Abstract
Purpose
To report arthroscopic second look as well as clinical results after arthroscopic autologous chondrocyte implantation (ACI) for articular cartilage repair at the knee joint.
Methods
A second-look assessment after arthroscopic ACI using spheroides was performed in 41 patients with 57 full-size articular cartilage defects of the knee. The median time from ACI to second-look arthroscopy was 10 (6–72) months. The ACI was assessed macroscopically and by probing according to the International Cartilage Repair Score (ICRS)–Cartilage Repair Assessment (CRA) to get information on the amount and quality of regeneration. Clinical follow-up with subjective outcome scores was performed an average of 34.5 ± 19.2 months after ACI. Twenty-seven (65.8 %) of ACI’s were combined with additional procedures.
Results
The ICRS-CRA was rated “normal” or “nearly normal” in 52 of 57 (91.3 %) and “abnormal” in 5 (8.8 %) of all cartilage defects. At follow-up, evaluation of KOOS was an average of 81.0 ± 12.9 for pain, 76.8 ± 16.6 for symptoms, 85.1 ± 14.9 for activities of daily living, 55.3 ± 27.7 for sport and recreation and 50.6 ± 23.8 for quality of live. IKDC was 63.0 ± 18.8, Lysholm score was 79.0 ± 18.0, and Tegner score was 4 (1–6). Subjective assessment according to the VAS scale was an average of 7.4 ± 2.1 for overall satisfaction and 6.7 ± 2.5 satisfaction for the operated knee. Seven patients (22.6 %) showed low subjective outcome scores at last follow-up—of these, 2 patients showed a CRA 3 and 5 a CRA 1 or 2.
Conclusion
At second-look arthroscopy, 52 (91.3 %) of all cartilage defects showed a normal or nearly normal macroscopic articular cartilage regeneration after arthroscopic ACI using spheroides. Twenty-four patients (77.4 %) showed good subjective clinical results. The high number of concomitant surgery reflexes the complex aetiology of cartilage lesions and complexity of treatment. Thus, a strict indication and surgical planing is necessary to avoid clinical failures.
Level of evidence
IV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current surgical treatment options for full-size articular cartilage injuries of the knee joint are limited. Possible options are marrow stimulation, osteochondral tissue transplantation or autologous chondrocyte implantation (ACI) [2, 4, 7, 11]. Marrow stimulating techniques such as microfracture have been shown to be effective in treating smaller lesions [4, 11, 35]. However, often the regenerated fibrocartilage lacks biomechanical quality and durability [16, 22]. Osteochondral transfer, e.g. mosaicplasty, has shown good clinical results, but the amount of donor tissue may limit the treatment of larger lesions [13]. Comparative trials were performed [15, 18, 19].
Since first performed 20 years ago, ACI has developed into a valuable treatment option for full-size articular cartilage lesions of the knee. Larger lesions can be treated, and clinical results are promising [3–5, 12, 17, 20, 23, 24, 26–30, 32, 33, 36]. A disadvantage of reported techniques is that the matrix-associated ACI has to be performed through an arthrotomy.
Since 2008, the senior author has used a matrix-associated ACI (Co.don AG, Teltow, Germany) which can be performed all-arthroscopically [10]. The clinical advantage is less soft tissue trauma and less postoperative pain, as well as fast recovery and rehabilitation. Therefore, the arthroscopic approach is an important new improvement, and clinical reports of its outcome were not yet published. Reports of second looks after ACI are generally rare [1, 6, 8, 12, 31], and results of arthroscopic-controlled ACI using spheroides have not been reported up today.
The aim of this study was to report arthroscopic second-look results and clinical results after all-arthroscopic ACI using spheroides at the knee joint.
Materials and methods
Between 2009 and 2014, a total of 41 patients with 57 full-thickness articular cartilage lesions of the knee had a second-look arthroscopy at our institution after ACI using spheroides (Co.don AG, Teltow, Germany). All patients were prospectively included in this study. Prior to surgery, patients were clinically and radiologically assessed for tibiofemoral and patellofemoral malalignment (Rosenberg view, lateral view, long leg axis radiographs, MRI). When pathologic, this was addressed simultaneously with, e.g. a high tibial osteotomy (HTO) or a medial tibial tubercle transfer, to offload the involved cartilage lesion and to optimise cartilage healing. Anterior cruciate ligament reconstruction and meniscal surgery were performed, too. Overall, concomitant surgery was necessary in 27 (65.8 %) of 41 patients. All surgeries were performed by the senior surgeon. Demographic data, characteristics of cartilage lesion and prior or concomitant surgeries are displayed in Table 1.
Surgical procedure of ACI
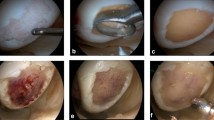
An arthroscopy was performed to assess the articular cartilage lesion and to reconfirm the indication for ACI. A cartilage biopsy was taken at the entrance of the intercondylar notch for cartilage cultivation. The chondrocytes were cultured in patients blood into spheroids (each about 200.000 autologous cartilage cells) (Co.don AG, Teltow, Germany) and were transferred into a sterile applicator with physiological saline solution for surgical implantation. The number of spheroides at time of implantation was between 7.5 and 96 per cm2 with an average of 33.7 ± 20.6 spheroids per cm2. After approximately 8 weeks of cartilage cultivation, the cells were ready for implantation. The mean size of the treated defects was 4.3 ± 3.4 cm2 Details of the cultivated spheroids are displayed in Table 2.
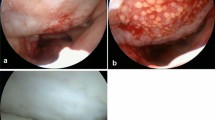
At time of ACI, the subchondral bone plate of the cartilage defect was carefully cleaned. The maximum damage tolerated for the cartilage shoulder was ICRS grade II–III. Severe degenerative cartilage damage ICRS III–IV, knee instability, significant subtotal meniscus loos or malalignment was exclusion criteria. The spheroids were introduced into the joint by a dry arthroscopy (Fig. 1a) and were allowed to set in place for 15 min to develop adhesions to the subchondral bone plate. Thus, there was no need to cover the ACI. Concomitant surgeries were performed at time of cartilage harvest or in combination with the ACI. Rehabilitation consisted of early passive motion as well as physiotherapy starting on the 2nd postoperative day. Weight bearing was restricted to 10 kg for 6 weeks, 20 kg in week 7 and 8 and with full weight bearing thereafter. Cycling and swimming were encouraged after 8 weeks, jogging after 1 year and strenuous stop-and-go sports earliest after 1.5 years depending on the degree of activity.
Second-look arthroscopy
Second-look arthroscopy was performed an average of 13.8 ± 13.2 months (6–72) after ACI. The quality of the ACI was assessed arthroscopically by the senior surgeon and two more orthopaedic surgeons by visual inspection and by using a probe according to the guidelines of the ICRS–Cartilage Repair Assessment (CRA). Arthroscopic examples of cartilage regeneration are displayed in Figs. 1 and 2. The reasons for second-look arthroscopy are displayed in Table 3. None of the secondary surgeries were done for cartilage evaluation only.
Clinical follow-up
Thirty-one patients completed the subjective questionnaires a mean of 34.5 ± 19.3 (6–72) months after ACI. Assessed were the knee injury and Osteoarthritis Outcome Score (KOOS), Lysholm score, subjective score of International Knee Documentation Committee (IKDC), Tegner activity scale, and VAS scale for satisfaction with the knee and overall (0–10, 0 means very unsatisfied and 10 very satisfied).
The current study was performed according to the Medical Association Declaration of Helsinki. The study protocol was approved by the regional ethical committee of the ATOS Clinic (ID-Nr. 4–15).
Statistical analysis
Mean, standard deviation (SD) and range were calculated. Due to ordinal data level for the CRA score (range 1–3), Chi-square tests were calculated to examine the association between CRA and predictors. Due to the approximate normal distribution, scatterplots and Pearson’s correlation coefficients for continuous values were applied in order to determine whether there is a correlation between predictors and metric outcome parameters. In addition, Kendall’s tau B coefficients were calculated for interval scaled (metric) and ordinal data, respectively. A two tailed p-value equal to or less than 0.05 was considered significant. Data analysis was performed with IBM SPSS for Windows (version 21). No priori sample size calculation was necessary as we included all second-look patients after ACI in a consecutive order.
Results
The cartilage status of the 57 lesions was rated “normal” (CRA I) in 12 lesions (21.1 %), “nearly normal” (CRA II) in 40 (70.1 %) and “abnormal” (CRA III) in 5 (8.8 %). None of the treated defects were rated as “severely abnormal” (CRA IV). Regarding the degree of defect repair, 40 lesions (70.2 %) were completely filled to the level of the surrounding cartilage shoulder and 9 lesions (15.8 %) reached only 75 % of the height of the surrounding cartilage shoulder. In terms of integration into the cartilage shoulder, 46 of 57 lesions (80.7 %) showed a complete integration into the surrounding cartilage. The macroscopic appearance was an “intact smooth surface” or (superficially) “fibrillated surface” in 43 lesions (75.4 %). Details of the ICRS-CRA assessment are displayed in Table 4.
The predictors BMI, size of defect, location of defect and follow-up time did not have a significant impact on the CRA. A significant correlation (p = 0.04) existed only between age and CRA, but without any trend. In the age group of 15–30 years, one defect (10 %) was CRA grade 1 and nine defects (90 %) were grade 2. The age group between 30 and 40 years resulted in eight defects (40 %) grade 1, nine (45 %) grade 2 and three (15 %) grade 3. In the age group 40 years and older, the assessment showed three defects (11.1 %) grade 1, 22 defects (81.5 %) grade 2 and two defects (7.4 %) grade 3.
The statistical evaluation of the subjective IKDC resulted in 63.0 ± 18.8, Lysholm score in 79.0 ± 18.0 and Tegner score in 3.5 ± 1.2 for the current level. The subjective assessment for satisfaction using the VAS scale showed an average of 7.4 ± 2.1 overall and 6.7 ± 2.5 for the operated knee. Results of KOOS subscales are displayed in a KOOS profile (Fig. 3). There was no correlation between the predictors age or BMI concerning the results of the subjective questionnaires. A positive correlation was found between the length of follow-up and subjective scores, e.g. KOOS for pain, symptoms, sport and recreation and quality of live, and the subjective IKDC with better results and longer follow-up time (Table 5) . There was no correlation found between CRA and clinical scores, which might be due to too the small number of patients.
Patients with insufficient cartilage regeneration
At second look, 4 patients with 5 cartilage lesions (8.8 %) were rated CRA grade 3 with an insufficient cartilage regeneration (CM 2–3 according to Outerbridge). All 4 patients have been complex cases (tibia plateau fracture with chronic kissing lesion, chronic cartilage lesion at the medial femoral condyle after mosaicplasty, chronic kissing lesion at patellofemoral joint, chronic retropatellar lesion).
Patients with low subjective scores
Seven patients showed low subjective outcome scores at their last follow-up. Two patients were rated CRA 3 and 5 patients CRA 1 or 2. Again, all patients were complex cases (2 × ACI + HTO, 1× subtotal loss of medial meniscus with joint space narrowing to 2–3 mm, 1× lateral meniscus allograft + ACI, 1× ACL revision, HTO and simultaneous ACT’s of 3 cartilage lesions, 1× new cartilage lesion grade 4 at other location).
Discussion
The most important finding of the present study was that arthroscopic ACI using spheroides is a reliable and safe procedure to repair full-size articular cartilage lesions of the knee joint. According to the ICRS-CRA, 52 defects (91.3 %) had a “normal” or “nearly normal” macroscopic cartilage regeneration assessed during second-look arthroscopy. Subjective clinical results were good in 24 patients (77.4 %).
At an average of 14 ± 13.2 (6–72) months postoperatively, 40 cartilage defects (70.2 %) were completely filled up to the height of the surrounding cartilage shoulder. The 17 defects (29.8 %) of the patients with a lower cartilage fill of 50–75 % of the depth had a shorter follow-up time of an average of 9.3 ± 3 (6–13) months. Overall, the ACI showed a complete integration into the surrounding cartilage shoulder in 46 of 57 lesions (80.7 %). Twenty-nine (50.9 %) of 57 repairs showed a superficial fibrillation of the cartilage regeneration and only 14 (24.6 %) a completely normal surface. The stiffness of the regenerated tissue tested with a probe was minimally softer than that of the surrounding cartilage shoulder in most cases, which might be related to the still maturating cartilage at short-term follow-up between 6 and 72 months after ACI.
In the current study, complete fill of the cartilage defects with regenerated “cartilage-like” tissue was achieved in 91.3 % of all lesions. In 77.4 %, the subjective clinical result was good or very good. Similar results were reported by Adachi et al. [1]. They showed that implantation of autologous tissue-engineered cartilage-like tissue was effective in the short- to midterm in 87.7 % according to the ICRS, and clinical rating had improved significantly. The arthroscopic finding was significantly correlated with the final clinical scores. Brun et al. [6] reported a multicentre study including 11 centres and reconfirmed above results. 63 s-look arthroscopies with 70 biopsies were taken 5–33 months after open implantation of a three-dimensional hyaluronic acid biomaterial (Hyalograft C Autograft, BioMed). Tissues taken from symptomatic patients were mainly fibrocartilage or mixed (hyaline–fibrocartilage) tissue, and biopsies taken from asymptomatic patients were hyaline cartilage in 83 % of biopsies. The amount of hyaline regenerated tissue was significantly higher in biopsies obtained more than 18 months after implantation compared to earlier biopsies. The authors concluded that cartilage maturation is still ongoing 18 months postoperatively and that persistence of symptoms might reflect the presence of a nonhyaline cartilage repair tissue. Enea et al. [8] reported a multicentre study of 33 s-look core biopsies in 30 patients. Patients were treated with an open matrix-associated ACI (MACI). At a mean follow-up of 15 months, 27 biopsies (81 %) were classified as normal or nearly normal according to the ICRS-CRA, and the overall median ICRS II histological score was 57 (41–75). However, 6 (18 %) were classified as abnormal or severely abnormal and only 21 % showed a hyaline-like cartilage of the examined biopsies. The authors concluded that the macroscopic appearance of the regenerated cartilage gave a good impression of the histological composition which was suggested to be still maturing after 15 months.
Good results were also reported by Gobbi et al. [12] at the patellofemoral joint. Clinical follow-up of 34 patients 5 years after second-generation ACI (hyaluronan-based scaffold seeded with autologous chondrocytes) demonstrated a significant improvement in IKDC, VAS and Tegner score. Second-look arthroscopy of eight patients an average of 14.8 months revealed the repaired surface to be nearly normal with hyaline-like biopsies. Meyerkort et al. [25] performed MRI’s of 23 patients 5 years after ACI (MACI) at the patellofemoral joint (9 combined with realignment procedure of the extensor mechanism). The mean weighted MOCART composite score improved to 3.4 at 5 years indicating an intact appearance in most grafts. Graft height measured >50 % of the cartilage shoulder in 82 % of patients and 91 % would undergo the procedure again. Petersen et al. [31] reported good-to-excellent clinical results and good repair tissue fill in midterm results of 94 patients 2–9 year (mean 4.2 years) after open ACI. Later, in a long-term evaluation with an average of 12.8 years follow-up, Petersen et al. [32] reported good results with a satisfaction rate of 92 % after open ACI, which was reconfirmed in other cohort studies recently with shorter follow-ups [9, 10, 14, 20, 32, 34].
In the current study, an overall decrease in activity level was observed. At follow-up, some patients were still recovering from the ACI and concomitant surgery. Similar to ACL patients, the decreased activity level may also reflect the fear of reinjury in this early phase of rehabilitation [21].
Four patients with persisting symptoms showed an insufficient cartilage regeneration of <50 % (CRA3) of defect fill. Another 5 patients showed low subjective outcome scores with—in contrast—a good cartilage regeneration CRA 1–2. These patients were either re-scoped within the first year after ACI in the early phase of cartilage regeneration or were very complex cases with early osteoarthritis, s/p partial meniscus resection, s/p meniscal allograft, s/p osteotomy, s/p ACL revision surgery or OATS. Especially in such complex cases, the indication for ACI must be decided very critically and was probably overstretched in above-described cases.
In the present study, the only correlation found was between follow-up time and subjective scores. The longer the clinical follow-up of our patients, the better their subjective scores. This finding is in line with the cartilage maturation and may also be influenced by the clinical results of concomitant surgeries. It seems to be very important to consider the aetiology of the individual cartilage lesions, e.g. alignment or instability, and to treat it accordingly.
A limitation of this study is that the second-look arthroscopies were performed an average of only 13 months after ACI, with a minimum second look time of 6 months. Therefore, the ICRS can only reflect an early stage of cartilage regeneration but not the final result. However, at this early stage, the morphologic results have been already very good. The subjective scores are also negatively influenced by the early follow-up and by the complexity of cases. Unfortunately, only one cartilage biopsy has been performed to assess the histological quality of the regenerated cartilage (Fig. 4a, b).
Conclusion
At second-look arthroscopy, 52 (91.3 %) of all cartilage defects showed a normal or nearly normal macroscopic articular cartilage regeneration after arthroscopic ACI using spheroides. Twenty-four patients (77.4 %) showed good subjective clinical results. The high number of concomitant surgery reflexes the complex aetiology of cartilage lesions and complexity of treatment. Thus, a strict indication and surgical planing is necessary to avoid clinical failures.
References
Adachi N, Ochi M, Deie M, Nakamae A, Kamei G, Uchio Y, Iwasa J (2014) Implantation of tissue-engineered cartilage-like tissue for the treatment for full-thickness cartilage defects of the knee. Knee Surg Sports Traumatol Arthrosc 22(6):1241–1248
Anderer U, Libera J (2002) In vitro engineering of human autogenous cartilage. J Bone Miner Res 17(8):1420–1429
Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G (2005) Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br 87(5):640–645
Bedi A, Feeley BT, Williams RJ 3rd (2010) Management of articular cartilage defects of the knee. J Bone Joint Surg Am 92(4):994–1009
Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331(14):889–895
Brun P, Dickinson SC, Zavan B, Cortivo R, Hollander AP, Abatangelo G (2008) Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther 10(6):R132
Cole BJ, Pascual-Garrido C, Grumet RC (2009) Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am 91(7):1778–1790
Enea D, Cecconi S, Busilacchi A, Manzotti S, Gesuita R, Gigante A (2012) Matrix-induced autologous chondrocyte implantation (MACI) in the knee. Knee Surg Sports Traumatol Arthrosc 20(5):862–869
Ferruzzi A, Buda R, Faldini C, Vannini F, Di Caprio F, Luciani D, Giannini S (2008) Autologous chondrocyte implantation in the knee joint: open compared with arthroscopic technique. Comparison at a minimum follow-up of five years. J Bone Joint Surg Am 90(Suppl 4):90–101
Fickert S, Gerwien P, Helmert B, Schattenberg T, Weckbach S, Kaszkin-Bettag M, Lehmann L (2012) One-year clinical and radiological results of a prospective, investigator-initiated trial examining a novel, purely autologous 3-dimensional autologous chondrocyte transplantation product in the knee. Cartilage 3(1):27–42
Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, Stokol T, Cheetham J, Nixon AJ (2010) Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am 92(10):1927–1937
Gobbi A, Kon E, Berruto M, Filardo G, Delcogliano M, Boldrini L, Bathan L, Marcacci M (2009) Patellofemoral full-thickness chondral defects treated with second-generation autologous chondrocyte implantation: results at 5 years’ follow-up. Am J Sports Med 37(6):1083–1092
Hangody L, Feczko P, Bartha L, Bodo G, Kish G (2001) Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res 391(Suppl):S328–S336
Harris JD, Siston RA, Pan X, Flanigan DC (2010) Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am 92(12):2220–2233
Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R (2003) Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am 85-A(2):185–192
Jones DG, Peterson L (2006) Autologous chondrocyte implantation. J Bone Joint Surg Am 88(11):2502–2520
Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O (2007) A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am 89(10):2105–2112
Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O (2004) Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am 86-A(3):455–464
Kon E, Filardo G, Berruto M, Benazzo F, Zanon G, Della Villa S, Marcacci M (2011) Articular cartilage treatment in high-level male soccer players: a prospective comparative study of arthroscopic second-generation autologous chondrocyte implantation versus microfracture. Am J Sports Med 39(12):2549–2557
Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M (2009) Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med 37(1):33–41
Kvist J, Ek A, Sporrstedt K, Good L (2005) Fear of re-injury: a hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 13(5):393–397
Libera J, Ruhnau K, Baum P, Lüthi U, Schreyer T, Meyer U, Wiesmann HP, Herrmann A, Korte T, Pullig O, Siodla V (2009) Cartilage Engineering. In: Meyer U, Handschel J, Wiesmann HP, Meyer T (eds) Fundamentals of tissue engineering and regenerative medicine. Springer, Berlin, pp 233–242
Manfredini M, Zerbinati F, Gildone A, Faccini R (2007) Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg 73(2):207–218
Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S (2006) Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol 57(1):16–23
Meyerkort D, Ebert JR, Ackland TR, Robertson WB, Fallon M, Zheng MH, Wood DJ (2014) Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc 22(10):2522–2530
Minas T (2001) Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res 391(Suppl):S349–S361
Mithofer K, Peterson L, Mandelbaum BR, Minas T (2005) Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med 33(11):1639–1646
Moradi B, Schonit E, Nierhoff C, Hagmann S, Oberle D, Gotterbarm T, Schmitt H, Zeifang F (2012) First-generation autologous chondrocyte implantation in patients with cartilage defects of the knee: 7 to 14 years’ clinical and magnetic resonance imaging follow-up evaluation. Arthroscopy 28(12):1851–1861
Ochi M, Uchio Y, Kawasaki K, Wakitani S, Iwasa J (2002) Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br 84(4):571–578
Peterson L, Brittberg M, Kiviranta I, Akerlund EL, Lindahl A (2002) Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 30(1):2–12
Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A (2000) Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 374:212–234
Peterson L, Vasiliadis HS, Brittberg M, Lindahl A (2010) Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med 38(6):1117–1124
Rosenberger RE, Gomoll AH, Bryant T, Minas T (2008) Repair of large chondral defects of the knee with autologous chondrocyte implantation in patients 45 years or older. Am J Sports Med 36(12):2336–2344
Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP (2009) Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med 37(Suppl 1):10S–19S
Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG (2003) Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 19(5):477–484
Zaslav K, Cole B, Brewster R, DeBerardino T, Farr J, Fowler P, Nissen C (2009) A prospective study of autologous chondrocyte implantation in patients with failed prior treatment for articular cartilage defect of the knee: results of the Study of the Treatment of Articular Repair (STAR) clinical trial. Am J Sports Med 37(1):42–55
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siebold, R., Karidakis, G., Feil, S. et al. Second-look assessment after all-arthroscopic autologous chondrocyte implantation with spheroides at the knee joint. Knee Surg Sports Traumatol Arthrosc 24, 1678–1685 (2016). https://doi.org/10.1007/s00167-015-3822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3822-2