhage and can be used as a diagnostic tool.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fragility fractures of the pelvis (FFP) and fragility fractures of the sacrum (FFS, also termed as sacral insufficiency fractures [1]) have become more prevalent [2] due to an increased elderly population and a continuous rise of people affected by osteoporosis [3]. There is only scarce information about the incidence of isolated FFS. They were found in 1.8% of women aged > 55 years presenting with lower back pain [4]. However, they are often part of FFP; a fracture of the posterior pelvic ring that is found in 82–98% of elderly presenting with an anterior pelvic fracture [5, 6]. FFS exhibit distinct patterns with the fracture lines being located uni- or bilaterally in the sacral ala, corresponding to Denis zone I [7]. Sometimes, there is a transverse fracture line, creating an H-type fracture pattern [1, 5, 8]. This is similar to the spinopelvic dissociation described by Roy-Camille et al. [9], but with different trauma mechanism and clinical circumstances [10]. H-type fractures have been found in 21% of FFS in a series including 245 patients with a FFP [5]. A possible explanation for these “unique and consistent locations” [8] are the large areas of low bone mass identified in the sacral ala extending from S1 cranially to S3 caudally. Furthermore, the bone mass is markedly lower in S2 compared to S1, which may explain the occurrence of an associated transverse fracture between S1 and S2. These results were demonstrated by a previous study using a 3D statistical modeling approach in individuals without fractures [11].
The primary therapeutic approach in non-displaced FFS is conservative treatment with pain-adapted mobilization and analgesic medication. In displaced fractures of the sacrum and in cases of long-standing pain, however, surgical stabilization is recommended. To minimize the surgical trauma in this frail patient group, the least invasive approach is preferred [1]. Nevertheless, when fixing sacral fractures with percutaneous iliosacral screws in elderly, loosening with backing out is seen in up to 14% [1, 12]. This can be prevented using cement augmentation [13] or trans-sacral implants [1, 14, 15].
Regarding the unique fracture pattern and challenges in the operative fixation of FFS, the goal of this study was to analyze the sacral bone mass in patients with a non-displaced FFS, using a 3D statistical modeling approach [16]. We further compared the data with the bone mass of elderly without sacral fracture [11]. We hypothesize that patients with sacral fractures exhibit a lower bone mass, especially in the sacral ala.
Materials and methods
A retrospective series of anonymized pelvic CT scans from 13 patients with a unilateral non-displaced FFS older than 60 years was included. Patients with bilateral or displaced sacral fractures, and such with signs of fracture consolidation or non-union were excluded on beforehand. The scans were acquired with following CT machines and protocols: nine with Philips Brilliance 64 (5 with protocol B, 4 with protocol D), one with Philips iCT 256 (protocol B), two with Siemens Somatom Zoom (B60s protocol), and one with Siemens Sensation 64 (B60s protocol). All scans were without intravenous contrast medium. A tube voltage of 120 kVp was applied. The mean voxel resolution in x and y direction was 0.7 mm (± 0.12), and 1.2 mm (± 0.6) in z direction.
60 individuals served as the control group with an age ≥ 60 years without fractures, which were part of a previous study [11]. They had a mean age of 68.3 years (± 5.3), 28 were males and 32 females. CT scanners SOMATOM Sensation 64 and SOMATOM Definition (Siemens AG, Erlangen, Germany) were employed. B45f convolution kernel was used, and a tube voltage of 120 kVp applied. Intravenous contrast was used in all the individuals.
To quantify the general bone mass of each patient, the average Hounsfield units (HU) in a circular region of interest in the mid-sagittal and mid-transverse plane of L5 was calculated using synedra View Personal (Version 1.1.0.1, synedra information technologies GmbH, Innsbruck, Austria) [11].
A 3D model of each sacrum was generated using Amira software (Amira 5.4.1, Visage Imaging, Inc., Berlin, Germany) as described previously [16]. The voxels of sacra with fractures on the right side were mirrored at the mid-sagittal plane to simulate fractures on the left side. Hence, after processing, the fractures were located on the left side in all sacra. The homologous voxels were averaged within the sacral mean shape [16].
To demonstrate sites of low bone mass, negative HU were segmented and visualized within the mean shape. A virtual bone probe with a diameter of 7 mm was placed centrally along trans-sacral corridors S1 and S2. These values were visualized using Excel (Version 2010, Microsoft Corporation, Redmond, CA, USA) [11].
To compare the values of the fractured and non-fractured side, the respective difference of HU along the virtual bone probe in S1 and S2 was calculated. Therefore, the values were mirrored at the sacral midline. The alar region was determined for S1 by the region with less than 50 HU in the control group and for S2 by negative HU in the control group.
Statistical analysis
Normal distribution of continuous data was tested using the Kolmogorov–Smirnov test. After confirming a significant probability of a normal distribution, we calculated the descriptive statistics and compared different groups using the unpaired t test and SPSS for Windows (Version 19, 2010, IBM Corp., Armonk, NY, USA). A p value of < 0.05 was considered as statistically significant.
Results
The mean age of the 13 patients included was 79.6 years (± 9.2, 61–95), 11 were female and 2 male. Three patients sustained a right-sided and ten a left-sided fracture of the sacral ala. This was accompanied by an ipsilateral fracture of the anterior pelvic ring in 10 patients, and one patient had bilateral anterior pelvic ring fractures (all FFP Type IIc [5]). Two patients presented with an isolated FFS (FFP Type IIa [5]).
The mean HU in L5 were significantly lower in the group with fractured sacra (mean 49 HU ± 31.7, range 10–115) when comparing to the non-fractured control group (mean 124 HU ± 51.1, range 14–259; p < 0.001; Fig. 1), indicating a lower vertebral bone mass.
The bone mass along the trans-sacral corridors was markedly lower in patients with a FFS when comparing to the control group (Fig. 2). The overall bone mass distribution showed a similar pattern, except to the comparably higher HU in the sacral ala at the fractured side (Denis zone I [7]). In the sacral body S1 (Denis zone III), the averaged values were as low as 40 HU with peaks of moderate HU being at the transition of the sacral body to the zone of the sacral (Denis zone II). In sacral body S2, the averaged values were only about 20 HU. Also, cortical bone at the auricular surfaces of the sacrum demonstrated lower HU in S1 and S2 when compared with non-fractured sacra.
Virtual bone probe along trans-sacral corridor S1 (a) and S2 (b). The fractured left side (in the figure right) has lower HU in the sacral ala comparing to the non-injured side. Overall, the values in the fractured sacra are lower than in the non-fractured control group. The HU in the sacral bodies of the fractured group are alarmingly low
The averaged bone model revealed a similar 3D distribution of negative HU when compared with the previously published data of bone mass in individuals with low general mass (< 100 HU in L5) without sacral fracture [11] (Fig. 3). The area of negative HU in the non-injured sacral ala was slightly larger with more negative HU in the sacral bodies. The fractured side, however, revealed a smaller area of negative HU.
Anterior semitransparent view of the sacral mean shape with negative HU in red. The fractured side is left, in the figure hence on the right. Extensive area of negative HU (in red) on the non-fractured side from S1 down to S3 with some extension in direction of sacral body S2. On the fractured left side (right) less negative HU especially in S1
The HU of the averaged model along the trans-sacral corridors S1 and S2 were assessed (Table 1). We found that the fractured side had significantly higher averaged HU in the sacral ala compared to the non-injured side (in S1 10 HU ± 8.3 vs. − 33 HU ± 15.1; in S2 − 17 HU ± 9.5 vs. − 53 HU ± 16.1; both p < 0.001). The HU of the ala on the fractured side were similar to the values in the control group (in S1 10 HU ± 8.3 vs. 20 HU ± 17.1, p 0.12; in S2 − 17 HU ± 9.5 vs. − 18 HU ± 10.3; p 0.76); however, the non-fractured side of the patients with FFS had significantly lower HU than the control group (in S1 − 33 HU ± 15.1 vs. 20 HU ± 17.1; in S2 − 53 HU ± 16.1 vs. − 18 HU ± 10.3; both p < 0.001).
Discussion
The sacra of elderly suffering from a unilateral FFS demonstrated an overall lower bone mass; the most markedly decrease was in the sacral bodies. The lateral limits of the sacral body S1 demonstrated moderate values of bone mass. Compared to the non-injured side, the gray values in the fractured zone were significantly higher.
The overall distribution of negative HU and bone mass along trans-sacral corridors in patients with FFS was similar to non-fractured sacra as reported previously [11]. The fractured sacra, however, revealed not only generally lower values, but there was a large difference in bone mass in the sacral bodies [17]. The values in the sacral bodies of patients with FFS were very low: approximately 40 HU in S1 and 20 HU in S2. This is clinically important as the threaded part of IS screws inserted for fracture fixation is located in the sacral body [18]. The stability achieved by these screws depends on a good holding power to exert compression on the fracture site. The low values of bone mass measured in the sacral bodies of the 13 patients with FFS may explain the higher risk of loosening of IS screws seen in osteoporotic patients [1, 12]. This may advocate the use of trans-sacral implants, as we suppose, that they do not depend on the sacral bone mass but on the density of iliac cortical bone [15, 19, 20]. However, the use of trans-sacral implants can be limited by the variable anatomy of the upper sacrum [21, 22]. Alternatively, this finding may support cement augmentation of iliosacral screws [13]. Their use led to significant pain reduction with a low complication rate [23]. Biomechanically, cement augmentation failed to show significant construct strengthening [24,25,26]. However, the mode of failure changed from screw backing out in non-augmented screws to breaching of the iliac cortex when using augmentation [26]. This may be caused by a better anchorage in the sacral body leading to less subsidence at the screw tip [25]. The mode of augmentation, before screw insertion or through perforations at the screw tip, did not reveal different pull-out strength [27].
The extent of negative HU in the non-injured ala of fractured sacra, representing mainly fatty bone marrow [28], may explain the typical pattern of FFS, which often are located in the sacral ala (corresponding to Denis I [7]) [1, 5, 8, 11].
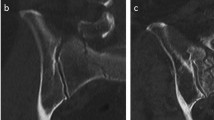
The higher HU observed in the fractured sacral ala could be explained by fracture-associated intraosseous hemorrhage, crush zones with increased intertrabecular interdentation, or also beginning, radiologically non-visible fracture consolidation. Following Henes et al. [29], a side difference of 36 HU in the sacral ala assessed by CT indicates an occult fracture located in the sacral ala confirmed by MRI. This method had a sensitivity of 79% and a specificity of 100% (Fig. 4). In our collective, similar values were observed with a difference between the fractured and non-injured side of 43 HU in S1 and 36 HU in S2. This may be a valuable diagnostic tool in the diagnostics of FFP and FFS. When a CT scan is performed in patients with sacral pain without evidence of a sacral fracture, HU measured in the sacral ala on both sides could confirm or exclude an occult sacral ala fracture [1].
82 years old female presenting with a isolated anterior pelvic ring fracture on the left side. As there was severe lower lumbar pain without evidence of a fracture in the CT (a), an MRI was obtained. This showed a bone bruise in the left sacral ala (b). On the left side in S1, there was an increase of 55 Hounsfield Units (a) as an indirect sign of an occult fracture
Sacroplasty is used in some centers for pain relief in FFS by injecting PMMA (polymethylmethacrylate) cement into the fractured sacral ala [30]. Sacroplasty is supposed “to restore structural stability to the weakened bone” [30]. The biomechanical data supporting this, however, is controversial [31,32,33]. When considering the large alar region containing negative HU in the fractured group, representing fatty bone marrow [28] (visualized in Fig. 3), as large cement volumes as 6 ml are used to fill the alar void [34].
We hypothesize that the moderate peaks at the outer limits of the sacral body S1 are trabeculae extending from the cranial endplate of S1 to the anterior sacral cortex [35]. They may persist with major bone loss similar to principal compressive trabeculae in the proximal femur [36], which would demonstrate their importance in weight transmission.
We demonstrated a noticeable lower sacral and vertebral bone mass in injured patients comparing to individuals without a FFS. As osteoporosis-associated fragility fractures are more prevalent in elderly and female gender [3], the non-fractured control group was significantly younger with more males. Hence, a lower general bone mass in patients with FFS was expected to some extent and confirmed in the sacrum as well as in L5. Similarly, in elderly with a fracture of the proximal humerus, a lower local bone mass was demonstrated in the contralateral humerus compared to a collective without fractures [37].
The limitations of this study were the use of multiple CT machines, which may have an influence on the level and calibration of HU. Using a tube current of 120 kV, a minor difference between scanners was shown for water (0–8 HU) and for diverse phantom measurements (between 0 and 10 HU and 0–18 HU) [38]. However, in cortical or cancellous bone, this was not yet demonstrated. The comparison with the control group is limited by the use of iv-contrast medium, but the studies remain controversial [39]. As mentioned above, the control group was not matched for gender or age. Another limitation was the comparable low number of patients with fractures, because we excluded displaced sacral fractures, patients with bilateral sacral fractures, and such with visible signs of fracture consolidation or non-union. We used clinical CT scans without calibration to a bone mineral density phantom, hence formal diagnosis of patients with osteoporosis or osteopenia was not possible. We consider clinical CT’s as relevant as their measured bone mass correlate with bone mineral density [40]. The use of clinical CT scans in the diagnosis of osteoporosis was advocated recently [41, 42]. Due to a lack of therapeutic consequence, patients in our center did not undergo a MRI, hence in our fractured patients, a contralateral occult fracture was not possible to rule out [1].
We showed that also cortical bone of patients with FFS had lower HU. However, with the resolution of clinical CT’s, it is not possible to differentiate a progressed trabecularization of the cortical bone from a cortical thinning [43]. Further research could aim at studying the thickness of cortical bone in sacra, especially in such with a FFS.
Conclusions
Sacra with non-displaced FFS demonstrated low bone mass especially in the alar region. The values in the sacral bodies were significantly lower than the values measured in a control group without FFS. These findings support the use of trans-sacral implants for the treatment of FFS or FFP, as the stability of these constructs does less depend on the screw purchase in the sacral body comparing to iliosacral screws. Alternatively, augmentation of iliosacral screws could be considered. The higher HU in the fractured area compared to the non-fractured side helps to diagnose occult fractures in the CT.
References
Wagner D, Ossendorf C, Gruszka D, Hofmann A, Rommens PM (2015) Fragility fractures of the sacrum: how to identify and when to treat surgically? Eur J Trauma Emerg Surg 41:349–362. https://doi.org/10.1007/s00068-015-0530-z
Sullivan MP, Baldwin KD, Donegan DJ, Mehta S, Ahn J (2014) Geriatric fractures about the hip: divergent patterns in the proximal femur, acetabulum, and pelvis. Orthopedics 37:151–157. https://doi.org/10.3928/01477447-20140225-50
Gauthier A, Kanis JA, Jiang Y, Martin M, Compston JE, Borgström F, Cooper C, McCloskey EV (2011) Epidemiological burden of postmenopausal osteoporosis in the UK from 2010 to 2021: estimations from a disease model. Arch Osteoporos 6:179–188. https://doi.org/10.1007/s11657-011-0063-y
Weber M, Hasler P, Gerber H (1993) Insufficiency fractures of the sacrum. Twenty cases and review of the literature. Spine 18:2507–2512
Rommens PM, Hofmann A (2013) Comprehensive classification of fragility fractures of the pelvic ring: recommendations for surgical treatment. Injury 44:1733–1744. https://doi.org/10.1016/j.injury.2013.06.023
Scheyerer MJ, Osterhoff G, Wehrle S, Wanner GA, Simmen H-P, Werner CML (2012) Detection of posterior pelvic injuries in fractures of the pubic rami. Injury 43:1326–1329. https://doi.org/10.1016/j.injury.2012.05.016
Denis F, Davis S, Comfort T (1988) Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res 227:67–81
Linstrom NJ, Heiserman JE, Kortman KE, Crawford NR, Baek S, Anderson RL, Pitt AM, Karis JP, Ross JS, Lekovic GP, Dean BL (2009) Anatomical and biomechanical analyses of the unique and consistent locations of sacral insufficiency fractures. Spine 34:309–315. https://doi.org/10.1097/BRS.0b013e318191ea01
Roy-Camille R, Saillant G, Gagna G, Mazel C (1985) Transverse fracture of the upper sacrum. Suicidal jumper’s fracture. Spine 10:838–845
Rommens PM, Wagner D, Hofmann A (2017) Fragility fractures of the pelvis. JBJS Rev. https://doi.org/10.2106/JBJS.RVW.16.00057
Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Rommens PM (2016) Sacral bone mass distribution assessed by averaged three-dimensional CT models: implications for pathogenesis and treatment of fragility fractures of the sacrum. J Bone Jt Surg Am 98:584–590. https://doi.org/10.2106/JBJS.15.00726
Reuther G, Röhner U, Will T, Dehne I, Petereit U (2014) CT-guided screw fixation of vertical sacral fractures in local anaesthesia using a standard CT. Rofo 186:1134–1139. https://doi.org/10.1055/s-0034-1366605
Wähnert D, Raschke MJ, Fuchs T (2013) Cement augmentation of the navigated iliosacral screw in the treatment of insufficiency fractures of the sacrum. A new method using modified implants. Int Orthop 37:1147–1150. https://doi.org/10.1007/s00264-013-1875-8
Beaulé PE, Antoniades J, Matta JM (2006) Trans-sacral fixation for failed posterior fixation of the pelvic ring. Arch Orthop Trauma Surg 126:49–52. https://doi.org/10.1007/s00402-005-0069-2
Mehling I, Hessmann MH, Rommens PM (2012) Stabilization of fatigue fractures of the dorsal pelvis with a trans-sacral bar. Operative technique and outcome. Injury 43:446–451. https://doi.org/10.1016/j.injury.2011.08.005
Wagner D, Kamer L, Rommens PM, Sawaguchi T, Richards RG, Noser H (2014) 3D statistical modeling techniques to investigate the anatomy of the sacrum, its bone mass distribution, and the trans-sacral corridors. J Orthop Res 32:1543–1548. https://doi.org/10.1002/jor.22667
Schönenberg D, Guggenberger R, Frey D, Pape H-C, Simmen H-P, Osterhoff G (2018) CT-based evaluation of volumetric bone density in fragility fractures of the pelvis—a matched case–control analysis. Osteoporos Int 29:459–465. https://doi.org/10.1007/s00198-017-4307-6
Routt ML Jr, Kregor PJ, Simonian PT, Mayo KA (1995) Early results of percutaneous iliosacral screws placed with the patient in the supine position. J Orthop Trauma 9:207–214
Vanderschot P, Kuppers M, Sermon A, Lateur L (2009) Trans-iliac-sacral-iliac-bar procedure to treat insufficiency fractures of the sacrum. Indian J Orthop 43:245–252. https://doi.org/10.4103/0019-5413.53454
Rommens PM, Wagner D, Hofmann A (2012) Surgical management of osteoporotic pelvic fractures: a new challenge. Eur J Trauma Emerg Surg 38:499–509. https://doi.org/10.1007/s00068-012-0224-8
Wagner D, Kamer L, Sawaguchi T, Richards RG, Noser H, Uesugi M, Ossendorf C, Rommens PM (2017) Critical dimensions of trans-sacral corridors assessed by 3D CT models. Relevance for implant positioning in fractures of the sacrum. J Orthop Res 35:2577–2584. https://doi.org/10.1002/jor.23554
Gras F, Gottschling H, Schröder M, Marintschev I, Hofmann GO, Burgkart R (2016) Transsacral osseous corridor anatomy is more amenable to screw insertion in males: a biomorphometric analysis of 280 pelves. Clin Orthop Relat Res 474:2304–2311. https://doi.org/10.1007/s11999-016-4954-5
Höch A, Pieroh P, Henkelmann R, Josten C, Böhme J (2017) In-screw polymethylmethacrylate-augmented sacroiliac screw for the treatment of fragility fractures of the pelvis: a prospective, observational study with 1-year follow-up. BMC Surg 17:132. https://doi.org/10.1186/s12893-017-0330-y
Höch A, Schimpf R, Hammer N, Schleifenbaum S, Werner M, Josten C, Böhme J (2017) Biomechanical analysis of stiffness and fracture displacement after using PMMA-augmented sacroiliac screw fixation for sacrum fractures. Biomed Tech (Berl) 62:421–428. https://doi.org/10.1515/bmt-2016-0235
Osterhoff G, Dodd AE, Unno F, Wong A, Amiri S, Lefaivre KA, Guy P (2016) Cement augmentation in sacroiliac screw fixation offers modest biomechanical advantages in a cadaver model. Clin Orthop Relat Res 474:2522–2530. https://doi.org/10.1007/s11999-016-4934-9
Grüneweller N, Raschke MJ, Zderic I, Widmer D, Wähnert D, Gueorguiev B, Richards RG, Fuchs T, Windolf M (2016) Biomechanical comparison of augmented versus non-augmented sacroiliac screws in a novel hemi-pelvis test model. J Orthop Res 35:1485–1493. https://doi.org/10.1002/jor.23401
Oberkircher L, Masaeli A, Bliemel C, Debus F, Ruchholtz S, Krüger A (2016) Primary stability of three different iliosacral screw fixation techniques in osteoporotic cadaver specimens-a biomechanical investigation. Spine J 16:226–232. https://doi.org/10.1016/j.spinee.2015.08.016
Schneider W, Bortfeld T, Schlegel W (2000) Correlation between CT numbers and tissue parameters needed for Monte Carlo simulations of clinical dose distributions. Phys Med Biol 45:459–478
Henes FO, Groth M, Bley TA, Regier M, Nüchtern JV, Ittrich H, Treszl A, Adam G, Bannas P (2012) Quantitative assessment of bone marrow attenuation values at MDCT: an objective tool for the detection of bone bruise related to occult sacral insufficiency fractures. Eur Radiol 22:2229–2236. https://doi.org/10.1007/s00330-012-2472-8
Gupta AC, Yoo AJ, Stone J, Barr JC, Brook A, Tutton S, Ortiz O, Hirsch AE, Larvie M, Frey ME, Jayaraman MV, Hirsch JA (2012) Percutaneous sacroplasty. J Neurointerv Surg 4:385–389. https://doi.org/10.1136/neurintsurg-2011-010136
Lyders EM, Whitlow CT, Baker MD, Morris PP (2010) Imaging and treatment of sacral insufficiency fractures. Am J Neuroradiol 31:201–210. https://doi.org/10.3174/ajnr.A1666
Mears SC, Sutter EG, Wall SJ, Rose DM, Belkoff SM (2010) Biomechanical comparison of three methods of sacral fracture fixation in osteoporotic bone. Spine 35:E392–E395. https://doi.org/10.1097/BRS.0b013e3181cb4fcd
Waites MD, Mears SC, Richards AM, Mathis JM, Belkoff SM (2008) A biomechanical comparison of lateral and posterior approaches to sacroplasty. Spine 33:E735–E738. https://doi.org/10.1097/BRS.0b013e31817ecc22
Andresen R, Radmer S, Wollny M, Andresen JR, Nissen U, Schober H-C (2017) CT-guided cement sacroplasty (CSP) as pain therapy in non-dislocated insufficiency fractures. Eur J Orthop Surg Traumatol 27:1045–1050. https://doi.org/10.1007/s00590-017-2001-1
Ebraheim N, Sabry FF, Nadim Y, Xu R, Yeasting RA (2000) Internal architecture of the sacrum in the elderly. An anatomic and radiographic study. Spine 25:292–297
Singh M, Nagrath AR, Maini PS (1970) Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. J Bone Jt Surg Am 52:457–467
Krappinger D, Roth T, Gschwentner M, Suckert A, Blauth M, Hengg C, Kralinger F (2012) Preoperative assessment of the cancellous bone mineral density of the proximal humerus using CT data. Skeletal Radiol 41:299–304. https://doi.org/10.1007/s00256-011-1174-7
Hammarstedt L, Thilander-Klang A, Muth A, Wängberg B, Odén A, Hellström M (2013) Adrenal lesions: variability in attenuation over time, between scanners, and between observers. Acta Radiol 54:817–826. https://doi.org/10.1177/0284185113482688
Gausden EB, Nwachukwu BU, Schreiber JJ, Lorich DG, Lane JM (2017) Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: a qualitative systematic review. J Bone Jt Surg Am 99:1580–1590. https://doi.org/10.2106/JBJS.16.00749
Vivanco JF, Burgers TA, García-Rodríguez S, Crookshank M, Kunz M, MacIntyre NJ, Harrison MM, Bryant JT, Sellens RW, Ploeg H-L (2014) Estimating the density of femoral head trabecular bone from hip fracture patients using computed tomography scan data. Proc Inst Mech Eng H 228:616–626. https://doi.org/10.1177/0954411914540285
Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG (2011) Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Jt Surg Am 93:1057–1063. https://doi.org/10.2106/JBJS.J.00160
Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N (2013) Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med 158:588–595. https://doi.org/10.7326/0003-4819-158-8-201304160-00003
Prevrhal S, Engelke K, Kalender WA (1999) Accuracy limits for the determination of cortical width and density: the influence of object size and CT imaging parameters. Phys Med Biol 44:751–764
Acknowledgements
We acknowledge the support of Dr. Karsten Schwieger. We thank Thomas Heldstab for his technical assistance. This study was partially co-funded by the TK System of the AO Foundation (Davos, Switzerland) and Depuy Synthes (Zuchwil, Switzerland).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any further conflicts of interests.
Ethics
The anonymous use of CT data was approved by local ethics committee (Landesärztekammer Rheinland-Pfalz, Mainz, Germany) for the group with fractured sacra. The non-fractured sacra were obtained from the CT database of the AO Research Institute Davos, Davos, Switzerland, registered at the “Eidgenössischer Öffentlichkeits- und Datenschutzbeauftragter”, Bern, Switzerland. All patients agreed to anonymous research use of their CT data.
Rights and permissions
About this article
Cite this article
Wagner, D., Hofmann, A., Kamer, L. et al. Fragility fractures of the sacrum occur in elderly patients with severe loss of sacral bone mass. Arch Orthop Trauma Surg 138, 971–977 (2018). https://doi.org/10.1007/s00402-018-2938-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-018-2938-5