Abstract
Objective
To determine the presence of a consistent osseous corridor through S1 and S2 and fluoroscopic landmarks thereof, which could be used for safe trans ilio-sacroiliac screw fixation of posterior pelvic ring disorders.
Study design
Computed tomography (CT) based anatomical investigation utilising multiplanar image and trajectory reconstruction (Agfa-IMPAX Version 5.2 software). Determination of the presence and dimension of a continuous osseous corridor in the coronal plane of the sacrum at the S1 and S2 vertebral levels.
Outcome measures
Determination of: (a) the presence of an osseous corridor in the coronal plane through S1 and S2 in males and females; (b) the dimension of the corridor with regard to diameter and length; (c) the fluoroscopic landmarks of the corridor.
Results
The mean cross-sectional area for S1 corridors in males and females was 2.13 and 1.47 cm2 , respectively. The mean cross-sectional area for the S2 corridor in males and females was 1.46 and 1.13 cm2, respectively. The limiting anatomical factor is the sagittal diameter of the sacral ala at the junction to the vertebral body. The centre of the S1 and S2 corridor is located in close proximity to the centre of the S1 and S2 vertebrae on the lateral fluoroscopic view as determined by the adjacent endplates and anterior and posterior vertebral cortices.
Conclusion
Two-thirds of males and females have a complete osseous corridor to pass a trans-sacroiliac S1 screw of 8 mm diameter. The S2 corridor was present in all males but only in 87 % of females. Preoperative review of the axial CT slices at the midpoint of the S1 or S2 vertebral body allows the presence of a trans-sacroiliac osseous corridor to be determined by assessing the passage at the narrowest point of the corridor at the junction of the sacral ala to the vertebral body.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Posterior pelvic ring disorders have different trauma mechanisms but their impact for patients is high. U-shaped sacral fractures are the result of high axial load leading to an additional horizontal fracture with rotational instability. The upper part of the lumbar spine is pivoted out of the posterior pelvic ring, whereas the caudal part is still connected, leading to the so-called spino-pelvic dissociation with high risk of sacral nerve root injuries and high-grade biomechanical instability [1]. These types of fractures are most commonly seen in high-energy injuries such as fall from a significant height. If the bone stock is weak like in osteoporosis or metastatic sacral infiltrations, insufficiency fractures may occur with destabilisation of the ring structure [2]. In the past, the surgical treatment included open procedures like lumbo-pelvic instrumented fusion or triangular osteosynthesis [3–5] with significant operative morbidity to the patient and time-consuming surgeries [6]. To reduce peri- and post-operative complications, several different percutaneous screw fixation techniques as treatment strategies were established in the last couple of years. These screws could be set either in the S1 or S2 vertebral body [7–10]. For U-shaped fractures-traumatic or insufficient- bilateral sacro-iliac screw fixation is necessary to stabilise the rotational and vertical instability [11]. Due to the complex anatomy of the sacrum and the high variability amongst individuals, sacro-iliac screw placement is still a challenge for surgeons [12–15], especially in acute trauma situations with unstable patients where CT guidance or computed navigation is often not possible. To facilitate minimal invasive treatment, trans ilio-sacroiliac fixation methods were introduced [16–18]. These methods offer a one step surgery with conventional fluoroscopy with good compression over both pelvic ring components. While these techniques show promising results, injury to neural and intra-pelvic structures during percutaneous screw placement remain a concern.
The aim of this study is the determination of the presence, dimensions and limits of a “safe zone” osseous corridor through which a trans-sacral screw can be placed across S1 or S2 in the male and female pelvis and the fluoroscopic landmarks thereof.
Materials and methods
The images of 38 adult male and 38 adult female patients who underwent computed tomography (CT) scans of their pelvis for unrelated medical reasons between October 2009 and December 2009 were included in the study. Investigations which revealed obvious degenerate, traumatic or osteolytic conditions were excluded. Investigations with slice thickness greater than 2.5 mm were excluded. Institutional review board approval was obtained.
Agfa-IPMAX Version 5.2 software was used for multiplanar reconstruction and trajectory placement, allowing unrestricted rotation of the pelvis and sacrum and virtual translation of the sacrum along the axis of the osseous corridor if present. Specifically, the presence and topography of a trans-sacral osseous corridor through the S1 and S2 vertebral bodies from one ilium to the other, which would safely accommodate a virtual trans ilio-sacroiliac screw of 8 mm, was determined.
The osseous corridor was defined as a complete osseous continuity from one ilium to the other of at least 8 mm in diameter without cortical breach of the anterior, posterior, superior or inferior borders of the S1 and S2 vertebral bodies.
The fluoroscopic landmarks determining the position of the corridor were extrapolated from the reconstructed sagittal CT images.
Specific measurements
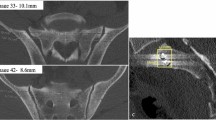
Sacroiliac screw positioning needs good three-dimensional imagination, because of unique anatomical features of the posterior pelvic ring: First, a screw placement in the sagittal plane should be achieved in the middle of the vertebral body of S1 or S2 to minimize the risk of lumbar and sacral root damage (Fig. 1). In addition, the sacrum cannot be explained with simple volumetric model due to the concave narrowing of the ala at pedicle level. This leads to a more double cone area at the first vertebral body with the narrowest point between the ala concavity, the S1 foramen and the sacral pedicle. This so called “vestibule”, as described by Carlson et al. [19], has to be passed on both sides if a trans ilio-sacroiliac screw is inserted. Where present the cross sectional areas of the S1 and S2 osseous corridors were determined. The distance of the corridor centres to the adjacent disc endplates and vertebral cortices were measured in the mid-sagittal plane. This represents the location of the corridor centre in the lateral radiological view of the S1 and S2 vertebra outlined by the endplates and cortices (Fig. 2).
In the transverse plane, the anterior–posterior length of S1 was measured adjusted for pelvic inclination at pedicle level to simulate the vestibule narrowing. In addition, anterior breaching through the promontorium was monitored. S2 was measured similar. The screw length was noted in the same plane (Fig. 3).
The distance of the S1 foramen to the sacral ala, as well as the interforaminal distance between S1 and 2 was measured in the inclination-adjusted coronal plane (Fig. 4). Again, all measurements were adjusted for pelvic inclination.
Statistical and descriptive analysis was performed using ANOVA analysis with SPSS for windows version 17.0 (SPSS, Chicago, IL, USA).
Results
The S1 foramen-alar distance of 15 mm in men and 13 mm in women was a significant difference. Women had a significantly smaller S1–S2 inter-foraminal distance. The anterior–posterior diameter of the S1 and S2 corridors was significantly smaller in women than in men. As a result of the dimensions of the S1 and S2 corridors being smaller in women than men the overall cross-sectional area of the S1 and S2 corridors were significantly smaller in women than men (Table 1).
S1
63 % of males and 66 % of females have a complete osseous corridor to pass a trans ilio-sacro-iliac screw. The mean cross-sectional area for S1 corridor in males and females was 2.13 and 1.47 cm2, respectively. The centre of the S1 corridor—as viewed on lateral fluoroscopy—is located at the 46 % mark between the midpoints of the superior and inferior endplates and at the 52 % mark between the midpoints of the anterior posterior cortices.
The limiting factor to the osseous canal was the narrow foraminal-alar distance of less than 8 mm.
S2
The S2 osseous corridor was present in 100 % of males but only present in 87 % of females. The mean cross-sectional area for the S2 corridor in males and females was 1.46 and 1.13 cm2, respectively. Similar to S1, the centre of the S2 corridor is located at the 45 % mark between superior and inferior endplates and at the 53 % mark between the midpoints of the anterior and posterior cortices.
The limiting factor placing S2 screws in women was the narrow inter-foraminal distance of S1 and 2.
Discussion
Posterior pelvic ring pathologies are still challenging surgical conditions. Traditional stabilization of sacral fractures with spino-pelvic dissociation such as in U-shaped sacral fractures, involves open lumbo-pelvic fixation using pedicle screw and pelvic fixation [3, 4, 20]. These are major procedures with significant soft tissue dissection and potentially considerable patient morbidity. Less invasive procedures utilising percutaneous iliosacral screws have been described with considerable success [11, 17, 21]. The percutaneous placement of screws across the sacroiliac joint into the sacrum is challenging due to the complex and variable anatomy [13, 15]. The specific difficulty in placing screws or bars across the sacrum through S1 or S2 is that slight anterior placement can lead to damage of the nerve roots particularly the L5 nerve root, which lies anterior to the sacral ala and common iliac artery and vein. Posterior placement through the S1 and S2 vertebral bodies can breach into the spinal canal. This has led to several virtual models for optimal screw placement [12]. Carlson et al. [19] introduced the “vestibule” concept for S1 screws, describing the safe corridor as a double cone with the “vestibule” as narrowest area to pass between the sacral pedicle, the sacral ala waist and the S1 foramen. The investigation of Day et al. showed that if the sacro-iliac screws were placed horizontally, the bony corridor is dramatically decreased in size [14]. CT guidance increases the placement accuracy [22, 23] but is of limited suitability in the acute setting.
Vanderschot et al. introduced navigated trans ilio-sacroiliac bar stabilization for bilateral sacro-iliac lesions as well as for sacral insufficiency fractures, whereby this technique has not completely prevented implant malplacement [16, 24, 25]. König et al. used a similar technique in U-shaped fractures [17]. In this study, a screw of 8 mm was placed using intraoperative fluoroscopy with standard sagittal plane, as well as inlet and outlet views only. This method could be used even in an acute setting, since only a unilateral pelvic approach is needed. Nevertheless, to date there is a lack of anatomical data on the exact topography of the sacrum with specific reference to trans ilio-sacroiliac screw placement.
In the presented study, 63 % of men and 66 % of women had a complete osseous corridor to safely pass a 8 mm trans ilio-sacroiliac screw across the S1 vertebral body (Fig. 5). In all cases where safe passage of a screw was not theoretically possible, a narrow foraminal-alar distance of less than 8 mm was present. This is shown in Fig. 6 where the sacral alae are relatively more concave than in patients were screw placement is possible. Here the alae are relatively less concave.
It was theoretically possible in 100 % of men and 87 % of women to pass a 8 mm S2 trans ilio-sacroiliac screw. Where it was not possible in women, it was due to a narrow S1–S2 inter-foraminal distance. At least six features of sacral dysmorphism have been described by Routt et al. [10], though there is no agreement of the true definition of sacral dysmorphism. Kaiser showed that the two most important characteristics for upper segment sacral dysplasia were coronal and axial angulation of the first sacral segment [26]. Sacrae in which S1 and S2 osseous tunnels were not present to pass 8 mm diameter screws, showed features of sacral dysmorphism. Sagittal sections of the sacral CT scans clearly show the osseous boundaries of the S1 and S2 vertebrae. Clear sagittal fluoroscopic images also show the boundaries of the S1 and S2 vertebrae. From these images, the location of the centre of the S1 and S2 osseous corridor can be identified. It is located approximately at the centre of the S1 and S2 vertebrae as seen on true sagittal fluoroscopy (Fig. 7).
As the relative dimensions of the S1 and S2 vertebral bodies were larger in men than women, the cross-sectional area of the S1 and S2 osseous corridors were significantly larger in men than women. Similarly, the lengths of the S1 and S2 screws were significantly larger in men than women.
Conclusion
This study demonstrates the presence of S1 and S2 osseous tunnels to allow passage of a 8 mm trans ilio-sarco-iliac in the majority of subjects. Where this is not possible, it is most likely due to sacral dysmorphism. Prior to performing a trans ilio-sacroiliac screw, accurate analysis of the CT scan of the sacrum in each patient must be performed to determine if an osseous tunnel is present. Failure to observe this may lead to compromise of the neurovascular structures anteriorly or penetration of the spinal canal posteriorly.
References
Roy-Camille R, Saillant G, Gagna G, Mazel C (1985) Transverse fracture of the upper sacrum. Suicidal jumper’s fracture. Spine (Phila Pa 1976) 10:838–845
Gibbons KJ, Soloniuk DS, Razack N (1990) Neurological injury and patterns of sacral fractures. J Neurosurg 72:889–893. doi:10.3171/jns.1990.72.6.0889
Bellabarba C, Schildhauer TA, Vaccaro AR, Chapman JR (2006) Complications associated with surgical stabilization of high-grade sacral fracture dislocations with spino-pelvic instability. Spine (Phila Pa 1976) 31:S80–S88. doi:10.1097/01.brs.0000217949.31762.be (discussion S104)
Mouhsine E, Wettstein M, Schizas C et al (2006) Modified triangular posterior osteosynthesis of unstable sacrum fracture. Eur Spine J 15:857–863. doi:10.1007/s00586-004-0858-2
Schildhauer TA, Bellabarba C, Nork SE et al (2006) Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma 20:447–457
König MA, Jehan S, Boszczyk AA, Boszczyk BM (2012) Surgical management of U-shaped sacral fractures: a systematic review of current treatment strategies. Eur Spine J 21:829–836. doi:10.1007/s00586-011-2125-7
Matta JM, Tornetta P (1996) Internal fixation of unstable pelvic ring injuries. Clin Orthop Relat Res 329:129–140
Gänsslen A, Hüfner T, Krettek C (2006) Die bildwandergestützte, perkutane transiliosakrale Schrauben-fixation instabiler Beckenverletzungen. Oper Orthop Traumatol 18:225–244. doi:10.1007/s00064-006-1173-3
Osterhoff G, Ossendorf C, Wanner GA et al (2011) Posterior screw fixation in rotationally unstable pelvic ring injuries. Injury 42:992–996. doi:10.1016/j.injury.2011.04.005
Routt M Jr (2006) Posterior pelvic-ring disruptions: iliosacral screws. In: Wiss D (ed) Fractures. Lippincott, Williams & Wilkins, Philadelphia, pp 649–667
Nork SE, Jones CB, Harding SP et al (2001) Percutaneous stabilization of U-shaped sacral fractures using iliosacral screws: technique and early results. J Orthop Trauma 15:238–246. doi:10.1097/00005131-200105000-00002
Mendel T, Appelt K, Kuhn P, Suhm N (2008) Bony sacroiliac corridor. A virtual volume model for the accurate insertion of transarticular screws. Unfallchirurg 111:19–26. doi:10.1007/s00113-007-1386-4
Noojin FK, Malkani AL, Haikal L et al (2000) Cross-sectional geometry of the sacral ala for safe insertion of iliosacral lag screws: a computed tomography model. J Orthop Trauma 14:31–35
Day CS, Prayson MJ, Shuler TE et al (2000) Transsacral versus modified pelvic landmarks for percutaneous iliosacral screw placement—a computed tomographic analysis and cadaveric study. Am J Orthop (Belle Mead NJ) 29:16–21
Karachalios T, Zibis AH, Zintzaras E et al (2010) An anatomical update on the morphologic variations of S1 and S2. Orthopedics 33:733. doi:10.3928/01477447-20100826-12
Vanderschot P, Meuleman C, Lefevre A, Broos P (2001) Trans iliac–sacral–iliac bar stabilisation to treat bilateral lesions of the sacro–iliac joint or sacrum: anatomical considerations and clinical experience. Injury 32:587–592
König M, Seidel U, Heini P (2013) Minimal-invasive percutaneous reduction and transsacral screw fixation for U-shaped fractures. J Spinal Disord Tech 00:1–7
Gardner MJ, Routt MLC (2011) Transiliac-transsacral screws for posterior pelvic stabilization. J Orthop Trauma 25:378–384. doi:10.1097/BOT.0b013e3181e47fad
Carlson DA, Scheid DK, Maar DC et al (2000) Safe placement of S1 and S2 iliosacral screws: the “vestibule” concept. J Orthop Trauma 14:264–269
Schildhauer TA, Bellabarba C, Nork SE et al (2006) Decompression and lumbopelvic fixation for sacral fracture-dislocations with spino-pelvic dissociation. J Orthop Trauma 20:447–457. doi:10.1097/00005131-200608000-00001
Fensky F, Schäffler A, Siebenlist S et al (2011) Percutaneous iliosacral screw fixation for pelvis insufficiency fracture after implantation of a pedestal cup: case report. Unfallchirurg 114:1115–1119. doi:10.1007/s00113-010-1908-3
Bale RJ, Kovacs P, Dolati B et al (2008) Stereotactic CT-guided percutaneous stabilization of posterior pelvic ring fractures: a preclinical cadaver study. J Vasc Interv Radiol 19:1093–1098. doi:10.1016/j.jvir.2008.04.006
Sciulli RL, Daffner RH, Altman DT et al (2007) CT-guided iliosacral screw placement: technique and clinical experience. AJR Am J Roentgenol 188:181–192. doi:10.2214/AJR.05.0479
Vanderschot P, Kuppers M, Sermon A, Lateur L (2009) Trans-iliac-sacral-iliac-bar procedure to treat insufficiency fractures of the sacrum. Indian J Orthop 43:245–252. doi:10.4103/0019-5413.53454
Papanastassiou ID, Setzer M, Eleraky M et al (2011) Minimally invasive sacroiliac fixation in oncologic patients with sacral insufficiency fractures using a fluoroscopy-based navigation system. J Spinal Disord Tech 24:76–82. doi:10.1097/BSD.0b013e3181df8e6b
Kaiser SP, Gardner MJ, Liu J et al (2014) Anatomic determinants of sacral dysmorphism and implications for safe iliosacral screw placement. J Bone Joint Surg Am 96:e120. doi:10.2106/JBJS.M.00895
Acknowledgments
The author would like to thank Spinegraphics Nottingham for the illustrations in this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No benefits or funds in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Rights and permissions
About this article
Cite this article
König, M.A., Sundaram, R.O., Saville, P. et al. Anatomical considerations for percutaneous trans ilio-sacroiliac S1 and S2 screw placement. Eur Spine J 25, 1800–1805 (2016). https://doi.org/10.1007/s00586-015-4327-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00586-015-4327-x