Abstract
Introduction
Although autologous chondrocyte implantation (ACI) has become well established for the treatment of full-thickness cartilage defects of the knee joint, nevertheless clinical results of retropatellar lesions are still inferior compared to those of defects located on femoral condyles. We report the clinical results obtained in 70 patients treated with ACI for full-thickness defects of the patella, with special reference to defect location and size, age, body mass index and sports activity.
Methods
At a follow-up of 38.4 months (range 14–64, follow-up rate 83.3%), patients’ subjective functional knee scores (IKDC, Lysholm) were analysed, as were the results of objective examination (according to ICRS).
Results
Mean patient age at the time of surgery was 34.3 years (±10.1). The mean Lysholm score at the time of follow-up was 73.0 (±22.4) and the subjective IKDC score was 61.6 (±21.5); normal and nearly normal clinical results according to the objective criteria of the International Cartilage Research Society (ICRS) were achieved in 67.1% of the patients, while abnormal results were achieved in 20.0% of the patients and severely abnormal results, in 12.9%. While different surgical techniques did not seem to have any significant influence on the treatment results, both defect size and defect location within the patella were found to be significantly associated with clinical outcome. The corollaries to this are that larger cartilage lesions of the patella are associated with an inferior outcome (p = 0.007) and that cartilage defects located on the lateral patellar facet are correlated with a better clinical outcome than those located on the medial facet or those involving both facets (p = 0.017).
Conclusion
This study demonstrates that within a group of patients treated with ACI for retropatellar cartilage lesion there are significant differences in clinical outcome, which are important and should be taken into account of when a decision has to be made on whether or not ACI is indicated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the introduction of autologous chondrocyte implantation (ACI) in 1994 by Brittberg et al. [4], it has been confirmed that this technique produces mechanically and functionally stable cartilage in patients with full-thickness cartilage defects [6, 32, 34] and even is cost effective from an economical point of view, even though cell expansion in vitro is very expensive [24, 27]. The cartilage regeneration induced by this technique seems biomechanically superior to regenerates induced by other techniques [33, 34] and the biomechanical properties seem to be comparable to those of the surrounding cartilage [17]. In various studies, reliable and satisfying clinical results have been obtained in the treatment of cartilage lesions located on the medial and lateral femoral condyles, while patellar defects are still a challenge with substantially lower good or very good results [4, 6, 22, 33, 34].
Although the lower proportion of satisfactory clinical results following treatment of defects in the retropatellar location is not restricted to the ACI technique and has also been observed with other cartilage repair techniques, such as microfracturing [21, 22, 40] and mosaicplasty [2, 15], the clinical results of ACI have been found to differ significantly with the site of the cartilage damage in most of the published studies. While good and very good treatment outcomes are reported in 80–90% of cases with damage in the region of the femoral condyle, the success rate for treatment of retropatellar defects is decidedly lower, at 60–70% [4, 28, 33, 34].
A possible explanation for the inferior results of treatment in the region of the posterior surface of the patella lies in the specific biomechanical properties of the anterior compartment of the knee joint. There are greater shearing forces here than in the medial and lateral compartments, which are less beneficial to the differentiation of transplanted cartilage cells than are the hydrostatic forces that are dominant in the region of the femoral condyle [10, 13, 23, 41], and during stair-climbing, for example, the forces arising in the femoropatellar compartment are in excess of seven times body weight [19].
All these thoughts are hypothetical, however, in most studies patients with retropatellar cartilage defects account for only a small proportion of the total patient population, and against this background it has not yet been possible to identify any prognostic factors connected with patellar defects alone that are associated with a good or a poor clinical outcome.
Only a few of the available studies published recently deal exclusively with ACI for defects in the retropatellar location [11, 18, 28], and in none of them has it been possible to identify defects or patient characteristics that can be significantly correlated with inferior or superior clinical outcome, as has been done in other studies.
The identification of such prognostic factors and a report of the clinical treatment outcomes referred to patient age, defect location, defect size, surgical procedure, number of concurrent and previous operations and other influential factors, such as nicotine use and body weight, in this highly selected patient population is the object of the present study.
Patients and methods
Patients
Between January 2001 and March 2005 a total of 95 patients with a retropatellar cartilaginous damage were treated with ACI in our department. Exclusion criteria in the current study were dysplasia of the femoropatellar joint (trochlea) indicated by preoperative X-ray investigation (axial view of the patellofemoral joint) and significant varus or valgus deformity (more than 5°) seen in the weight-bearing AP view of the whole leg. After exclusion of 11 patients with either of these conditions 84 patients were available for the study; 70 of them were available for follow-up investigations and were ultimately included in the current study with informed consent (follow-up rate 83.3%).
During surgery, location, grade and size of the defect, surgical technique, intraoperative complications and accompanying surgical procedures were evaluated according to the recommendations of the International Cartilage Society (ICRS) [3, 7].
Surgical technique
In all cases ACI was preceded by arthroscopy of the affected knee joint in our department to check that later ACI was indeed indicated and to extract the chondrocytes from the area of unstressed cartilage in the region of the intercondylar fossa with a standard instrument set. Cell expansion was accomplished by Cellgenix (Freiburg, Germany) for cell suspensions in the periosteum-covered and Chondrogide-covered groups and by BioTissue Technology (BioSeed C®, Freiburg, Germany) for the matrix-associated ACI group. After expansion, ACI was performed under general anaesthesia in a second operation.
All surgical operations were performed in an open technique via arthrotomy of the knee joint. Once a medial skin incision had been made from 1 to 2 cm proximal to the cranial pole of the patella to approximately 2 cm distal to the patellar tip, exposure of the knee joint capsule and arthrotomy at the medial patellar rim followed. The patella was luxated and turned back on itself to the side, after which first of all the cartilage damage was assessed and then the damaged area of cartilage was debrided; the area debrided was extended sideways until it was entirely surrounded by healthy cartilage, and in this area the full depth of cartilage down to the subchondral bone was removed, care being taken to avoid perforating the latter; for this stage of the operation a sharp spoon, a sharp curette and, in the region of the rim, a scalpel were used in a recurring sequence. This procedure was followed in all groups. The surgeon operating performed ACI using the conventional technique (periosteum patch and Chondrogide® membrane: Surgeon 1) or the matrix-associated procedure (Surgeon 2), according to individual preference. Thus, the study design in the work described in the present paper was that of a retrospective cohort study with two treating surgeons.
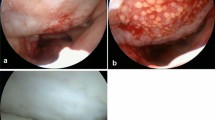
In the membrane group, a Chondrogide® membrane (Geistlich, Wolhusen, Switzerland) was cut to size and was sutured in a circular manner with PDS suture material (6-0, Ethicon, Germany; Fig. 1) the chondrocyte suspension (1 million cells per cm² debrided defect) was then injected [39]. Once the transplant had been sealed with fibrin glue (Tissuecol, Baxter, Deutschland) a redon suction drain was placed and the wound was closed layer by layer. The same suture technique and further procedure were used in the periosteal patch group. In this group, the periosteal patch was harvested from the proximal tibia via an additional incision, as described by Brittberg et al. [4]. In the matrix group, after in vitro expansion, cells were cultured on a three-dimensional (3D) poly-lactic-co-glycolic acid (PLGA) fleece and afterwards implanted using a transpatellar fixation technique that has already been described elsewhere [8, 31].
The same postoperative treatment was performed in all groups. Postoperatively, the patients were advised to limit weight-bearing on the affected side to 15 kg for the first 6 weeks, after which, if there was no inflammation in the knee joint, weight-bearing load was gradually increased, to reach full weight-bearing 8–12 weeks after surgery. To reduce the retropatellar pressure each patient was fitted with an orthotic device set to limit flexion to 30° for 2 weeks and then to 60° for the next 2 weeks. Ice was applied perioperatively, and the use of passive motor movement splints (CPM, Ormed, Germany) was continued during aftertreatment from the first day after surgery onward, as was physiotherapy with isometric exercises designed to strengthen the joint.
Intraoperatively, defect size and location were mapped according to the recommendations of the ICRS [3, 5]. All treated defects were grade III and IV lesions of the patella. For further evaluation according to the surgical protocol, the location was grouped according to the Fulkerson classification [35]. This classification distinguishes between defects involving the lateral (Type II lesions) or medial (Type III) facet alone and those involving both facets (Type I and IV). Since in this classification type I lesions (involving the inferior pole of the patella including the patella ridge) and type IV lesions (involving the medial and lateral facet and the patella ridge) cannot be reliably distinguished, type I and type IV lesions were taken together as one group, and differentiated from lesions involving the lateral facet only and those restricted to the medial facet.
Follow-up investigation
Standard measuring instruments were used in the present study for the follow-up investigation. At the time of the follow-up investigation the patients’ subjective impressions of outcome were elicited by means of the Lysholm score [26] and the IKDC score [3, 7], while objectively the patients were classed according to the criteria of the ICRS score as having results that were “Normal (A)”, “Nearly normal (B)”, “Abnormal (C)” and “Severely abnormal (D)” [3, 7]. A physical examination was performed by an independent examiner blinded to specific case data, including surgical technique, defect location, size, and other details. Since MRI was not conducted consistently (example given in Fig. 2), it has not been included in the current study.
To allow a comparative statement on the physical stamina of the patients before and after the operative treatment, the Cincinnati Sports Activity Scale was used to ascertain the patients’ level of activity before the operation (retrospectively) and at the time of follow-up [29].
Statistical evaluation
SPSS for windows version 11.0 (SPSS Inc. Chicago, IL.) was used for the statistical analysis designed to work up the data ascertained in this study. Differences between the treatment groups were assessed with the aid of ANOVA analysis of variance and ANCOVA analysis of correlation. p values < 0.05 were considered statistically significant and p values < 0.01 were considered to be statistically highly significant. p values between 0.05 and 0.10 were considered to indicate a statistical trend. For further investigation of the influences of different factors (including defect size, defect location, age, nicotine use, surgical technique) on the treatment results, a multivariate data analysis for correlation was performed in addition.
The Spearman–Rank correlation coefficient was calculated to describe the correlation between the subjective IKDC and Lysholm scores at the time of follow-up.
Results
The mean length of follow-up was 38.4 (SD ± 15.6, min. 14.3) months, and the average age of patients involved in the current study was 34.3 (SD ± 10.1) years.
The average body mass index (BMI) was 25.05 kg/m² (SD ± 5.80). Patients’ tobacco use was quantified as packages of cigarettes per day and year (pack-years), and the average was 2.00 (SD ± 4.93). For the statistical evaluation patients were divided into smoking (12 patients = 17.1%) and nonsmoking subgroups (82.9%). Patients had a mean of 1.55 (SD ± 1.4) previous operations: in 9 patients the operation was performed following failed microfracturing. One patient had already been treated with ACI on the lateral facet in isolation. The average defect size (cumulated defect size of the medial and lateral facets) was 4.41 (SD ± 2.15) cm².
The athletic activity level before the operation was an average of 34.44 (SD ± 33.98) points on the Cincinnati Sports Activity Scale. According to the sports activity levels 30 patients only performed sports at level IV, while 20 patients were assigned to level III. A total of 20 further patients were assigned to levels II (12 patients) and I (8 patients).
The patient characteristics are shown referred to defect location in Table 1.
In response to the question about any improvement in symptoms compared with their condition “before the operation” 59 (84%) of the patients said the symptoms were “better”; 2 patients (2.9%), “the same”; and 9 patients (12.9%), “worse”. Most of the patients (57, or 81.4%) said they would give consent for the same operation again if they were to suffer the same symptoms as before.
The subjective treatment outcomes expressed by the Lysholm and IKDC scores at the time of the follow-up investigation can be seen in Fig. 3 and were 73.0 points (SD ± 22.4) on the Lysholm score (max. 100 points) and 62 points (SD ± 21.5) on the IKDC score (max. 100 points). When these results were broken down by location, statistical analysis revealed a significantly better outcome—for both the Lysholm and the IKDC score—for defects located on the lateral facet than for those located on the medial facet and those involving both facets. The statistical significance was also observed after multivariate statistical analysis (p (Lysholm) = 0.017; p (IKDC) = 0.008). Treatment results are displayed by defect location in Fig. 3. Furthermore, there was a significant correlation between evaluated IKDC and Lysholm score values (r = 0.844).
Clinical results at time of follow-up in dependence of defect location. Lysholm scores are represented by grey columns; IKDC by black columns. Statistical significance between different groups (p < 0.05) are indicated by asterisks. (Fulkerson classification of retropatellar cartilage defects [35]: Type II defects involving the lateral alone; Type III isolated defects of the medial facet; Type I and IV defects involving both facets)
Comparison of the pre- and postoperative scores for athletic activity showed a significant improvement from 34.4 (SD ± 33.9) to 61.5 (SD ± 21.5) points according to the Cincinnati Activity Scale for the entire treatment group (p < 0.001), and 58 (82.9%) of the 70 patients even recovered the level of sports activity they had enjoyed before injury (in the case of chronic lesions, the level before the knee symptoms started). Detailed Cincinnati sports activity levels before injury and after surgery are given in Table 2.
Multivariate statistical analysis for factors influencing the clinical treatment outcome revealed a significant influence, defect location as described above [p (Lysholm) = 0.017] and patient age [p (Lysholm) = 0.048], while the influence of tobacco use [p (Lysholm) = 0.064] and defect size (smaller defects were correlated with better outcome; [p (Lysholm) = 0.068]) just failed to reach statistical significance. Other factors (number of surgical operations performed before ACI [p (Lysholm) = 0.894], body mass index [p (Lysholm) = 0.546], preoperative sports activity [p (Lysholm) = 0.821], surgical technique [p (Lysholm) = 0.879] and length of follow-up [p (Lysholm) = 0.767] did not seem to have any significant influence on clinical outcome.
According to the criteria of the ICRS score, the objective follow-up examination revealed treatment results that were “normal” in 21 cases, “nearly normal” in 26 cases, “abnormal” in 14 cases and “severely abnormal” in 9 patients. Percentages and objective treatment results are displayed referred to defect location in Table 3.
In the case of the patients in whom outcome was classified as “Severely abnormal” the treatment was considered to have failed on the grounds of a limited range of motion in five cases, of painful retropatellar crepitations in three cases and of persistent effusion in one case. In the patients with painful retropatellar crepitations MRI revealed an incomplete or total absence of defect filling; the patient with total lack of defect filling had undergone another operation. In two cases MRI revealed transplant hypertrophy as a minor complication, which has successfully been treated by arthroscopic debridment. Both of these patients were initially treated with a periosteum patch covered ACI. The only other complication noted was a minor wound healing problem in one patient.
Discussion
In full-thickness cartilage defects of the medial and lateral femoral condyle treated with autologous chondrocyte implantation (ACI) the percentage of patients with good and excellent clinical results varies between 75 and 85%, depending on the study population [4, 6, 9, 16, 33, 34]. While this proportion of satisfactory results has made ACI a well-established therapy for such patients, in the subgroup with patella defects the proportion who achieve good clinical results is lower. While in Brittberg’s original work describing the technique of ACI the success rate for retropatellar lesions was only two out of seven patients, i.e., only 29% [4], in recently published larger studies the success rates varies between 60 and 85%, but this means the clinical results are still unsatisfactory in approximately a third of the patients treated [2, 6, 12, 18, 28, 34].
It has been suggested that this high proportion of failures of ACI in patella defects can be explained with reference to the biomechanical characteristics of the anterior knee compartment, in which shearing forces—which are prejudicial to chondrogenic differentiation and cartilage regeneration—are greater than in the medial and lateral compartments of the knee, in which hydrostatic pressure leading to ultimate differentiation of the repaired cartilage is predominant [10, 13, 23, 41].
Nevertheless, since even in the patellofemoral joint of the knee good results can be achieved in two thirds of the patients treated with ACI for full-thickness cartilage defects, ACI seems appropriate in many cases [6, 28]. Far fewer patients are treated with ACI for cartilage defects in the anterior compartment than for cartilage defects on the femoral condyles. In most studies only a small proportion of the patients are described as having defects located on the patella [6, 34]. Minas and Bryant [28] are the only workers in this sector who have been able to describe an association between more detailed characteristics (such as defect location) of retropatellar cartilage lesions and clinical outcome in 36 patients treated with ACI for retropatellar cartilage defects. However, in this study, many patients received an additional ACI at another location, which makes it difficult to compare their results with ours. Although the patient population in this study included a greater proportion of patients with retropatellar defects than have those in other ACI studies, it was still not possible to identify either risk factors or prognostic factors that could be correlated with poor or with good clinical results. This also applies to the studies published by Gobbi et al. [12] who reported clinical and in some cases core spin tomography and histology results of 22 patients who had undergone retropatellar matrix-associated ACI and whose average IKDC score was 73.6 points and the work of Henderson who described the results of a total of 44 patients in dependence of patellar tracking treated with ACI for retropatellar cartilage defects [18].
In the present study a total of 70 patients with retropatellar cartilage defects were enrolled and followed up for a mean of 38 months (range 14.7–64.3 months). No correlation was found between clinical outcome and length of follow-up (p = 0.767), which is worth mentioning, since the length of follow-up varied from patient to patient, as is typical when a retrospective study design is used. Nevertheless, length of follow-up did not differ significantly between the different groups when the data were broken down by defect location (Fig. 3).
On analysis of the different scores used in this study it becomes obvious that the subjective IKDC score assesses the treatment outcome seems to be more critically than the Lysholm score. The average IKDC values are lower in all subgroups; at the same time the variance is larger in the IKDC compared to the Lysholm score. This is in keeping with the observations published in other studies [20, 30, 36, 38], although to our knowledge it has not been analysed in detail. Although objective investigation such as was conducted in this study is very important, when both scoring systems are used a good distinction can nonetheless be made between satisfactory and poor treatment outcomes, which is reflected in the wide standard deviation in the scores. The coefficient of correlation between each of the two scores used and outcome was 0.844, which indicates a close correlation, and thus they seem well suited to the assessment of patients who have undergone ACI. The correlation factor between subjective IKDC and Lysholm score equals the results published by other studies [38].
Overall, in 67.1% of our cases excellent and good clinical outcomes were achieved and severely abnormal results were obtained in only 12.9%. These observations are compatible with the results of studies of ACI of the patella published earlier [11, 12, 18, 28]. These satisfactory overall results are also reflected in the fact that 58 (82.9%) of the 70 patients followed up in this study recovered the level of sports activity they had enjoyed prior to injury and overall 84.3% reported clinical improvement compared with their condition prior to surgery. Most (81.4%) said they would have the same surgery again, which is also a good indicator of patient satisfaction. Nevertheless, the results also demonstrate that for a significant proportion of patients it was not possible to achieve a return to their earlier status. However, the results are within the range reported for the treatment of retropatellar cartilage defects in other studies, though it is difficult to compare studies in which other cartliage repair techniques (microfracturing, periosteum transplantation) have been used for the treatment of retropatellar cartilage lesions, as few studies dealing with this highly selected patient population are available. There does seem to be a tendency for our results to be better than those reported by Schonholtz and Ling [37] to have been achieved by chondroplasty in patients with retropatellar cartilage lesions and although they seem to be in the same range as early results reported by Lorentzon [25] following treatment of 26 patients by periosteum transplantation and slightly better than later results of the same group who further demonstrated the importance of adequate rehabilitation protocols following operative cartilage repair [1]. It is difficult in these cases to draw any conclusions or to make comparisons, owing to the differing study designs and small case numbers; in our opinion quantitative comparisons are not permissible, so that only trends can be desribed. Nevertheless, Gobbi [11] described a clear advantage of matrix-associated ACI but we have been unable to confirm this in our patient population.
One of the most crucial points, when treating retropatellar cartilage lesions is the decision how to accomodate the biomechanical abnormalities (such as patella maltracking or malalignment) that might led to the clinical problem of cartilage damage on the patella. There are several studies that strongly favor to correct these abnormalities in any cases in which biomechanical problems have been observed using surgical techqniues such as anteromedialisation of the tibial tuberosity or the Fulkerson procedure [28, 35]. Although we generally agree whith this, in the current study trochlea dysplasia as well as significant valgus deformity which both lead to a relevant lateralisation of the patella have been defined as a exclusion criteria in order to be able to exclusively obersve the results of ACI and in order to obtain a homogenous study population. Nevertheless, the fact that these problematic patients who certainly are associated with inferior clinical outcomes have been excluded of the current study needs to be kept in mind when the clinical results of the present study are compared to those of other studies. Exclusion of patients with severe biomechanical abnormalities might result in a higher rate of statisfying results in this study.
With reference to the clinical results in the current study, it is interesting that the different surgical techniques used, such as periosteal patch-covered conventional ACI, collagen membrane-covered ACI and 3D matrix-associated ACI, seemed not to have any influence on the results. With the exception, that the rate of transplant hypertrophy was highest in the group of periosteum patch covered ACI, which has already been described by other studies [14], the functional score led to similar results in all groups, and the small differences in the proportions of patients with good, excellent, abnormal and severely abnormal objective results were far from reaching statistical significance, so that we can assume it would not be possible to detect any differences in results between these surgical techniques even in a larger study population (p value(Lysholm) = 0.879, p value(IKDC) = 0.577).
In contrast to surgical technique, defect size was a major factor influencing the clinical outcome in patients treated for retropatellar defects with ACI (p value(Lysholm) = 0.007). Specifically, small defects were associated with a better outcome than were larger defects, an observation that has also been described by other authors reporting on cartilage defects located on the femoral condyles. Comparison of different locations of defects on the retropatellar side revealed that location of a defect on the lateral facet of the patella was significantly associated with a better clinical outcome than was location on the medial facet or location extending to both medial and lateral facets (p value(Lysholm) = 0.014, p value(IKDC) = 0.008). The deficits in these last-mentioned locations also differ in size (mean 4.4 cm² in the lateral facet, as against 6.2 cm² for deficits involving both lateral and medial facets), so that it is difficult to draw any conclusion on whether the observed differences in clinical outcome are due to the location of the defect or to the defect size alone. In the comparison between defects located on the lateral and on the medial facet the groups concerned are similar—notwithstanding a tendency for defects located on the lateral facet to be larger (see also Table 1)—and any difference revealed therefore seems more likely to have some clinical relevance.
In contrast to our initial theory—we assumed that well-trained, athletic patients with low BMI would have a good clinical outcome—body mass index (p value(Lysholm) = 0.548, p value(IKDC) = 0.883) and preoperative sports activity (p value(Lysholm) = 0.821, p value (IKDC) = 0.214) did not have any great influence on the clinical results. It has to be said, however, that the generally higher expectations of athletically active patients and the greater physical stress they expose themselves to do result in a tendency to dissatisfaction in the face of restrictions that are, objectively seen, quite tolerable. This tendency to dissatisfaction in fitter patients has a negative influence on the subjective scores, such as the Lysholm and IKDC scores, in this patient subgroup.
In the assessment of the patients’ athletic ability following ACI it is striking that, as expected, it is closely correlated with the postoperative function scores (p value(Lysholm) and p value(IKDC) < 0.001). In 58 of the 70 patients the level of sporting activity before their injury was recovered, which we regard as a completely satisfactory result. It must be said, however, that recovery of the preoperative level of athletic activity obviously depends on what the preoperative level was and the percentage of patients who do get back to their original level depends heavily on how active the whole patient population was. Patients with a preoperative level I sporting activity recovered this in 6 cases (50%), while 5 (62.5%) of the 8 with level II athletic activity before surgery recovered their original level. A large proportion of the patients did little or no sport before surgery (level III and IV), which is something that must be given due consideration in view of the high proportion of patients in the total population who recovered their original level.
In summary, we can conclude that the results of retropatellar ACI are excellent or good in approximately 70% of patients even when larger patient populations are considered. In contrast to these studies, detailed consideration of the patient population makes it possible to identify subgroups that are associated with better or worse clinical outcomes, which is highly useful in consultations when patients need sound advice on what treatment is indicated. This means that defect size acquires a decisive role in the patient’s prognosis and suggests that defects in the lateral facet have a better prognosis than those in the medial facet and than lesions extending beyond the patellar ridge. The prognosis of circumscribed cartilage lesions of the lateral facet following ACI, with 77% of good and excellent treatment outcomes, is in the same range as that of circumscribed lesions of the medial and lateral condyles of the femur, while large defects involving the region of the patellar ridge are associated with good results in only approximately 50% of cases. According to our data, these facts are more significant in the assessment of prognosis than what technique is applied for ACI. These observations should be borne in mind when decisions have to be made on whether or not ACI is indicated.
References
Alfredson H, Lorentzon R (1999) Superior results with continuous passive motion compared to active motion after periosteal transplantation. A retrospective study of human patella cartilage defect treatment. Knee Surg Sports Traumatol Arthrosc 7:232–238
Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, Skinner JA, Pringle J (2003) A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br 85:223–230
Brittberg M (2000) ICRS clinical cartilage injury evaluation system. 3rd ICRS Meeting Göteborg, Sweden
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331:889–895
Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A (2003) Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am 85-A (Suppl 3):109–115
Brittberg M, Tallheden T, Sjogren-Jansson B, Lindahl A, Peterson L (2001) Autologous chondrocytes used for articular cartilage repair: an update. Clin Orthop Relat Res 391:S337–S348
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A (Suppl 2):58–69
Erggelet C, Sittinger M, Lahm A (2003) The arthroscopic implantation of autologous chondrocytes for the treatment of full-thickness cartilage defects of the knee joint. Arthroscopy 19:108–110
Erggelet C, Steinwachs MR, Reichelt A (2000) The operative treatment of full thickness cartilage defects in the knee joint with autologous chondrocyte transplantation. Saudi Med J 21:715–721
Fitzgerald JB, Jin M, Grodzinsky AJ (2006) Shear and compression differentially regulate clusters of functionally related temporal transcription patterns in cartilage tissue. J Biol Chem 281:24095–24103
Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M (2006) Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med 34:1763–1773
Gobbi A, Nunag P, Malinowski K (2005) Treatment of full thickness chondral lesions of the knee with microfracture in a group of athletes. Knee Surg Sports Traumatol Arthrosc 13:213–221
Grodzinsky AJ, Levenston ME, Jin M, Frank EH (2000) Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng 2:691–713
Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, Cannon SR, Briggs TW (2004) The use of chondrogide membrane in autologous chondrocyte implantation. Knee 11:51–55
Hangody L, Feczko P, Bartha L, Bodo G, Kish G (2001) Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res 391:S328–S336
Henderson I, Francisco R, Oakes B, Cameron J (2005) Autologous chondrocyte implantation for treatment of focal chondral defects of the knee—a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee 12:209–216
Henderson I, Lavigne P, Valenzuela H, Oakes B (2007) Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res 455:253–261
Henderson IJ, Lavigne P (2006) Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee 13:274–279
Huberti HH, Hayes WC (1984) Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am 66:715–724
Koukoulias N, Papastergiou S, Kazakos K, Poulios G, Parisis K (2007) Clinical results of meniscus repair with the meniscus arrow: a 4- to 8-year follow-up study. Knee Surg Sports Traumatol Arthrosc 15:133–137
Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Uhl M, Sudkamp N (2006) Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy 22:1180–1186
Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N (2006) Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage 14:1119–1125
Lane Smith R, Trindade MC, Ikenoue T, Mohtai M, Das P, Carter DR, Goodman SB, Schurman DJ (2000) Effects of shear stress on articular chondrocyte metabolism. Biorheology 37:95–107
Lindahl A, Brittberg M, Peterson L (2001) Health economics benefits following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 9:358–363
Lorentzon R, Alfredson H, Hildingsson C (1998) Treatment of deep cartilage defects of the patella with periosteal transplantation. Knee Surg Sports Traumatol Arthrosc 6:202–228
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10:150–154
Minas T (1998) Chondrocyte implantation in the repair of chondral lesions of the knee: economics and quality of life. Am J Orthop 27:739–744
Minas T, Bryant T (2005) The role of autologous chondrocyte implantation in the patellofemoral joint. Clin Orthop Relat Res 436:30–39
Noyes FR, Barber SD, Mooar LA (1989) A rationale for assessing sports activity levels and limitations in knee disorders. Clin Orthop Relat Res 246:238–249
Nyland J, Hester P, Caborn DN (2002) Double-bundle posterior cruciate ligament reconstruction with allograft tissue: 2-year postoperative outcomes. Knee Surg Sports Traumatol Arthrosc 10:274–279
Perka C, Schultz O, Sittinger M, Zippel H (2000) Chondrocyte transplantation in PGLA/polydioxanone fleece. Orthopade 29:112–9
Petersen L, Brittberg M, Lindahl A (2003) Autologous chondrocyte transplantation of the ankle. Foot Ankle Clin 8:291–303
Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30:2–12
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 374:212–34
Pidoriano AJ, Weinstein RN, Buuck DA, Fulkerson JP (1997) Correlation of patellar articular lesions with results from anteromedial tibial tubercle transfer. Am J Sports Med 25:533–7
Risberg MA, Holm I, Steen H, Beynnon BD (1999) Sensitivity to changes over time for the IKDC form, the Lysholm score, and the Cincinnati knee score. A prospective study of 120 ACL reconstructed patients with a 2-year follow-up. Knee Surg Sports Traumatol Arthrosc 7:152–9
Schonholtz GJ, Ling B (1985) Arthroscopic chondroplasty of the patella. Arthroscopy 1:92–6
Sernert N, Kartus J, Kohler K, Stener S, Larsson J, Eriksson BI, Karlsson J (1999) Analysis of subjective, objective and functional examination tests after anterior cruciate ligament reconstruction. A follow-up of 527 patients. Knee Surg Sports Traumatol Arthrosc 7:160–165
Steinwachs MR, Kreuz PC (2003) Clinical results of autologous chondrocyte transplantation (ACT) using a collagen membrane. In: Nöth U, Hendrich C, Eulert J (eds) Cartilage surgery and future perspectives, Chap 5. Springer, Heidelberg, pp 37–47
Steinwachs MR, Kreuz PC, Krause SJ, Lahm A (2003) Klinische Ergebnisse nach Mikrofrakturierung bei der Behandlung von Gelenkknorpeldefekten. Sportorthopädie Sporttraumatologie 19:291–294
Torzilli PA, Deng XH, Ramcharan M (2006) Effect of compressive strain on cell viability in statically loaded articular cartilage. Biomech Model Mechanobiol 5:123–32
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niemeyer, P., Steinwachs, M., Erggelet, C. et al. Autologous chondrocyte implantation for the treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg 128, 1223–1231 (2008). https://doi.org/10.1007/s00402-007-0413-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-007-0413-9