Abstract
We investigate whether there is any association between the Braak neurofibrillary tangle (NFT) stage and clinical and MRI features in definite primary age-related tauopathy (PART). We analysed 52 cases with a Braak NFT tangle stage >0 and ≤IV, and a Thal phase of 0 (no beta-amyloid present). Twenty-nine (56%) were female. Median age at death was 88 years (IQR 82–92 years). Fifteen (29%) were TDP-positive (75% TDP stage I), 16 (31%) had argyrophilic grain disease and three (6%) had alpha-synuclein-positive Lewy bodies. TDP-43 inclusion when present were rare and predominantly perivascular. Of the 15 with TDP-43, three showed a moderate number of inclusions and also had hippocampal sclerosis, neuronal intranuclear inclusions and fine neurites of the CA1 region of the hippocampus. Four cases (8%) had an apolipoprotein epsilon 4 (APOE4) allele. There was a significant correlation between age at death and Braak NFT stage (r = 0.32, p = 0.02). After accounting for age at clinical examination, there were significant associations between Braak NFT stage, and WAIS-R Block Design and Trail Making Tests A and B, with higher Braak stage associated with poorer performances. Thirty of the 52 cases had completed an antemortem volumetric head MRI. Two separate MRI analyses revealed an association between higher Braak NFT stage and grey matter atrophy in the head of the left hippocampus. There were no significant clinical or radiologic associations with TDP-43. Findings from this study demonstrate that aggregated tau distribution is associated with poorer cognitive performance, as well as atrophy, in the absence of beta-amyloid. These findings support the parcellation of definite PART as a useful construct. The relatively low frequencies of APOE4, TDP-43, Lewy bodies, and hippocampal sclerosis, and the rarity and morphology of TDP-43 lesions are noted contrasts to what is typically observed in Alzheimer’s disease of the old.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, a panel of leading experts published a consensus paper describing a common pathology associated with human aging. They referred to this pathology as primary age-related tauopathy (PART) [12]. PART is pathologically characterized by the presence of tau-positive neurofibrillary tangles (NFTs) with minimal to absent beta-amyloid pathology. A diagnosis of PART requires a Braak NFT stage ≤IV (usually III or lower) and a Thal beta-amyloid phase ≤2 [12]. PART can be further subclassified pathologically as definite PART and possible PART depending on whether beta-amyloid is absent or present, respectively. That is, cases with a Thal phase of 0, meaning no beta-amyloid is present in the brain, are categorized as definite PART while cases with a Thal phase of 1 or 2 in which beta-amyloid is present, are categorized as possible PART [12]. Further non-pathologic classification of PART may also be undertaken, but requires knowledge about the clinical diagnosis prior to death. That is, PART can be further subclassified as asymptomatic PART (no clinical signs of cognitive decline or dementia) or symptomatic PART (cognitive decline or dementia is present) [33]. Therefore, there are four different categories of PART: asymptomatic definite PART; symptomatic definite PART; asymptomatic possible PART and symptomatic possible PART. Symptomatic definite and possible PART subsumes diseases that were known by the following labels: senile dementia with tangles [65]; neurofibrillary tangle-predominant form of senile dementia [7]; and neurofibrillary tangle-predominant dementia [34]. True prevalence of definite PART is unknown, although evidence suggests that PART alone may account for approximately 18% of pathologies of cognitively normal elderly [43] and 5% of cognitively impaired elderly [6].
Since the publication of pathological criteria defining PART, there have been debates [11, 21] as to whether PART is an aging phenomenon that differs from Alzheimer’s disease [12, 33] or whether PART is a part of Alzheimer’s disease [9, 16]. There is also debate as to whether PART is more akin to a variant of Alzheimer’s disease referred to as limbic predominant Alzheimer’s disease [53] in which there is relatively more tau burden in allocortex compared to isocortex. PART has also become relevant in the discussion of cognitively normal people with suspected non-Alzheimer pathophysiology (SNAP) [28]. Little is known about PART, yet the construct of definite PART allows us to study the effects of tau in the absence of beta-amyloid. With this in mind, the main aim for this study is to determine whether aggregated tau distribution, as defined by the Braak NFT stage, is associated with specific clinical or imaging features, in definite PART. Hence, we limited our analysis to definite PART, i.e. a Thal beta-amyloid phase 0, and Braak NFT tangle stage >0 and ≤IV. We hypothesized that increasing Braak NFT stages would be associated with more hippocampal atrophy and poorer cognitive performance in definite PART.
Methods
Subject selection
We identified all cases in our pathological database that had been recruited and prospectively followed in the Mayo Clinic Alzheimer’s Disease Research Center, Alzheimer’s Disease Patient Registry or the Mayo Clinic Study of Aging who had died with a brain autopsy between 1999 and 2012, had a Braak neurofibrillary tangle stage >0 and ≤IV [8] and a low likelihood of Alzheimer’s disease according to NIA-Reagan criteria [68]. Cases with a pathological diagnosis of a frontotemporal lobar degeneration [47], progressive supranuclear palsy [26], corticobasal degeneration [14], or Lewy body disease [51], were excluded. A total of 106 cases were identified. All cases had undergone apolipoprotein epsilon E genotyping as previously described [13].
This study was approved by the Mayo Clinic IRB. Prior to death, all subjects or their proxies had provided written consent for brain autopsy examination.
Pathological analysis
All 106 cases had undergone pathological examination according to the recommendations of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) [52] and each assigned a Braak neurofibrillary tangle stage [8] using modified Bielschowsky silver stain. Modified Bielschowsky stain was utilized for Braak staging in order to maintain consistence within our database. The left hemi-brain was fixed per protocol and paraffin block sections were stained with hematoxylin and eosin and modified Bielschowsky. Immunohistochemistry was performed using antibodies for alpha-synuclein (LB509; 1:200; Zymed, San Francisco, CA, USA), phospho-tau (AT8; 1:1000; Endogen, Woburn, MA, USA) and beta-amyloid (6F/3D; 1:10; Novocastra VectorLabs, Burlingame, CA, USA). In addition, all cases were screened for the presence of TDP-43 immunoreactivity [40]. Specifically, the amygdala and hippocampus were screened using a polyclonal antibody (MC2085) that recognizes a peptide sequence in the 25-kDa C-terminal fragment) with a DAKO-Autostainer (DAKO-Cytomaton, Carpinteria, CA, USA) and 3,3′-diaminobenzidine as the chromogen. Sections were reviewed (by DWD and KAJ) to assess for the presence of any TDP-43 immunoreactive lesions including neuronal cytoplasmic inclusions, dystrophic neurites, or neuronal intranuclear inclusions. We also determined TDP-43 stage based on published criteria [36, 37]. A diagnosis of hippocampal sclerosis was made if neuronal loss in the subiculum and CA1 regions of the hippocampus was out of proportion to the burden of neurofibrillary tangles [15] while a diagnosis of argyrophilic grain disease was made if there was silver and tau-positive spindle-shaped lesions, coiled bodies and balloon neurons in transentorhinal and entorhinal cortex, amygdala or temporal allocortex [31].
Thal phase determination
We determined Thal beta-amyloid phase [61] for all 106 cases. Our method for the determination of Thal phase has been previously published [54]. Briefly, Thal amyloid phase was assigned for all cases retrospectively by assessing for senile plaques in neocortex (i.e. mid-frontal, inferior parietal or superior temporal cortex), hippocampus, basal ganglia and cerebellar molecular layer using beta-amyloid staining (clone 6F/3D, 1:10 dilution; Novacastra Vector Labs, Burlingame, CA, USA). The maximum Thal amyloid phase was determined as follows: phase 0, no amyloid in any brain regions; phase 1, beta-amyloid limited to neocortex; phase 2, beta-amyloid involves CA1/subiculum of the hippocampus; phase 3, beta-amyloid present in basal ganglia; phase 4, beta-amyloid present in C4 region of the hippocampus or midbrain; and phase 5, beta-amyloid present in the cerebellar molecular layer. Of the 106 cases, 52 cases had a Thal phase 0. We analyse the C4 region of the hippocampus for Thal phase 4 determination given that the C4 region of the hippocampus was noted to perform as good as, if not better than, midbrain [61]. Hence, 52 cases met pathological criteria for definite PART and were utilized for the clinical analyses. Of these 52 cases, a subset of 30 had also completed a volumetric head MRI prior to death and was utilized for imaging analyses.
MRI analyses
Voxel-level comparisons of grey matter volume were performed using voxel-based morphometry (VBM) in SPM5 on the 30 cases that had completed an antemortem head MRI. All MRI were normalized to a customized template and segmented using customized priors and unified segmentation. Grey matter images were then modulated and smoothed using an 8-mm full-width-at-half-maximum smoothing kernel. A multiple regression analysis was used to assess correlations between grey matter volume and Braak stage, including age at death as a covariate and results were assessed uncorrected for multiple comparisons using a threshold of p < 0.001. Based on the VBM results, we also performed atlas-based parcellation, an independent technique, using the automated anatomical labelling (AAL) atlas to measure volumes of the total hippocampus [64], as well as volumes of the head, body and tail of the hippocampus, for each subject. The AAL hippocampal region-of-interest was manually edited to separate head, body and tail sections. The head included the hippocampal digitations and the uncus. The tail section included the posterior transverse segment of the hippocampus, and the segment in between the head and the tail was labelled as the body of the hippocampus. Given the concern that TDP-43 might be a confounder for any association identified between atrophy and with Braak NFT stage we further determined whether there were any significant differences in MRI features between those with and without TDP-43.
Clinical and neuropsychological assessments
For this study the following clinical and neuropsychological variables were analysed: Mini-Mental State Examination [19], Clinical Dementia Rating Scale Sum of Boxes [49], Wechsler Memory Scale-Revised Logical Memory II [66], Boston Naming Test [41], Control Oral word Association Test [45], Wechsler Adult Intelligence Scale-R Block Design [66], Auditory Verbal Learning Test Delayed Recall [57], Trailmaking Test Parts A and B [59], and a modified version of the Unified Parkinson’s Disease Rating Scale [17]. For some variables, we also converted the raw score to a Mayo’s Older American Normative Studies (MOANS) score [60] which provides an age-adjusted score. The average MOANS score is 10 with a standard deviation of 3. To further account for any confounding effect of duration from examination to death, we limited the analyses to only those cases with <4.0 years duration to death (n = 44 cases). Given the concern that TDP-43 might also be a confounder for any association identified with clinical variables and Braak NFT stage we further determined whether there were any significant differences in clinical features between those with and without TDP-43.
Statistical analyses
We used linear regression to evaluate the association between Braak stage at death—treated as a continuous predictor taking values 1, 2, or 3—and an individual’s last clinical or neuropsychological assessment or hippocampal volume treated as the response. All regression models included an adjustment for age, except for models involving MOANS scores, since MOANS by definition is an age-adjusted score. We summarize associations with Braak stage by reporting the regression coefficient and 95% bootstrap confidence intervals which can be interpreted as the estimated change in mean response for a one-unit increase in Braak. We used the bootstrap procedure with 10,000 replicates because of skewness in some response variables. This allowed us to make valid inferences without assuming approximate conditional normality. It is known that there is a relationship between hypothesis tests and confidence intervals (CIs) such that a 95% CI that excludes the null value of zero can be interpreted as corresponding to a two-sided p value <0.05. Using this relationship, we report percentile-based bootstrap CIs for the Braak effect and calculate a two-sided p value by identifying the coverage probability corresponding to the widest percentile-based CI that excluded the null value. The corresponding p value is the complement of this coverage probability. We interpret these p values as an analysis result from a “trend test” providing indications of how well our observed data fit the model under the null hypothesis and making the strong assumption that all aspects of our statistical model, including random sampling, are correct [23]. Smaller p values indicate a greater degree of incompatibility between our data and what we would expect given the null hypothesis.
Results
Fifty-two cases met inclusion criteria for the study. Demographics and clinical features and their associations with Braak NFT stage are shown in Table 1. Of the 52 PART cases, 29 (56%) were female. The median age at the time of clinical examination was 87 years (IQR 81–91) with median age of death being 88 years (IQR 82–92). The median time from last clinical evaluation to death was 1.0 year (IQR 0.5–2.6). Fifteen cases (29%) were TDP-positive. The distribution of TDP-43 in those with immunoreactivity was limited to TDP-43 stage 3, with the vast majority (n = 9; 75%) being TDP-43 stage 1 (amygdala only). In the majority of cases, there were rare-scant TDP-43 immunoreactive inclusions, often a single inclusion was observed, and in many cases inclusions were often perivascular (Fig. 1). Of the 15 cases with TDP-43, three cases (20%) had fine neurites in the CA1 region of hippocampus [38], neuronal intranuclear inclusions and hippocampal sclerosis. Sixteen cases (31%) had argyrophilic grain disease, and three (6%) had alpha-synuclein-positive brainstem Lewy bodies consistent with a diagnosis of incidental Lewy body disease [22]. Of the 52 definite PART cases, only four (8%) had an APOE4 allele. The median score and interquartile range for general cognition on the Mini-Mental State Examination at the time of last clinical evaluation (28 points; IQR 27–29 points) was within the typically considered normal range. There was a significant rank correlation between age at death and Braak NFT stage (r = 0.32, p = 0.02). After correcting for age at clinical examination and education with regression or via MOANS, there were evidence for associations between Braak NFT stage and WAIS-R Block Design, and Trailmaking Test Parts A and B, with higher Braak stage being associated with poorer performances (Table 2). There were no other significant associations including associations with either of two different measures of recall, or with the modified Unified Parkinson’s Disease Rating Scale, a measure of motoric function.
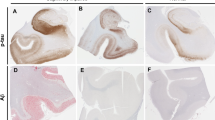
TDP-43 immunoreactive inclusions in PART. Variable lesion morphologies were observed, including perivascular associated TDP-43 (a) (v vessel), as well as neuronal cytoplasmic inclusions and dystrophic neurites in subpia (b) and amygdala (c), and neuronal intranuclear inclusions (d, arrow). Magnification ×40
There were no significant differences in demographics between the entire cohort of 52 cases and the subset that had completed an antemortem head MRI (n = 30). Demographics and clinical features of the 30 cases that had completed an antemortem volumetric head MRI scan are shown in Table 3. The VBM analysis showed correlations between Braak NFT stage and grey matter volume in the left anterior hippocampus, as well as in scattered regions of the cerebellum (Fig. 2). The region-of-interest level MRI analysis demonstrated associations between Braak stage and the left head of the hippocampus (p = 0.002), with an estimated volume decline of 6.02% for one-unit increase in Braak NFT stage (Fig. 3). The association between left hippocampal head and Braak stage survived a Bonferroni corrected p value of <0.006. No associations were identified between Braak NFT stage and volume loss of total hippocampal volume, or with volumes of the body or tail of the hippocampus (p > 0.05 for all).
Anatomic correlates of increasing Braak stage in definite PART. Focal atrophy (red) was identified in the left anterior hippocampus. Results are shown on coronal slices of the customized template through the anterior and posterior hippocampus and on a sagittal slice showing the left hippocampus. Results are shown uncorrected for multiple comparisons at p < 0.001
Those with TDP-43 were older at death (91 vs 87 years; p = 0.007) (Table 4). We found no significant differences in clinical features between cases with and without TDP-43. There were no significant differences in grey matter volume on VBM at an uncorrected threshold of p < 0.001 between the TDP-positive cases with MRI (n = 11), and an age, gender, and Braak NFT stage-matched group of TDP-negative cases (n = 11).
Discussion
This study focused on definite PART which allowed us to assess the effect of aggregated tau in the absence of beta-amyloid. The observed associations between the Braak NFT stage and demographics, clinical and MRI metrics, suggest that aggregated tau, in the absence of beta-amyloid and defined as PART, is not silent.
We found evidence of an association between Braak NFT stage and atrophy of the left head of the hippocampus. The finding of focal atrophy involving the hippocampus is not surprising given that the Braak NFT distribution was essentially capped at stage III (only 2 cases were stage IV) in our cohort which is in keeping with involvement of the hippocampus but not beyond. The presence of an association in the left hippocampus could be due to the fact that Braak staging was only performed on the left hemisphere. This is important since asymmetric lesions do occur in Alzheimer’s disease and also may occur in PART. Unlike in typical Alzheimer’s disease where atrophy of the hippocampus does not show an anterior and posterior gradient, atrophy in definite PART showed an anterior predominance. This is important as there is evidence that the anterior and posterior hippocampus have different network connections [1], with the posterior hippocampus involved with retrieval and encoding aspects of episodic memory [18, 24, 58]. The lack of involvement of the posterior hippocampus in definite PART, therefore, likely explains why we did not observe any association between tau and the Auditory Verbal Learning Test Delayed Recall or the WMS-R Logical Memory Delayed Recall Test. Similarly, observations have been made in another neurodegenerative disease, semantic dementia, in which the hippocampus is involved, but involvement is limited to the anterior hippocampus [10]. Patients with semantic dementia also show relative sparing of episodic memory [27] and hence it would not be surprising for PART to be associated with a semantic dementia like phenotype. Therefore, while studies have shown that NFT biochemistry is identical between PART and Alzheimer’s disease with both characterized by NFTs immunoreactive to 3R + 4R tau [34], further neuropathological studies are required to determine whether the distribution of NFTs in the hippocampus differ between PART and Alzheimer’s disease, as has been suggested [30]. In fact, it has been observed that extra-cellular tangles, so-called ghost tangles, are more frequent in PART compared to Alzheimer’s disease [34]. Hence, it would also be important to compare the distributions of ghost tangles in the hippocampus and other limbic areas between PART and Alzheimer’s disease. The findings from this study are also informative regarding the neuroimaging designation of SNAP. As previously discussed [42], the results suggest that NFT tauopathy can be associated with beta-amyloid negative hippocampal atrophy.
The Braak NFT stage was also associated with poorer performance on tests of cognitive speed, executive function, and visuospatial skills, although it is possible that the poorer performance on Trailmaking Test Part B and the WAIS-R Block Design could have been due to cognitive slowing, since both are timed tests [59, 66]. Hence, with this in mind, as well as the lower performance on Trailmaking Part A, it appears that tau in definite PART may be particularly associated with cognitive slowing. The anatomic correlate of cognitive slowing in this study requires further analysis. However, it is possible that cognitive slowing is related to atrophy of the hippocampus. In fact, studies have identified an association between cognitive slowing and damage to the hippocampus in cognitively normal elderly [5, 56]. It should also be noted that although there was evidence for an association between Braak NFT stage and poorer performance on these measures, the patients in this cohort, on average, performed much better than would be expected for Alzheimer’s disease. The finding that the vast majority in this cohort were relatively unimpaired at the time of last evaluation is consistent with PART being more associated with death without cognitive impairment than with dementia [44].
There is one large study on symptomatic PART (tangle-dominant dementia) that assessed demographic and clinical features although beta-amyloid status not accounted for [34]. Nothing is known about the demographics, clinical and pathological features of definite PART. This study therefore sheds light on, and increases our understanding of, definite part. We found that, similar to Alzheimer’s disease, there was an older age at death and a slight female predominance. Different from Alzheimer’s disease, however, was the identification of a low frequency of the APOE 4 allele. The frequency of the APOE4 allele in this study of 8% is similar to the calculated frequency of 10% in the PART consensus criteria that utilized NACC data [12]. While it is known that the APOE4 allele frequency decreases with age [35], the APOE4 allele frequency was found to be 32% in an Alzheimer’s disease cohort with identical mean age of death of 88 years [35]. This is 3–4 times higher than what we observed in definite PART. This finding is not all that surprising though as the APOE4 allele has been linked to beta-amyloid, and beta-amyloid by design was absent in the 52 cases in this study. Lewy bodies were identified in only 6% of our cases which is not unexpected given the frequency of incidental Lewy bodies reported in normal aging cohorts [22]. The low frequency of Lewy bodies in definite PART differs from that reported in Alzheimer’s disease which has been as high as 56% [63], even in Alzheimer’s disease cases with lower Braak stages [32]. The frequency of argyrophilic grain disease was relatively high in this cohort, being present in almost a third of the cases. Therefore, since argyrophilic grain disease is a 4R tauopathies [62], many cases of definite PART would be expected to have both 4R tau and 3R + 4R tau lesions. Our finding also supports argyrophilic grain disease being an age-related phenomenon as previously suggested [43, 48]. Surprisingly, we found argyrophilic grain disease to be present in almost 50% of those with TDP-43, a finding that although not reaching significance, requires further investigating.
We assessed the frequency, distribution, morphology and associations of TDP-43 in PART. The frequency of TDP-43 was observed at 29% which is lower than the frequency of TDP-43 reported in studies of Alzheimer’s disease when the amygdala is screened, where the range is between 42 and 74% [4, 36, 50]. In addition, unlike in Alzheimer’ disease where TDP-43 distribution covers all stages (stages 1–6) [36, 37] with only 16% of Alzheimer’s disease cases being TDP-43 stage 1, we did not observe any definite PART cases in which TDP-43 distribution was beyond stage 3. In fact, 75% of PART cases with TDP-43 were TDP-43 stage 1. This may be important since we and others previously found little evidence for a difference between TDP-43 stage 0 and stage 1 in Alzheimer’s disease [29, 36]. In PART we also observed that TDP-43 when present was rare to scant and in many cases only one inclusion was identified. This contrasts with Alzheimer’s disease where TDP-43 deposition is on average moderate, at times even frequent [4]. In terms of morphology, the majority of the inclusions were perivascular and not the typical neuronal cytoplasmic inclusions or dystrophic neurites or tangle-associated TDP-43 observed in Alzheimer’s disease [2]. Perivascular-type inclusions have previously been observed in frontotemporal degeneration and familial Lewy body disease [46], as well as in elderly non-demented adults [20]. Therefore, TDP-43 morphology in PART appears to be different from TDP-43 morphology in Alzheimer’s disease, but more similar to TDP-43 in diseases without amyloid. The differences observed between TDP-43 in PART and TDP-43 in Alzheimer’s disease may explain the absence of any association between clinical and/or imaging features and TDP-43 in definite PART compared to Alzheimer’s disease where TDP-43 is strongly associated with memory loss and hippocampal atrophy [40].
Of the 15 definite PART cases with TDP-43, three cases are worth discussing separately. These three cases all had fine neurites in the hippocampus [38], and all three also had hippocampal sclerosis. All three cases could be considered to have frontotemporal lobar degeneration especially given the presence of neuronal intranuclear inclusions. However, given that all three cases were 90 years old at the time of death, one could argue that a more appropriate interpretation for the presence of TDP-43 in these three cases is cerebral age-related TDP-43 with sclerosis [55]. Hence, the most appropriate pathological diagnosis for these three cases would be definite PART with cerebral age-related TDP-43 with sclerosis. We did not have any case in which the presence of TDP-43 would be considered a pathologic precursor to hippocampal sclerosis [3, 25] since there were no cases with TDP-43 and fine neurites of the CA1 region of the hippocampus without hippocampal sclerosis, or even TDP-43 deposition outside of the amygdala, other than the three with hippocampal sclerosis.
One of the current debates centres on whether PART is more akin to the limbic predominant form of Alzheimer’s disease in which NFT’s burden is relatively greater in limbic regions compared to neocortical regions [53]. Operationally, to be diagnosed as limbic predominant Alzheimer’s disease, the ratio of the average hippocampal to cortical NFT count must be greater than 75th percentile of the entire Alzheimer’s disease cohort, hippocampal NFT densities must be greater than the median values for CA1, subiculum, and CA1-subiculum average, and at least three of the cortical NFT densities must be less than or equal to the median values [53]. Two independent cohorts showed that the average age of death of limbic predominant Alzheimer’s disease cases were 86 and 89 years [53, 67], similar to the median age of death of our definite PART cohort, yet the APOE4 allele frequencies were 65 and 70% which is in stark contrast to the APOE4 allele frequency of 8% in PART. Similarly, the relatively low frequency of TDP-43 in definite PART (29%) is also in contrast to the very high frequency of TDP-43 in limbic predominant Alzheimer’s disease (67%) [39]. These differences argue against PART being more akin to limbic predominant Alzheimer’s disease.
One of the central debates concerning PART and Alzheimer’s disease is whether PART represents early Alzheimer’s disease, and, therefore, with time patients with PART will develop Alzheimer’s disease. That is, patients with PART over time will show beta-amyloid deposition. While pathology cannot answer this question of progression given its cross-sectional nature, future studies with tau-PET may be useful to determine the proportion of cases of definite PART that will later develop amyloid deposition and other features of Alzheimer’s disease. Regardless, however, of the outcome of such studies, we show that a substantial number of patients die with PART who do not have beta-amyloid. Perhaps it is a matter of semantics, but if medial temporal tauopathy is dissociable from Alzheimer’s disease (i.e. NFT and plaque disease), it seems reasonable to conclude the former should be denoted as a distinct entity.
We did find a correlation between Braak NFT stage and age at death suggesting that with definite PART, tau deposition progresses as individuals age. Hence, it appears that NFT-tau increases in abundance over time in the absence of beta-amyloid. The cases included in this study were old, with an average age at death of 88 years; hence, if PART represented early AD, one would assume that beta-amyloid deposition in such patients would be occurring in the 9th–10th decade of life, which although not impossible, seems a less likely phenomenon.
In summary, we have demonstrated that in definite PART, the Braak NFT stage is associated with poorer performance on neuropsychological tests, and left anterior hippocampal atrophy further supporting PART as a useful construct, separate from Alzheimer’s disease.
References
Adnan A, Barnett A, Moayedi M, McCormick C, Cohn M, McAndrews MP (2016) Distinct hippocampal functional networks revealed by tractography-based parcellation. Brain Struct Funct 221:2999–3012. doi:10.1007/s00429-015-1084-x
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61:435–445. doi:10.1002/ana.21154
Aoki N, Murray ME, Ogaki K, Fujioka S, Rutherford NJ, Rademakers R, Ross OA, Dickson DW (2015) Hippocampal sclerosis in Lewy body disease is a TDP-43 proteinopathy similar to FTLD-TDP Type A. Acta Neuropathol 129:53–64. doi:10.1007/s00401-014-1358-z
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117:125–136. doi:10.1007/s00401-008-0480-1
Aribisala BS, Royle NA, Maniega SM, Valdes Hernandez MC, Murray C, Penke L, Gow A, Starr JM, Bastin ME, Deary IJ et al (2014) Quantitative multi-modal MRI of the Hippocampus and cognitive ability in community-dwelling older subjects. Cortex 53:34–44. doi:10.1016/j.cortex.2013.12.012
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Bancher C, Jellinger KA (1994) Neurofibrillary tangle predominant form of senile dementia of Alzheimer type: a rare subtype in very old subjects. Acta Neuropathol 88:565–570
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K (2014) Are cases with tau pathology occurring in the absence of Abeta deposits part of the AD-related pathological process? Acta Neuropathol 128:767–772. doi:10.1007/s00401-014-1356-1
Chan D, Fox N, Rossor M (2002) Differing patterns of temporal atrophy in Alzheimer’s disease and semantic dementia. Neurology 58:838
Crary JF (2016) Primary age-related tauopathy and the amyloid cascade hypothesis: the exception that proves the rule? J Neurol Neuromed 1:53–57
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. doi:10.1007/s00401-014-1349-0
Crook R, Hardy J, Duff K (1994) Single-day apolipoprotein E genotyping. J Neurosci Methods 53:125–127
Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, Jellinger K, Lantos PL, Lippa CF, Mirra SS et al (2002) Office of rare diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol 61:935–946
Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (> or =80 years of age) humans. Acta Neuropathol 88:212–221
Duyckaerts C, Braak H, Brion JP, Buee L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M et al (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756. doi:10.1007/s00401-015-1390-7
Fahn S, Elton RL, Committee UD (1987) The unified Parkinson’s disease rating scale. Recent developments in Parkinson’s disease. MacMillian Healthcare Information, Florham Park, NJ, pp 153–163, 293–304
Fernandez G, Weyerts H, Schrader-Bolsche M, Tendolkar I, Smid HG, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR et al (1998) Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci 18:1841–1847
Folstein MF, Robins LN, Helzer JE (1983) The mini-mental state examination. Arch Gen Psychiatry 40:812
Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM et al (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and without severe mental illness. Arch Neurol 67:1238–1250. doi:10.1001/archneurol.2010.254
Giaccone G (2015) The existence of primary age-related tauopathy suggests that not all the cases with early Braak stages of neurofibrillary pathology are Alzheimer’s disease. J Alzheimers Dis 48:919–921. doi:10.3233/JAD-150435
Gibb WR (1986) Idiopathic Parkinson’s disease and the Lewy body disorders. Neuropathol Appl Neurobiol 12:223–234
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350. doi:10.1007/s10654-016-0149-3
Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V (2003) Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus 13:164–174. doi:10.1002/hipo.10064
Hatanpaa KJ, Bigio EH, Cairns NJ, Womack KB, Weintraub S, Morris JC, Foong C, Xiao G, Hladik C, Mantanona TY et al (2008) TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. J Neuropathol Exp Neurol 67:271–279. doi:10.1097/NEN.0b013e31816a12a6
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I (1994) Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44:2015–2019
Hodges JR, Patterson K (2007) Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol 6:1004–1014. doi:10.1016/S1474-4422(07)70266-1
Jack CR Jr, Knopman DS, Chetelat G, Dickson D, Fagan AM, Frisoni GB, Jagust W, Mormino EC, Petersen RC, Sperling RA et al (2016) Suspected non-Alzheimer disease pathophysiology–concept and controversy. Nat Rev Neurol 12:117–124. doi:10.1038/nrneurol.2015.251
James BD, Wilson RS, Boyle PA, Trojanowski JQ, Bennett DA, Schneider JA (2016) TDP-43 stage, mixed pathologies, and clinical Alzheimer’s-type dementia. Brain. doi:10.1093/brain/aww224
Jellinger K (2003) Plaque-predominant and tangle-predominant variants of Alzheimer’s disease. In: Dickson D (ed) Neurodegeneration: the molecular pathology of dementia and movement disorders, pp 66–68
Jellinger KA (1998) Dementia with grains (argyrophilic grain disease). Brain Pathol 8:377–386
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm (Vienna) 111:1219–1235. doi:10.1007/s00702-004-0138-7
Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR Jr et al (2015) PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 129:757–762. doi:10.1007/s00401-015-1407-2
Jellinger KA, Attems J (2007) Neurofibrillary tangle-predominant dementia: comparison with classical Alzheimer disease. Acta Neuropathol 113:107–117. doi:10.1007/s00401-006-0156-7
Jicha GA, Parisi JE, Dickson DW, Cha RH, Johnson KA, Smith GE, Boeve BF, Petersen RC, Knopman DS (2008) Age and apoE associations with complex pathologic features in Alzheimer’s disease. J Neurol Sci 273:34–39. doi:10.1016/j.jns.2008.06.008
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450. doi:10.1007/s00401-013-1211-9
Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, Liesinger AM, Petersen RC, Parisi JE, Dickson DW (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131:571–585. doi:10.1007/s00401-016-1537-1
Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118:349–358. doi:10.1007/s00401-009-0547-7
Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE et al (2015) TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol 78:697–709. doi:10.1002/ana.24493
Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF et al (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127:811–824. doi:10.1007/s00401-014-1269-z
Kaplan E, Goodglass H, Weintraub S (1978) The Boston Naming Test. Veterans Administration Medical Center, Philadelphia, PA
Knopman DS, Jack CR Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe VJ, Kantarci K, Gunter JL, Senjem ML, Mielke MM et al (2013) Brain injury biomarkers are not dependent on beta-amyloid in normal elderly. Ann Neurol 73:472–480. doi:10.1002/ana.23816
Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE et al (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62:1087–1095
Kryscio RJ, Abner EL, Jicha GA, Nelson PT, Smith CD, Van Eldik LJ, Lou W, Fardo DW, Cooper GE, Schmitt FA (2016) Self-reported memory complaints: a comparison of demented and unimpaired outcomes. J Prev Alzheimers Dis 3:13–19. doi:10.14283/jpad.2015.74
Lezak M (1995) Neuropsychological Assessment. Oxford University Press, New York, NY
Lin WL, Castanedes-Casey M, Dickson DW (2009) Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol 68:1167–1176. doi:10.1097/NEN.0b013e3181baacec
Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4. doi:10.1007/s00401-009-0612-2
Martinez-Lage P, Munoz DG (1997) Prevalence and disease associations of argyrophilic grains of Braak. J Neuropathol Exp Neurol 56:157–164
Mattis S (1988) Dementia rating scale: professional manual. Psychological Assessment Resources, Odessa, FL
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2016) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. doi:10.1111/bpa.12424
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872. doi:10.1212/01.wnl.0000187889.17253.b1
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796. doi:10.1016/S1474-4422(11)70156-9
Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, Rawal B, Parisi JE, Petersen RC, Kantarci K et al (2015) Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138:1370–1381. doi:10.1093/brain/awv050
Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang WX, Kryscio RJ et al (2016) “New Old Pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 75:482–498. doi:10.1093/jnen/nlw033
Papp KV, Kaplan RF, Springate B, Moscufo N, Wakefield DB, Guttmann CR, Wolfson L (2014) Processing speed in normal aging: effects of white matter hyperintensities and hippocampal volume loss. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 21:197–213. doi:10.1080/13825585.2013.795513
Rey A (1964) L’examen clinique en psychologie. Presses Universitaires de France, Paris, France
Schacter DL, Wagner AD (1999) Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus 9:7–24. doi:10.1002/(SICI)1098-1063(1999)9:1<7:AID-HIPO2>3.0.CO;2-K
Spreen O, Strauss E (1998) Compendium of Neuropsychological tests, second edition: administration, norms and commentary. Oxford University Press, New York, NY
Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ (2005) Mayo’s older Americans normative studies: age- and IQ-adjusted norms for the Wechsler memory scale-revised. Clin Neuropsychol 19:378–463. doi:10.1080/13854040590945201
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Togo T, Sahara N, Yen SH, Cookson N, Ishizawa T, Hutton M, de Silva R, Lees A, Dickson DW (2002) Argyrophilic grain disease is a sporadic 4-repeat tauopathy. J Neuropathol Exp Neurol 61:547–556
Tsuang DW, Wilson RK, Lopez OL, Luedecking-Zimmer EK, Leverenz JB, DeKosky ST, Kamboh MI, Hamilton RL (2005) Genetic association between the APOE*4 allele and Lewy bodies in Alzheimer disease. Neurology 64:509–513. doi:10.1212/01.WNL.0000150892.81839.D1
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289. doi:10.1006/nimg.2001.0978
Ulrich J, Spillantini MG, Goedert M, Dukas L, Stähelin HB (1992) Abundant neurofibrillary tangles without senile plaques in a subset of patients with senile dementia. Neurodegeneration 1:257–284
Wechsler D (1987) Wechsler memory scale-revised (manual). Psychological Corporation, New York, NY
Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC et al (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11:868–877. doi:10.1016/S1474-4422(12)70200-4
WorkingG roup (1997) Consensus recommendation for the post-mortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for Neuropathologic Assessment of Alzheimer’s disease. Neurobiol Aging 18:S1–S2
Acknowledgements
This study was supported by the US National Institute of Aging (NIA) Grants R01 AG037491 (PI: KAJ), P50 AG16574 (PI: RCP), U01 AG006786 (PI: RCP), R01 AG11378 (PI: CRJ) and R01 AG041851 (PI: CRJ). We wish to thank Kris Johnson, Linda Rousseau, Virginia Phillips and Monica Casey-Castenedes for pathological support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Josephs, K.A., Murray, M.E., Tosakulwong, N. et al. Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133, 705–715 (2017). https://doi.org/10.1007/s00401-017-1681-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1681-2