Abstract
Alzheimer’s disease (AD), the most common form of dementia worldwide, is a mixed proteinopathy (β-amyloid, tau and other proteins). Classically defined as a clinicopathological entity, AD is a heterogeneous, multifactorial disorder with various pathobiological subtypes showing different forms of cognitive presentation, currently referred to as the Alzheimer spectrum or continuum. Its morphological hallmarks are extracellular β-amyloid (amyloid plaques) and intraneuronal tau aggregates forming neurofibrillary tangles and neurites, vascular amyloid deposits (cerebral amyloid angiopathy), synapse and neuronal loss as well as neuroinflammation and reactive astrogliosis, leading to cerebral atrophy and progressive mental/cognitive impairment (dementia). In addition to “classical” AD, several subtypes with characteristic regional patterns of tau pathology have been segregated that are characterized by distinct clinical features, differences in age, sex distribution, disease duration, cognitive status, APOE genotype, and biomarker levels. In addition to four major subtypes based on the distribution of tau pathology and brain atrophy (typical, limbic predominant, hippocampal sparing, and minimal atrophy), several other clinical variants (non-amnestic, corticobasal, behavioral/dysexecutive, posterior cortical variants, etc.) have been identified. These heterogeneous AD variants are characterized by different patterns of key neuronal network destructions, in particular the default-mode network that is responsible for cognitive decline. Other frequent age-related co-pathologies, e.g., cerebrovascular lesions, Lewy and TDP-43 pathologies, hippocampal sclerosis, or argyrophilic grain disease, essentially influence the clinical picture and course of AD, and can challenge our understanding of this disorder including the threshold and causal relevance of each individual pathology. Unravelling the clinico-morphological heterogeneity among the AD spectrum entities is important for better elucidation of the pathogenic mechanisms affecting the aging brain that may enable a broader diagnostic coverage of AD as a basis for implementing precision medicine approaches and for developing preventive and ultimately disease-modifying therapies for this devastating disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), the most common form of dementia in adults that currently affects around 50 million people worldwide, is clinically featured by a multidomain cognitive impairment with progressive decrease of daily living abilities. Its principal risk factor is age, with disease incidence increasing from 2/1000 at age 65–74 years to 337/1000 at age 85+, and doubling every 5 years after age 65, with peaks in the tenth decade and slight decrease afterwards. The overall AD duration varies between 24 years (age 66) and 15 years (age 80), with estimated preclinical durations of 10 years (prodromal 4 years and dementia 6 years) (Vermunt et al. 2019). With the disproportional increase of the elderly population, the prevalence of AD will approach around 132 million worldwide by 2050, and, thus, has become a tremendous public health and socioeconomic challenge of the twenty-first century (Alzheimer’s-Association 2021). The scientific approach to AD suffers from mismatches between clinical, pathological and technological data, and from heterogeneity in cohort datasets impeding the reproducibility of results (Birkenbihl et al. 2021) and causing difficulties in conceiving diagnostic gold standards and creating models for drug discovery and screening (Ferrari and Sorbi 2021). As available treatments only target symptoms and neither slow nor reverse the progression of the disease, the development of disease-modifying therapies is urgent (Long and Holtzman 2019). The value of aducanumab, a monoclonal antibody targeting aggregated amyloid-β (Aβ), recently approved for AD treatment by the US Food and Drug Administration, is a matter of current discussion (Karlawish 2021; Barenholtz Levy 2021; Liu and Howard 2021; Musiek and Morris 2021; Nisticò and Borg 2021; Planche and Villain 2021; Schulman et al. 2021).
AD was originally defined as a clinicopathological entity, clinically characterized by progressive memory deficit involving multiple cognitive domains or skills, and a defining pathological substrate with deposition of Aβ in extracellular plaques and cerebral vasculature (cerebral amyloid angiopathy/CAA), neuritic plaques defined by the presence of phosphorylated tau protein (p-tau), intraneuronal aggregations of p-tau as neurofibrillary tangles (NFTs) in cell soma, and neuropil threads (NT), mainly in dendritic compartments and, to a lesser degree, in the axonal domain. These changes are accompanied by early synaptic loss, activated microglia, mitochondrial dysfunction causing energy loss, neuroinflammation, neurovascular dysfunction, disruption of the blood–brain barrier, neuronal loss, reactive astrogliosis, and brain atrophy (DeTure and Dickson 2019; Jellinger 2020; Trejo-Lopez et al. 2021). Clinical diagnosis of AD, according to the recommendations of the International Working Group, is to be restricted to people with positive biomarkers and specific AD phenotypes (Dubois et al. 2021), but despite the advent of more specific neuroimaging and reliable biomarkers, the final diagnosis of AD rests with postmortem neuropathology, using the updated National Institute on Aging/Alzheimer’s Association (NIA/AA) “ABC” criteria (Montine et al. 2012). This score for AD neuropathological changes (ADNC) combines “A” for the phase of amyloid plaques (Thal et al. 2002), “B” for the NFT stages (Braak and Braak 1991), and “C” for the CERAD neuritic plaque score, which describes the density of neuritic plaques in certain key regions in the neocortex (Mirra et al. 1993). From this combination, it gives a likelihood for the degree of ADNC in an individual case. Table 1 shows how each of the three scores are transformed to state the level of ADNC on a four-tiered scale (non, low, intermediate, and high). Thus, the entire process of the neuropathological diagnosis of AD can be followed along the pathways shown in Fig. 1. Standard metrics for tangles and neuritic plaques are usually semi-quantitative (Hyman et al. 2012; Mirra et al. 1993; Montine et al. 2012) and, according to the BrainNet Europe consortium, good agreement can be reached in the diagnosis only when the lesions are substantial, e.g., when having reached isocortical structures (Braak stage V–VI with absolute agreement 91%), while for mild lesions the agreement was poorer (Braak stage I–II, agreement 50%) (Alafuzoff et al. 2008), thereby limiting the ability to make accurate correlation of antemortem cognitive status and morphological findings (Jellinger 2011; Nelson et al. 2012). These changes, which involve brain regions and neuronal cell types in a characteristic pattern as a result of selective cellular and regional vulnerability to pathological proteins and their spread through the brain (Fornari et al. 2020; Mattsson et al. 2016; Mrdjen et al. 2019), present clinically as progressive decline of cognition and other brain functions, including non-cognitive, behavioral and psychological symptoms, like agitation, anxiety, depression, apathy, delusions, sleep and appetite changes (Casanova et al. 2011; Cerejeira et al. 2012; Gottesman and Stern 2019). Neuropsychiatric symptoms such as agitation, aggression, psychosis and activity disturbances increase as the dementia progresses, while affective symptoms (anxiety, depressive symptoms) remain stable throughout the cognitive spectrum, except for an increase in depression-dementia score in severe dementia (Wiels et al. 2021). Neuropsychiatric and cognitive symptoms are both prevalent across the Alzheimer clinical spectrum, but show different evolution during the course of disease. Cognitive function shows a uniform gradual decline, while large intraindividual heterogeneity of neuropsychiatric symptoms was seen over time across all AD groups (Eikelboom et al. 2021). These symptoms have been linked to multiple areas of neurodegeneration, including frontal and/or limbic regions as well as involvement of brainstem areas (serotonergic dorsal raphe nucleus, noradrenergic locus ceruleus), which may be early affected in AD (Grinberg et al. 2009; Matchett et al. 2021; Simic et al. 2009). However, not all persons who meet neuropathological criteria for AD exhibit cognitive impairment, and new criteria recognize the presence of preclinical pathology (Mehta and Schneider 2021). More detailed approaches for assessing comorbid conditions, such as Lewy and TDP-43 pathologies, vascular brain injury, and others, that frequently co-exist with ADNC and can complicate the pathological diagnosis, have also been considered (Montine et al. 2012). Current practice used in the AD Centers Program achieved an excellent agreement for the revised NIA/AA guidelines for the severity score for ADNC and, together with the National Alzheimer’s Coordinating Center (NACC) database offers a robust and rapid method to evaluate newly identified conditions and provides carefully curated neuropathological information (Besser et al. 2018; Mock et al. 2020). Meta-analysis of 20 (out of 1189) studies to distinguish autopsy-verified AD from other dementias or healthy controls showed a sensitivity of 85.4% (95% CI 80.8–90) and a specificity of 77.7% (95% CI 70.2–85.1). Values were higher for neuroimaging procedures and slightly lower for cerebrospinal fluid (CSF) biomarkers, while the combination of both resulted in higher values (Plassman et al. 2006). These data are related to two factors: (1) the various subtypes or variants of AD, as described later, and (2) the high frequency of comorbidities in elderly persons that may cause difficulties in the clinical diagnosis of AD.
Pathway of the combination of different pathological features that allows a classification of ADNC according to the NIA-AA guidelines (Jellinger 2020)
AD, a mixed proteinopathy (Aβ, tau, TDP-43, and others), is increasingly recognized as a pathologically heterogeneous, multifactorial and biologically multilayered disorder, currently referred to as the Alzheimer continuum (Jack et al. 2019), with several pathobiological phenotypes and various co-pathologies (Ferreira et al. 2020; Mehta and Schneider 2021). There are growing genetic, transcriptional, and proteomic data pointing to the complexity of AD pathogenesis, indicating that multiple distinct pathways can drive AD pathology (Wisniewski and Drummond 2019). Its variability in age and clinical presentation is well known, such as the differences between early-onset AD (EOAD) and the more common late-onset AD (LOAD), both showing a different burden of ADNC, with significant heterogeneity in cognitive presentation and patterns of atrophy and hypometabolism (Phillips et al. 2019). Individuals with early AD show heterogeneity in disease progression, which increases when stratifying on risk factors associated with progression (Jutten et al. 2021). EOAD is associated with greater likelihood with an atypical, non-memory-dominant clinical presentation, including visual, language, executive, behavioral or motor dysfunctions (Graff-Radford et al. 2021), especially in the absence of APOE ε4, but it has faster cognitive and functional decline than LOAD, regardless of the APOE genotype (Smirnov et al. 2021), although APOE genotype varies by age at onset and clinical phenotype, highlighting the heterogenic nature of AD (Whitwell et al. 2021). Clustering analysis identified two clusters of patients in an EOAD cohort: cluster 1 had a predominant memory impairment and more frequent APOE ε4 allele; cluster 2 showed faster cognitive and functional decline and more severe posterior brain abnormalities (Pollet et al. 2021).
Clinicopathological features of AD variants
The main clinical phenotype of AD is the amnestic type targeting episodic memory, but the clinical spectrum of AD extends well beyond the classical amnestic-predominant syndrome (Villain and Dubois 2019). With the new definition of AD as a biological spectrum, using the NIA/AA research framework (Jack et al. 2019), it is now possible to review the different pathophysiologically defined subtypes of AD (Ferreira et al. 2020). Neuropathological criteria align with revised clinical framework for considering ADNC in living individuals according to a biologically based categorization termed “ATN” (Jack et al. 2019), which uses flexible combinations of in vivo biomarkers for Aβ deposition (A), tau pathology (T), and neurodegeneration (N). They include CSF or plasma biomarkers, positron emission tomography (PET), and functional and structural magnetic resonance imaging (MRI). The biomarker profiles and categories of the Alzheimer continuum referring to ADNC have been summarized recently (Jellinger 2020). The risk of progressive cognitive deterioration differs considerably between the various phenotypes: A−N− < A+N− < A+T−N+ < A+T+N+, the A+T−N+ cases with worse prognosis than the negative (A−T−N−) ones (Knopman et al. 2018). Some contributions are associated with preclinical AD (A+T+N−), symptomatic stages (A+T+N+), or non-AD diseases (A−T±N+) (Jack et al. 2018b). Several main diagnostic pathways with distinct biomarker sequences have been proposed. Neuroimaging can track neurodegeneration and other sources of network impairments, metabolomics provides a molecular snapshot that is sensitive to ongoing pathological processes, and genomics characterizes genetic risk factors representing key pathways associated with AD (Badhwar et al. 2020). In addition to amyloid and tau PET as well as (18)FFDG-PET assessing glucose metabolism, emerging biomarkers able to measure synapse injury and loss in the brain are placed in different positions in the order of diagnostic evaluations, depending on clinical presentation (Chételat et al. 2020; Colom-Cadena et al. 2020; Dronse et al. 2017; Hanna Al-Shaikh et al. 2020). Comparison of the diagnostic accuracy of PIB (amyloid) PET versus 18F-FDG-PET in a series of autopsy-confirmed dementia cases revealed a higher sensibility of PIB-PET for intermediate-high ADNC, with similar specificity. When both modalities were congruent, sensibility and specificity approached 100% (Lesman-Segev et al. 2021). A recent study demonstrated that tau-PET is a promising prognostic marker in preclinical/prodromal stages of AD (Ossenkoppele et al. 2021) and can be used to identify the density and distribution of AD-type tau pathology, supporting a neuropathological diagnosis of AD (Fleisher et al. 2020).
A seminal clinicopathological study distinguished three AD subtypes based on postmortem NFT density: typical AD (tAD) with balanced NFT counts in the neocortex and hippocampus (75%), hippocampal-sparing (HcSP) forms with predominant involvement of association cortices (11%), and a limbic-predominant (LP) type with predominant involvement of the hippocampus (14%) (Murray et al. 2011). They corresponded to different clinical phenotypes with different ages at onset and progression rates: HcSP patients had earlier age at disease onset, higher proportion of men, and more rapid disease progression than tAD, while LP-AD patients were older at onset, involved more women, and progressed more slowly than the other groups. The disease duration of LP-AD and tAD cases was similar, age at death for LP-AD was highest and for HcSP forms lowest, which was coherent with the possible contribution of TDP-43 pathology, hippocampal sclerosis (HS) and the MAPT (microtubule-associated protein tau) H1H1 genotype to LP-AD, factors related to atrophy related to temporal lobe, older age and slower disease progression. APOE ε4 carriers more frequently had LP-AD and tAD than HcSP-AD in noncarriers, suggesting that APOE-negativity may increase resistance of the hippocampus against tau pathology (Mattsson et al. 2018). Vascular co-pathology was highest in LP-AD and lowest in HcSP-AD, while Lewy co-pathology was lowest in the latter subtype. These data were largely confirmed by a personal study of 933 autopsy cases of AD, all with Braak NFT stage > 4, although tAD was slightly more frequent than in the Mayo series (82.5 vs 75%) (Jellinger 2012). In both series, age at disease onset and death was significantly higher in LP-AD than in tAD (p < 0.01) and lower in HcSP-AD (Charil et al. 2019). While the final Mini Mental State Examination (MMSE) scores in the Mayo series were lowest in HcSP-AD, in the Vienna series they were lowest in tAD. Among co-pathologies, cerebrovascular lesions in our cohort were most frequent in LP-AD and Lewy pathology in HcSP-AD, but slightly less frequent than in the Mayo series. Minimal AD was not considered in both series.
292 participants with autopsy-confirmed AD from the Religious Orders Study and Memory and Aging Project were categorized by neuropathological subtype based on regional NFT counts. There were 20% LP-AD, 8% HcSP-AD, and 73% tAD. People with HpSp-AD declined rapidly, but might not have a memory-sparing syndrome. Compared to tAD, they performed significantly worse in all cognitive domains and declined faster in memory, language, and globally, but did not have relatively preserved memory performance (Uretsky et al. 2021).
The AD subgroups, based on the distribution of tau pathology and corresponding brain atrophy have also been identified by neuroimaging studies using structural MRI (Byun et al. 2015; Park et al. 2017; Risacher et al. 2017; Sintini et al. 2020; Zhang et al. 2021b). Most severe medial-temporal atrophy was seen in LP-AD > tAD > HcSP-AD, and most severe cortical atrophy in HcSP-AD > tAD > LP-AD. tAD and non-amnestic forms had a significantly higher ratio of hippocampal to cortical volumes than those with an amnestic presentation (Whitwell et al. 2012). Recent MRI studies consistently identified four categories of brain atrophy: both hippocampal and neocortical forms, hippocampal only and no or minor gray matter atrophy (minimal atrophy AD/MA-AD), which shows comparable clinical severity (Dong et al. 2017; Ferreira et al. 2019; Oppedal et al. 2019; Poulakis et al. 2018), while others showed diffuse atrophy (32.2%), bilateral parietal, frontal and temporal atrophy (occipital sparing; 29.2%), left temporal dominant (22.4%), and MA-AD (16.1%) (Zhang et al. 2021a). Compared with the other groups, MA-AD showed an intermediate age at onset and a slower rate of disease progression. However, despite absence of significant brain atrophy, this group, due to positive AD CSF biomarker levels (increased p-tau and decreased Aβ), fulfilled the diagnostic criteria for AD, which was concordant with other studies (Hwang et al. 2015; Noh et al. 2014; Shiino et al. 2006). The MA-AD subtype was associated with an increased risk of developing clinical AD over time (Planche et al. 2019).
In prodromal AD, also four atrophy patterns were identified: (1) largely normal neuroanatomical profile with least abnormal cognitive and CBS biomarker profiles and slowest clinical progression; (2) classical AD with fastest clinical progression; (3) diffuse pattern of atrophy, less pronounced in medial-temporal lobe and greater executive impairment; (4) notable focal involvement of medial-temporal lobe and slow steady progression (Dong et al. 2017). Another study of prodromal AD using structural MRI identified the following four atrophy types: (1) medial-temporal predominant atrophy with worst memory and language function; (2) parieto-occipital atrophy with poor executive/attention and visuospatial functioning; (3) mild atrophy with best cognitive performance and language; (4) diffuse cortical atrophy with intermediate cognitive functions. The medial-temporal subtype showed fastest decline in memory and language, the parieto-occipital one declined fastest in the executive/attention domain and the diffuse subtype in visuospatial functioning, while the mild subtype showed intermediate decline in all subtypes (Ten Kate et al. 2018). FDG-PET data identified only three main hypometabolic AD subtypes: (1) typical AD (48.6%), showing classical posterior temporal-parietal patterns; (2) “limbic predominant” (44.6%), characterized by old age and a memory-predominant cognitive profile; and (3) a relatively rare “cortical-predominant” subtype (6.8%), characterized by younger age and more severe executive dysfunction (Levin et al. 2021).
A study using MRI, tau PET and amyloid PET also distinguished three subtypes of AD: medial temporal dominant (53%), parietal dominant (23%), and diffuse (24%), although Aβ deposition did not differ across the subtypes (Jeon et al. 2019), suggesting a typical pattern of tau spread with NFTs developing in the entorhinal cortex and then progressing to the association cortices. The MA-AD type shows the earliest presentation of the disease, which progresses via LP-AD finally to typical AD (Fig. 2).
Main factors and characteristics of the four major subtypes of AD. AD Alzheimer’s disease, NFT neurofibrillary tangle, WMH white matter hyperintensity, CAA cerebral amyloid angiopathy, EOAD early-onset Alzheimer’s disease, LOAD late-onset Alzheimer’s disease, LP-AD limbic-predominant Alzheimer’s disease (Jellinger 2020)
According to studies on the cognitive relevance of atrophy subtypes in 2083 patients with mild cognitive impairment (MCI), typical/diffuse brain atrophy was associated with faster cognitive decline and higher risk of developing AD over time; HcSP and LP atrophy were also associated with incidental dementia with faster decline in the L-P atrophy group. DLB was more frequent in the HcSP- and minimal/no atrophy group (Planche et al. 2021). LP-AD and MA-AD subtypes show A+T−N− or A+T−N+ profiles (Cedres et al. 2020). Four tau atrophy subtypes were distinguished: medio-temporal predominant, LP, diffuse, and MA-AD. The medio-temporal predominant and diffuse subtypes showed intra-network connectivity reduction in the default-mode visual and limbic network and pronounced reduction of “global efficiency”; LP-AD showed only marginal global network failure, while MA-AD, in contrast, showed limited impairment in cognitive scores but prominent global network failure (Rauchmann et al. 2021). Recent studies in Aβ-negative and Aβ-positive individuals, all cognitively unimpaired, with and without MCI suggested that tau pathology may affect memory performance in cognitively unimpaired individuals via reduced functional connectivity in critical medial temporal lobe-cortical networks, while memory impairment in patients with MCI was associated with posterior hippocampal atrophy (Berron et al. 2021).
Tau PET studies in 260 Aβ-positive patients with MCI or dementia revealed the highest tau load in HcSP-AD and tAD, while LP patients showed significantly higher entorhinal tau load (Ossenkoppele et al. 2020), indicating that tau pathology is closely related to neurodegeneration (Das et al. 2021; Iaccarino et al. 2017; Tetzloff et al. 2018). They can significantly interrupt key brain networks, which could induce symptoms in the absence of overt cortical atrophy in the medial temporal lobe in MA-AD that shows memory impairment comparable to LP-AD and tAD (Ferreira et al. 2017, 2019; Risacher et al. 2017).
Distinct patterns of network destruction, which parallel the NFT (Braak stage V) and atrophy pattern of the four AD subtypes, have been documented (Ferreira et al. 2019), indicating that disruption of the default network is a consistent damage in heterogeneous AD groups (Byun et al. 2015; Varol et al. 2017). There are associated mechanisms underlying large-scale network disruption linking known molecular biomarkers and phenotypic variations in the AD spectrum (Wang et al. 2021). Amyloid is a partial mediator of the relationship between functional network failure and tau deposition in functionally connected brain regions, causing a cascading network failure in AD. Younger age of disease onset was associated with ‘non-Braak-like’ patterns of tau, suggesting an association with atypical clinical phenotypes (Jones et al. 2017). There is a close relationship between gray matter network disruptions and tau pathology in individuals with abnormal amyloid, which may reflect a reduced communication between neighboring brain areas and an altered ability to integrate information from distributed brain regions with tau pathology, indicative of a more random network topology across different AD stages (Pelkmans et al. 2021).
Investigation of associations of 18F-florbetapir (FBP) retention in the white matter with MRI-based markers of white matter degeneration AD, clinical progression, and fluid biomarkers showed that FBP retention in the white matter was associated with large-caliber axon degeneration. FBP uptake in normal-appearing white matter predicted clinical decline in preclinical or prodromal AD. Demyelination levels progressed across the AD continuum and were associated with clinical progression at early stages, suggesting that this process might be a relevant degenerative feature in the disease course (Moscoso et al. 2021). A meta-analysis of 24 studies calculated the pooled frequency of the four AD subtypes. tAD, characterized by tau pathology and atrophy of both hippocampus and association cortex, was most frequent with a pooled frequency of 55%; LP-AD, HcSP-AD and MA-AD had pooled frequencies of 21, 17 and 15%, respectively (Ferreira et al. 2020). These data depended on the algorithm used for biological and nonbiological subtyping, such as age at onset, disease duration, education years, MMSE, clinical dementia rating, APOE status, and CSF biomarkers.
These and other findings support the distinct subtype hypothesis, with pathology spreading through the brain in a different manner in these subtypes, as opposed to the staging hypothesis of brain pathology (Ferreira et al. 2019; Murray et al. 2011; Zhang et al. 2016). The assembly of mixtures of Aβ subtypes into different Aβ seeds leads to the formation of distinct subtypes having distinct physiochemical and biological properties which result in the generation of distinct AD molecular subgroups (Di Fede et al. 2018). Several studies suggest that conformational diversity of misfolded Aβ is a leading factor for clinical variability in AD, supporting the notion that AD subtypes are encoded in disease-associated Aβ-sheet heterogeneities (Confer et al. 2021; Duran-Aniotz et al. 2021). Moreover, certain types of Aβ aggregates exhibit key hallmarks of prion strains including divergent biochemical attributes and the ability to induce distinct pathological phenotypes when intracerebrally injected into mouse models. The evidence demonstrates that Aβ can assemble into distinct strains of aggregates and how such strains may be primary drivers of the phenotypic heterogeneity in AD (Lau et al. 2021). Amyloid spatially exceeds tau and neurodegeneration, with individual heterogeneities. Molecular pathology and neurodegeneration usually show progressive overlap along the AD course, indicating shared vulnerabilities or synergistic toxic mechanisms (Iaccarino et al. 2021). Although Aβ and tau might have differential downstream effects on synaptic and axonal function in a stage-dependent matter, recent findings suggest that amyloid-related changes occur first, followed by tau-related axonal degeneration (Pereira et al. 2021).
In PSEN1-carriers, altered γ-secretase activity and resulting Aβ accumulation are prerequisites for EOAD, However, tau hyperphosphorylation pattern, and its degradation by the proteasome, drastically influences disease onset in individuals with otherwise similar Aβ pathology, hinting towards a multifactorial model of familial AD (Sepulveda-Falla et al. 2021). Autosomal dominant AD (AD-AD), present in a younger population, is more likely to manifest with non-amnestic features in the prodromal phase or neurological signs, such as seizures or paralysis. The large variety of mutations associated with AD-AD explains the wide range of phenotypes, while in LOAD they are explained by age-associated co-morbidities (Rujeedawa et al. 2021). In sporadic AD, a wide range of heterogeneity, also influenced by tau pathology, has been identified (Dujardin et al. 2020; Murray et al. 2015). Cortical NFT burden was higher among atypical clinical variants relative to the amnestic syndrome.
Other recent studies evaluating cortical thickness and tau-PET in a cohort of cognitively impaired, amyloid-positive (A+) individuals revealed a T–N mismatch correlated with several underlying factors such as age and burden of WMH lesions. These data also indicate that tau-atrophy variability represents different biologically relevant subtypes, thus documenting the phenotypic heterogeneity of AD (Das et al. 2021). The propensity of a tau monomer to adopt distinct conformations appears to be linked to defined local motifs that expose different patterns of amyloidogenic amino acid sequences (Vaquer-Alicea et al. 2021). However, the molecular link between Aβ plaques and NFTs is still unclear. Recent studies have shown that PAX6 (a transcription factor essential for eye and brain development and increased in AD brain) signaling pathways plays a key role between Aβ toxicity and hyperphosphorylation (Zhang et al. 2021b).
HcSP-AD cases showed the highest levels of education, while MA-AD patients had the lowest, suggesting that high education may be one of the factors protecting the hippocampus and contributing to the fact that LP-AD may be unmasked and may contribute more to its clinical manifestation. Based on the cognitive reserve theory suggesting that education is beneficial for the brain by forming more pathology-resistant networks, it may act as surrogate for or act in synergy with cognitive or social engagement (Giovacchini et al. 2019; Stern 2012). Among Aβ-positive individuals, greater cognitive reserve relates to attenuated clinical progression in preclinical stages of AD, but accelerated cognitive decline after the onset of dementia (van Loenhoud et al. 2019). More severe neurodegeneration and more aggressive disease progression in HcSP-AD (Byun et al. 2015; Murray et al. 2011; Na et al. 2016) and the absence of hippocampal atrophy in MA-AD, which has a lower level of reserve, support this hypothesis (Ferreira et al. 2020).
Higher functional MRI-assessed system segregation was associated with less decrement in global cognition and episodic memory per unit increase of temporal lobe tau PET, indicating that higher segregation of functional connections into distinct large-scale networks supports cognitive resilience in AD (Ewers et al. 2021).The AD-resilient group (pathological AD without cognitive impairment) showed preserved densities of synaptophysin-positive presynaptic terminals and dendritic spines and increased densities of GFAP-positive astrocytes compared to the AD-dementia group and normal controls (Arnold et al. 2013). Hence, greater amounts of presynaptic proteins and distinct protein–protein interactions may be components of cognitive reserve reducing the risk of dementia with aging (Honer et al. 2012). Data from a large population-based sample of older adults discovered genetic factors associated with differential cognitive resilience to brain amyloidosis determined by PET, supporting the hypothesis that genetic heterogeneity is one of the factors that explains differential cognitive resilience to brain amyloidosis (Ramanan et al. 2021).
All AD subtypes show reduced basal forebrain volumes: LP-AD had the fastest atrophy rate, while MS-AD did not show any significant volume decline over time; the atrophy rates of the hippocampus and precuneus also differed among subtypes (Machado et al. 2020). NFT accumulation in the nucleus basalis of Meynert (NBM) was lowest in HcSP-AD, higher in tAD, and highest in LP-AD. Both changes may underlie more severe cholinergic deficits in EOAD individuals, in particular in patients with HcSP-AD (Hanna Al-Shaikh et al. 2020).
In conclusion, AD is heterogenous in each aspect, such as amyloid composition, tau distribution, relation between Aβ and tau, resulting brain atrophy, clinical symptoms, and genetic background. Thus, it is impossible to explain AD with a single pathological process, but the pathogenic factors for its heterogeneity need further elucidation.
Rapidly progressive Alzheimer’s disease
Among AD subtypes, a rapidly progressive form (rpAD), is characterized by rapidly progressive cognitive decline and/or short disease duration, and the possible occurrence of early focal neurologic signs (Schmidt et al. 2010, 2011). Early impairment in executive functions or language, motor disturbances, such as myoclonus (66–75%), gait impairment (66–887%), pyramidal (53–6%) or extrapyramidal (54%) signs, visual signs, such as hallucinations (44–62%), or psychiatric symptoms, frequently occurred in rpAD, either alone or in combination (Schmidt et al. 2012; Tosto et al. 2015). The mean duration of disease is between 2 and 3 years. Patients are younger at the time of death (60.0 vs 81.8 years) (Pillai et al. 2018).
Individuals with rpAD have more severe pathology, more comorbidities and lower baseline neuropsychology test scores of language and executive functions (Nance et al. 2019). Due to the rapid course of disease, rpAD mimics Creutzfeldt–Jakob disease (CJD), and represents a relatively frequent alternative diagnosis among cases referred to as possible or probable CJD to surveillance centers for prion diseases worldwide (Abu-Rumeileh et al. 2018a). The early clinical differential diagnosis between these two disorders can be challenging given the partial overlap in clinical features, although CSF biomarkers are within the range expected for classical AD except for protein 14-3-3, which was detectable in 43% of rpAD cases (Schmidt et al. 2012), whereas CSF neurofilament light chain protein (NfL) performs better than t-tau in the discrimination between rpAD and prion disease (Abu-Rumeileh et al. 2018b). Although rpAD and tAD seem to share the neuropathological core features, recent evidence suggests that a distinctive molecular signature involving the structure of Aβ aggregates and the proteomic landscape of amyloid plaques may distinguish rpAD from tAD. Studies using solid-state nuclear magnetic resonance measurements demonstrated a specific Aβ-40 fibril structure in t-AD and posterior cortical atrophy variant AD, whereas in rpAD they exhibited a significantly greater proportion of additional structures indicating differences in Aβ structure between different subtypes of AD (Qiang et al. 2017). Plaques from rpAD patients were abundant in synaptic proteins, in particular these involved in synaptic vesicle release, indicating the importance of synaptic dysfunction in accelerated plaque development in rpAD (Drummond et al. 2017). Recent proteomic studies indicated that the disregulation and dislocation factor proline and glutamine-rich protein (ASPQ), the subsequent DNA-related anomalies and aberrant function of TIA-A-positive stress granules in association with pathological tau represent a critical pathway which contributes to rapid progression of AD (Younas et al. 2020). Studies using conformation-dependent immunoassay (CDI) revealed distinct special attributes of diffuse and cored plaques in the temporal cortex of rapidly and slowly progressive AD, indicating a major conformational diversity of Aβ accumulating in the neocortex, with the most notable differences in the temporal cortex of rpAD (Liu et al. 2021), while spectroanalytic studies of Aβ proteoforms extracted from brain tissue detected the presence of highly hydrophobic Aβ seed in rpAD brains that seeded secretion at a slowed pace in comparison to t tAD (Noor et al. 2021). Previous studies showed a significant 1.2 decrease of di-glycosylated prion protein (PrP) isoforms in rpAD, suggesting distinct PrP involvement in association with the altered PrP interacting protein in rpAD (Zafar et al. 2017). Involvement of high-density oligomers of PrP were isolated in frontal cortex tissues from rpAD brains that are suggested to be involved in destabilization of the neuronal actin/tubulin infrastructure, as a contributing factor for the rapid progression of rpAD (Shafiq et al. 2021).
Specific dementia phenotypes (PART and others)
The current guidelines for the pathological diagnosis of AD only consider the classical “plaque and tangle” phenotype, but not other forms such as the “plaque only but without tangle formation predominant” type with abundant amyloid or the “little or no tau pathology” type limited to the hippocampus and abnormal p-tau pathology in neocortical pyramidal cells. This latter type observed in 3–8% of demented subjects over age 85 years (Tiraboschi et al. 2004) represents a specific type of dementia with Lewy bodies (DLB-AD) (Hansen et al. 1990). Another phenotype is the recently described “primary age-related tauopathy” (PART), originally described as NFT-predominant dementia (Jellinger and Attems 2007), predominantly involving people aged 85 years and associated with MCI. It is morphologically characterized by tau pathology restricted to the medial temporal lobe (Braak NFT stages 0–4), relative absence of amyloid (Thal Aβ phases 0–2), and total absence of neuritic and rare CAA (Josephs et al. 2017), while NFT changes in PART with both 3 and 4 repeat (3R and 4R) tau isoforms are identical to those in tAD (Jellinger and Attems 2007). MAPT H1H1 genotype frequency is high in both PART and LP-AD and similar to tAD, while APOE ε4 is rare in PART (Bancher et al. 1997). In PART, the lack of Aβ oligomers is suggested to be responsible for lesser tau aggregation, lower Braak NFT stages, and less cognitive impairment, since they have been shown to potentiate tau aggregation by promoting tau seed uptake (Shin et al. 2019). The pattern of hippocampal tau pathology differs significantly between PART and AD (Jellinger 2018; Zhang et al. 2020). Whereas tau pathology in tAD usually displays relative sparing of the hippocampal subregion CA2 until later stages, early involvement and greater NFT intensity in this subregion than in CA1 has been demonstrated in PART that also frequently shows significant asymmetry of hippocampal tau pathology (Walker et al. 2021a, b). Positive correlations were reported in PART between the Braak NFT stage and TDP-43 stage and density (Zhang et al. 2019). AD and putative PART cases exhibited similar patterns of p-tau seeding activity that anticipated histopathology across all NFT stages. This suggests that pathological tau seeding activity begins in the transentorhinal/entorhinal region rather than in the locus ceruleus (Kaufman et al. 2018). PART is considered either a prodromal form or a subtype of AD (Jellinger et al. 2015) or a distinct tauopathic entity separate from tAD (Hickman et al. 2020) (Table 2). In contrast to tAD showing a slight decrease after age 85, the frequency of PART increases later (Jellinger and Attems 2010).
Analysis of PET data suggested that tau pathology is common among individuals without significant amyloidosis, since 45% of a sample of the Alzheimer’s Disease Neuroimaging Initiative (ADNI) were categorized A−/T+ and only 6% as A+/T−, contrary to the amyloid cascade and ATN frameworks. A rather poor cognitive performance in the A−/T+ group and the association of Braak NFT I/II levels and cognition in these groups indicated that tau pathology is confined to the medial temporal lobe, and the absence of Aβ could be part of the AD developmental cascade rather than a feature of aging with no or only minor cognitive deficits, i.e., PART (Weigand et al. 2020). Mesial temporal tau in Aβ-negative, cognitively normal individuals, which are likely PART, is related to worse cognitive performance and greater neocortical tau load (Groot et al. 2021). Differentiating PART from tAD remains a diagnostic challenge (Nelson et al. 2016). Biomarker and neuroimaging studies will be important to define PART antemortem and to follow its natural history (Hickman et al. 2020). LP-AD cases shared some morphological features with PART (Crary et al. 2014), although later studies revealed significant differences versus LP-AD, emphasizing that PART may not be a variant of AD (Janocko et al. 2012; Jellinger 2016), whereas others suggested that PART is a part of AD (Duyckaerts et al. 2015).
Another recently described disease entity mainly involving elderly people (age > 75 years), the limbic-predominant age-related TDP-43 encephalopathy (LATE), is associated with an amnestic dementia syndrome that may mimic AD (Besser et al. 2020; Nelson et al. 2019). It shows pathogenic mechanisms of both frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) and AD, but there are different molecular patterns of TDP-43 pathology in various clinical phenotypes with a higher chance of frontotemporal dementia-like symptoms in AD + full-length TDP-43 cases (Tomé et al. 2020).
LATE-NC is predominantly age-related TDP-43 encephalopathy without other neuropathological changes. The recommended grading was divided into three: (1) amygdala only, (2) hippocampus, (3) middle frontal cortex. When compared with FTLD-TDP, it has a later age of onset and limbic predominance of pathological changes (Nelson et al. 2011). Clinically, LATE-NC was more associated with an amnestic syndrome rather than the behavioral aphasic syndrome typical for FTLD-TDP. Like AD, this entity correlated with the presence of APOE ε4 allele (Robinson et al. 2018; Yang et al. 2018). When comparing “pure” LATE-NC with “pure” AD, regarding neuropathological changes, those with “pure” LATE-NC showed a more gradual clinical decline (Murray et al. 2014). It is not uncommon that AD pathology occurs together with Lewy and TDP-43 pathologies causing neuropsychiatric syndromes (Bayram et al. 2019). Recent findings suggest that neocortical Lewy bodies (LBs) are associated with LATE-NC, specifically in the younger old and in women. Limbic/neocortical type LBs have additive effects on cognitive function in AD (Agrawal et al. 2021). Recent studies indicated that in most cases LATE-neuropathological changes (LATE-NC) and FTLD-TDP can be differentiated by applying single neuropathological criteria, e.g. the severity of cortical TDP-43 inclusions (Robinson et al. 2020). However, reliable biomarkers for antemortem diagnosis of LATE are currently not available (Teylan et al. 2019).
Pathobiology of other AD variants
The AD continuum shows a broad spectrum of clinical manifestations beyond the classical amnestic-predominant syndrome. Factor mixture analysis of neurocognitive changes in 230 patients with clinically diagnosed AD identified four groups: visuospatial AD, typical cognitive pattern (tAD), less impaired memory (mild AD), and non-amnestic AD with language/praxia deficits and relatively spared memory (Zangrossi et al. 2021).
The original ADNI distinguished five diagnostic clinical groups: AD (n = 110), late MCI (n = 133), early MCI (n = 148), significant memory concern (n = 94), and cognitive normal (n = 173) (Galili et al. 2014), while later studies of the same cohort even differentiated six clinical subtypes (Mitelpunkt et al. 2020). Another study classified more than 4000 people with LOAD into six subgroups according to their cognitive functions and genetic data, indicating that AD is not a single homogenous condition (Mukherjee et al. 2020 ). Based on patterns of grey matter (GM) volumes and hypometabolism, the following 4 AD subgroups were differentiated: AD-memory, AD-executive, AD-language, and AD-visuospatial. AD-memory showed mediotemporal lower GM volume and hypometabolism than all the subgroups; AD-language asymmetric GM volumes in temporal lobe (left > right), with prominent hypometabolism in lateral temporal lobe. AD-executive had lower fronto-parietal GM volumes, AD-visuospatial lower GM volumes in posterior areas and hypometabolism in parietal regions and precuneus compared to AD-memory. Thus, cognitively defined AD subgroups show specific reduction of GM volumes and hypometabolism pattern with differences in trajectories of metabolism over time (Groot et al. 2021b).
A number of non-amnestic syndromes generally referred to as atypical or focal AD have been identified (Galton et al. 2000; Lam et al. 2013). With regard to variable clinical presentation, a number of phenotypes have been determined based on consensus and accepted guidelines: besides the “typical” AD (the amnestic syndrome being more common than the non-amnestic one) (Sahoo et al. 2018), posterior cortical atrophy (PCA) (Crutch et al. 2017), showing greater NFT burden in the occipital and parietal lobes but lower in hippocampus (Levine et al. 1993), and a 4-R tauopathy clinically presenting as PCA (Jellinger et al. 2011). Higher NFT burden in the cingular gyrus and CA1 sector of hippocampus were independently associated with worsening visuospatial dysfunction, suggesting domain-specific functional consequences of regional NFT accumulation (Petersen et al. 2019). LPPA shows higher NFT density in the superior temporal gyrus but amyloid plaques similar to amnestic AD (Ahmed et al. 2012; Spinelli et al. 2017), the corticobasal syndrome subtype of AD (Di Stefano et al. 2016; McMillan et al. 2016), with atypical distribution of ADNC (a higher NFT density in the perirolandic cortices and greater neuronal loss in substantia nigra), which may contribute to parkinsonism that is uncommon in tAD (Sakae et al. 2019). Non-amnestic AD with TDP-43 pathology, showing little evidence that clinical or anatomical features are related to TDP-43 (Sahoo et al. 2018), and the rather rare behavioral variant of AD (bvAD) (Ossenkoppele et al. 2015) also showed heterogeneous distribution of ADNC (Singleton et al. 2021). It revealed temporo-parietal predominant atrophy in the Mayo series, where PCA, LPPA and bv-forms were more common in HcSP-AD than in LP and tAD (Josephs et al. 2015; Murray et al. 2011). The presence of left-sided NFT predominance and higher neocortical-to-entorhinal NFT ratio in primary progressive aphasia (PPA) establishes clinical concordance of ADNC with the aphasic phenotype, although these concordant clinicopathological relationships are not universal. PPA is a language disorder characterized by cortical atrophy, accumulation of both NFTs and hypertrophic microglia associated with lower neuron density in language-associated hemispheres targeting the language network (Ohm et al. 2021). PPA is subdivided into 3 subtypes: semantic variant (svPPA), non-fluent (nfvPPA), and logopenic variant (lvPPA). The latter progressed with broader language problems, nfvPPA to mutism, while semantic impairment was the major problem in svPPA. 83% of lvPPA were consistent with AD, while 54% of nfvPPA progressed to other tauopathies (Ulugut et al. 2021). Lobar cerebral microbleeds (CMB) in lvPPA affected temporal, frontal and parietal lobes with left side predominance, while CMB volume decreased in the left temporal area. Aberrant brain perfusion in lvPPA may be derived from brain atrophy and may involve aberrant microcirculation caused by lobar CMBs and cerebrovascular injuries (Ikeda et al. 2021). However, individual PPA cases with ADNC exist where distribution of plaques and NFTs do not account for this specific phenotype (Gefen et al. 2012).
The behavioral/dysexecutive variant of AD is a rare, atypical young-onset variant defined clinically by early impairments in executive and behavioral domains associated with multi-domain cognitive impairment. While it is hardly distinguishable from the behavioral variant of frontotemporal dementia (bvFTD) using clinical and cognitive features alone, CSF biomarkers and temporoparietal hypometabolism with relative sparing of mediotemporal (vs. amnestic), occipital (vs. visual phenotype) and left temporal (vs. language phenotype) help predict underlying pathology during life (Bergeron et al. 2020; Townley et al. 2020). Frontal variant of AD is a rare non-amnestic syndrome characterized by behavioral and/or dysexecutive impairments, mimicking bvFTD. Brain imaging showed diffuse Aβ deposition across the cerebral cortex, substantial tau pathology and hypometabolism in frontal and temporal lobes, while plasma biomarkers indicated an AD profile (Li et al. 2020; Paquin et al. 2020). PPA-AD is characterized by cortical atrophy and NFT densities concentrated in the language-dominant hemisphere. Stereological methods showed accumulation of NFTs and activated microglia associated with reduced neuronal densities, suggesting that they may collectively contribute to focal neurodegeneration characteristic of PPA-AD.
There is evidence linking different large-scale functional network abnormalities to distinct AD phenotypes: specifically, executive deficits in EO-AD link with dysfunctions of networks that support attention and executive functions. PCA, most commonly associated with loss of complex visuospatial functions, relates to the breakdown of visual and dorsal attentional circuits, while the PPA variant of AD is associated with dysfunction of the left-lateralized language network (Mesulam et al. 2021; Pini et al. 2021; Wong et al. 2019). Atypical clinical variants of AD often show a younger age at onset and fewer associations with the APOE ε4 genotype compared to typical amnestic forms (Murray et al. 2011), suggesting that APOE is a selective risk factor that increases the vulnerability of memory-related medial temporal areas, rather than language-related neocortices (Weintraub et al. 2020). Older age and APOE ε4 seem to affect expression by promoting a more medial-temporal lobe-prominent pattern of tau pathology (La Joie et al. 2021). APOE genotype contributes to heterogeneity in the rate of AD progression. Compared to APOE ε3/ε3 genotype, APOE ε2 and ε4 have opposite (slowing and accelerating, respectively) effects on the rate of cognitive decline, which are largely independent of the differential APOE allele effects on AD and co-morbid pathologies (PART and others) (Qian et al. 2021),
The pathogenic factors underlying AD subtypes are unclear and cannot be explained by Aβ pathology alone, because the distribution of Aβ PET retention is quite similar in all subtypes (Lehmann et al. 2013), with only subtle differences across phenotypes, while they are associated with differential patterns of tau pathology (La Joie et al. 2021). However, solid-state nuclear magnetic resonance measurements showed quantitative differences between Aβ-40 and Aβ-42 in the brain tissue of patients with two atypical clinical subtypes—PCA variant and a typical long duration AD—indicating that there are structural variations in Aβ fibers from clinical AD subtypes (Walker 2020). MA-AD, although Aβ positive, shows less tau pathology. Furthermore, it has been suggested that Aβ enhances tau pathology development in AD through increased tau spreading (Vergara et al. 2019), which may be accelerated via cellular prion protein (Gomes et al. 2019). The variations in sites of tau seeding between individuals could underlie differences in the clinical presentation and course of AD (Stopschinski et al. 2021). There is increasing evidence that Aβ, similar to prion protein, can assemble into distinct strains of aggregation, which may be the primary driver of the phenotypic heterogeneity of AD (Lau et al. 2021). These and other data indicate that AD “subtypes” may be linked to different tau protein modifications, suggesting that these patients may have multiple molecular drivers of an otherwise common phenotype.
A recent study using tau PET scans from 1612 individuals of the ADNI study identified four distinct spatiotemporal trajectories of tau pathology, ranging in prevalence from 18 to 33%. It replicated previously described LP and HcSP patterns, while also discovering posterior and lateral-temporal patterns resembling atypical clinical variants. These “subtypes” presented with distinct demographic and cognitive profiles and differing longitudinal outcomes. In addition, network diffusion models implied that pathology originates and spreads through distinct corticolimbic networks in the different subtypes, suggesting that variation in tau pathology is common and systematic. These and other data question whether “tAD” is a quantifiable entity, rather suggesting that several AD subtypes exist that may be influenced in their expression by genetic, cellular, developmental and other factors (Dujardin et al. 2020; Vogel et al. 2021).
Recent studies of 222 AD patients from The Religious Orders Study and Memory and Aging Project (ROSMAP) Study of molecular profiling and gene expression data, using consensus non-negative matrix factorization, identified two subtypes—synaptic and inflammatory: the synaptic type was more relevant in AD with APOE ε3/ε4 genotype, the inflammatory type more with the APOE ε3/ε3 genotype, being more prevalent in females (Zheng and Xu 2021). Molecular profiling measuring the brain levels of 25 inflammatory factors involved in neuroinflammation allowed a stratification of AD patients in three distinct “neuroinflammatory clusters”. These findings strengthen the relevance of neuroinflammation in the pathogenesis of AD and that the differential involvement of neuroinflammatory molecules released by microglial cells during disease development may contribute to modulate the neuropathological changes, driving at least in part the AD phenotype diversity (Sorrentino et al. 2021). Recent proteomic studies highlight the molecular heterogeneity of AD and the relevance of neuroinflammation as major players in AD pathology (Velásquez et al. 2021).
Analysis of 1543 transcriptomes across five brain regions in two AD cohorts, using an integrative network approach, identified three major molecular subtypes of AD corresponding to different combinations of multiple dysregulated pathways, such as susceptibility to tau-mediated neurodegeneration, Aβ neuroinflammation, synaptic signaling, immune activity, mitochondrial organization, and myelination. This molecular subtyping of AD using RNA sequencing revealed novel mechanisms and targets (Neff et al. 2021). Gene expression data of 222 AD patients identified two molecular subtypes—synaptic and inflammatory: the synaptic type is characterized by disrupted synaptic vesicle priming and synaptic plasticity; the inflammatory type by disrupted IL2, interferon-α and -γ pathways. The synaptic type was more prevalent in males and the inflammatory type in females (Zheng and Xu 2021). Using CSF proteomics, three distinct pathophysiological subtypes of AD were discovered: one with neuronal hyperplasticity, a second with innate immune activation, and a third with blood–brain barrier dysfunction. These AD proteomic subtypes may already manifest in cognitively normal individuals and may predispose to AD before amyloid has reached abnormal levels (Tijms et al. 2021). This suggests that these multiple phenotypes are part of the same AD continuum (Ossenkoppele et al. 2015), which may have consequences for future personalized therapeutic approaches.
In conclusion, research into typical AD has revealed previously unrecognized neuropathological heterogeneity across the AD spectrum. Neuroimaging, genetics, biomarkers, and basic science studies provide key insights into the features that might drive selective vulnerability of differing brain networks, with potential implications for understanding typical and atypical forms of AD.
The impact of co-pathologies
The aging brain is vulnerable to a wide array of neuropathologies. AD pathology rarely occurs in isolation, while complex co-pathologies influence the clinical picture and may increase disease progression. The number of co-pathologies increases in the aging brain, causing complex mixed pathologies (Jellinger and Attems 2015; Matej et al. 2019; McAleese et al. 2021a; Power et al. 2018; Rahimi and Kovacs 2014; Robinson et al. 2021; Thomas et al. 2020; Tomé et al. 2020). However, highly prevalent co-morbidities are not restricted to the oldest-old but are common even in EOAD (Beach and Malek-Ahmadi 2021). The challenges of pathological mimics and concomitant pathologies in the neuropathological diagnosis of AD have been critically reviewed recently (King et al. 2020). The most frequent co-lesions are cerebrovascular diseases, Lewy- and TDP-43 proteinopathies (Agrawal et al. 2021; Besser et al. 2020; Boyle et al. 2021; DeTure and Dickson 2019; Schneider et al. 2007).
TDP-43 pathology was noted in particular in a very elderly subgroup of AD cases and was associated with an apparent worsening of cognitive decline (Josephs et al. 2014). A staging system was devised, where the earliest TDP-43 pathology in the context of AD was the amygdala, then entorhinal cortex and subiculum, then dentate gyrus of occipitotemporal cortex (stage 3), followed by the inferior temporal gyrus (stage 4), substantia nigra, inferior olive and midbrain tectum (stage 5), and finally, basal ganglia and middle frontal cortex (stage 6) of a different pattern and density than the typical FTLD-TDP. The different TDP-43 patterns were associated with typical AD symptoms in 80–100% of AD cases (Josephs et al. 2016; Tomé et al. 2020). A study of aged subjects (range 80–89 years) with cognitive impairments found 66% with “mixed pathology”, i.e., ADNC combined with TDP-43, α-synuclein or vascular brain lesions (Alafuzoff and Libard 2020).
In a consecutive autopsy series of 2060 elderly patients, ADNC was present in 82.9% of all demented and in 92.8% of clinically diagnosed patients, but only 33.6 and 47.6%, respectively, showed pure AD (ABC 3/3/3); atypical forms including PART (7 and 6%); additional cerebrovascular disease (CVD (24.3%), Lewy (12.5%) or other mixed pathologies. Vascular dementia accounted for 12.2 and 3.3%, respectively, while other non-AD pathologies were present in 7.2 and 3.3%, respectively (Jellinger 2006).
While a small study of demented elderly persons reported pure ADNC in only 31% and multiple pathologies in 63% (Wang et al. 2012), another one found at least one non-AD pathological diagnosis in 98% of patients with EOAD and in 100% in those with LOAD, with the number of co-pathologies predicting worse cognitive performance (Spina et al. 2021). A review of 12 clinico-pathological studies with 3,574 patients, irrespective of the clinical symptoms, reported ADNC between 19 and 67%, Lewy pathologies in 6–39%, vascular changes in 28–70%, TDP-43 proteinopathy in 19–78%, HS between 3 and 13%, and mixed pathologies between 8 and 70% (Rahimi and Kovacs 2014).
Among 447 patients with clinically probable AD, only 3.3% showed pure ADNC, 27.3% AD + CVD + other lesions, 7.6% ADNC + other degenerative lesions, and 47% AD + CVD and other neurodegenerative lesions (Kapasi et al. 2017). Neuropathological examination of 1164 deceased participants from two longitudinal clinicopathological studies provided 11 pathological including markers of AD and non-AD neurodegenerative diseases (like LATE-NC, HS, LBs, cerebrovascular lesions), most of them accounting for a large proportion in late life cognitive decline (Boyle et al. 2021). There is increasing association with LATE-NC, in younger age with LBs and increased Braak stage with CAA (Robinson et al. 2021). Among 670 aged individuals from the Brains for Dementia Research Program, more than three-quarters had multiple brain pathologies, ranging from low/intermediate levels of additional lesions (69.9%), to mixed severe pathology (7.5%), whereas only 22.7% had pure ADNC (McAleese et al. 2021a). In a data collection from the NACC assessing those at 39 NIA AD centers across the USA, 924 individuals had complete neuropathological data. 63% of individuals given the “gold standard” diagnosis of AD possessed either TDP-43 proteinopathy or CAA of sufficient severity to independently explain the majority of their cognitive impairment (Thomas et al. 2020). Aβ and/or tau burden, particularly in Braak NFT stages IV–VI, and small cerebral vessel disease may synergistically affect cognitive decline (Jang et al. 2020), and a significant interaction was found between Braak NFT stages, CAA status and cognitive decline (Malek-Ahmadi et al. 2020). Based on data from the NACC, 1854 participants with a clinical diagnosis of AD and ADNC at autopsy (confirmed AD) were studied. 204 with the clinical diagnosis of AD had no ADNC (AD-mimics), while 253 participants with negative clinical AD diagnosis had ADNC (unidentified AD). Compared to confirmed AD cases, AD mimics (FTLD, HS, cerebrovascular pathology, etc.) had less severe cognitive impairment (Gauthreaux et al. 2020).
Analysis of 522 individuals (> 50 years) from the Center for Neurodegenerative Disease Research (CNDR) autopsy and the NACC database found widespread distribution of CAA, LATE-NC and LBs that progressively accumulate alongside plaques and NFTs in AD. CAA interacted with plaques and NFTs especially in APOE ε4-positive cases, associated with higher NFT stages later in the disease course, while most LBs associated with moderate to severe plaques and NFTs (Robinson et al. 2021). Increased TDP-43 pathology in tAD and LP-AD compared to HcSP-AD (Murray et al. 2011) was due to a strong association between HS and TDP-43, but clinical presentation seemed to be driven by morphological subtypes rather than by TDP-43 pathology (Josephs et al. 2015). Among 172 autopsy-confirmed AD cases, 69% of which had typical ADNC, 31% were HcSP-AD and 36% TDP-43 positive, while there were no LP-AD cases (Sahoo et al. 2018).
Recent studies have revealed considerably different co-morbidities between EOAD and LOAD, the latter showing more frequent LATE, HS and argyrophilic grain disease (AGD), while Lewy pathology and CAA were common in both EOAD and LOAD. Thus, these co-pathologies may play an important role in the clinical phenotype of both forms, particularly in EOAD (Spina et al. 2021), although concomitant LATE in AD has shown not to be associated with increased tau or Aβ pathological burden or greater neuropsychiatric impairment (Liu et al. 2020; McAleese et al. 2020; Teipel et al. 2021). On the other hand, LATE-NC was independently associated with dementia and strongly associated with arteriolosclerosis in the oldest old (Harrison et al. 2021).
Several other pathological changes occur in the aging brain and may be associated with ADNC. AGD, a limbic predominant 4R tauopathy, with grain-like deposits in neuritic dendrites, oligodendroglial inclusions (“coiled bodies”), ramified astrocytes, and ballooned neurons in the amygdala, hippocampus and medial temporal lobes (Togo et al. 2002), has been reported in up to 25% of AD cases (Togo et al. 2002). It rarely occurs before the age of 75 years (Wurm et al. 2020). Granulovacuolar degeneration (GVD), characterized as 3–5 γm vesicles, occurs in the pyramidal neurons of the hippocampus, usually in association with NFTs. Their origin and significance are unclear. Despite the strong association between tau aggregation and GVD body formation (Wiersma et al. 2019), intracellular aggregates of proteins other than tau can also induce GVD formation, which needs further elucidation (Wiersma et al. 2020). They correlate with NFT density, suggesting that they may represent a cellular response to neuronal damage of late-state autophagic vacuoles (Hou et al. 2018). Necrosome complex detected in granulovacuolar degeneration has been shown to be associated with neuron loss in AD (Koper et al. 2020).
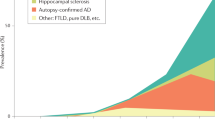
Several co-morbidities may impact the integrity of cerebral white matter. In amyloid-negative amnestic type dementia, medial temporal atrophy was associated with high WMH due to greated CVD burden that may be important for development of hippocampal atrophy (Wong et al. 2019). In a recent study of 206 cases with ADNC, only 33 were without co-morbidities. White matter demyelination was more marked in AD with co-pathologies, which showed early alterations in oligodendrocytes and transcription of genes linked to myelin proteins, suggesting that oligodendrogliopathy is part of AD (Ferrer and Andrés-Benito 2020). Demyelination progresses across the AD continuum, suggesting that this pathologic process might be a relevant degenerative feature in the disease course (Moscoso et al. 2021). Recent studies indicated that anterior white matter lesions (WMLs) could be associated with both small vessel disease-associated and AD-associated cortical pathologies, while posterior WMLs may be associated with degenerative mechanisms secondary to AD pathology (McAleese et al. 2021b). Taken together, these and other recent findings highlight the relevance of multiple pathologies in the development of age-related dementias and suggest that they are “partners in crime”. These concomitant pathologies may be harmful to individuals with low cognitive reserve such as patients with MA-AD. They can cause a number of challenges including the evaluation of the significance of each pathological entity in the manifestation of the clinical symptoms and the threshold of each individual pathology to cause dementia (King et al. 2020). The total burden of comorbid pathological abnormalities, rather than single lesions, is the most important cause of cognitive impairment often despite the clinical diagnosis of “only” or “pure” AD (White et al. 2016). The co-pathologies, multiple players, which may each account for so-called AD (Fig. 3), should be considered in the diagnosis and treatment of dementia in the elderly as part of an increasingly personal approach. Nevertheless, it should be borne in mind that all the additional pathologies may interact, although their mutual impact often remains unclear. Therefore, the reliability and clinical relevance of the current diagnostic criteria need better qualification and validation.
Diagram showing the overlap between AD and other (co-) pathologies. Only a subset of individuals with the clinical symptoms of AD demonstrate “pure” ADNC. Other co-pathologies also contribute to dementia and may overlap with ADNC, while some persons with ADNC may not demonstrate dementia. Modified after (Mehta and Schneider 2021). AD Alzheimer disease, ADNC Alzheimer neuropathological changes, CVD cerebrovascular disease, HS hippocampal sclerosis, LBD Lewy body disease, VBI vascular brain injury, LATE-NC limbic-predominant age-related TDP-43 neuropathological changes, PART primary age-related tauopathy
Conclusions and perspectives
AD is a heterogeneous, multifactorial disorder, manifesting clinically and morphologically as several pathobiological phenotypes that have a distinctive signature of neuronal network disruptions associated with specific brain atrophy patterns and reflecting the different spread of NFT/tau pathology and neuronal loss due to different vulnerability patterns of affected brain regions, which relates to specific molecular-functional properties of the affected neuronal systems (Grothe et al. 2018; Wang et al. 2020). This is substantially related to three important factors, i.e., risk, protective factors, and concomitant pathologies. The balance between risk and protective factors determines brain cellular and regional vulnerability, which contributes to differential spatial manifestations of pathologies and potential disease-relevant lesion patterns, leading to divergent clinicopathological presentations of AD. The severity of lesions corresponds to the “N” category in the new A/T/N classification of biomarkers (Jack et al. 2018a), indicating that the AD clinical syndrome includes several pathobiologically defined entities or subtypes, which show both clinical and morphological differences. This heterogeneity of the Alzheimer continuum is due to multiple pathogenic factors, e.g., the “upstream” genotypes causing comorbidities, “downstream” disease-modifying gene variants (MAPT, H2 haplotype, PSEN1, APE, TREM, GRN and other variants), which induce misfolding tau, Aβ, TDP-43 and others, the synergetic or additive action of which results in various disease phenotypes (Nelson et al. 2016). Several factors such as brain resilience may help to compensate for these pathologies up to a certain level, although their relevance is still poorly understood. It has been suggested that individuals should be identified who have connectivity patterns resistant to the initiation or spread of neurodegeneration. Furthermore, compensation strategies could be active in HcSP-AD and MA-AD, because the learning and encoding capacity in these subtypes is partially spared (Ferreira et al. 2017). The prevalence of biological AD with the various subtypes and variants is greater than clinical probable AD at any age, in particular at age 85 + (Jack et al. 2019). These problems and the increasing incidence of AD illustrate its consequences on public health and the resulting challenges for future medicine. Increased sensitivity and specificity of new A/T/N markers and more extensive clinicopathological studies in well-documented populations are needed, with postmortem studies using the updated NIA-AA criteria. The recent advent of tau PET and novel imaging and fluid-based (CSF and plasma) biomarkers will allow us to study the temporal progression of tau and other pathologies in vivo (Hampel et al. 2021; Koychev et al. 2020; Ricci et al. 2020; Wesenhagen et al. 2020). Although the current techniques do not yet have the resolution to assess the molecular mechanisms of Aβ and tau at the single neuron level, recent in vivo studies support the hypothesis that Aβ and tau have toxic effects on synaptic function and axonal integrity over the course of AD. By combining several established methods, it could be shown that early disease stages are characterized by Aβ-induced synaptic damages, memory impairment and functional connectivity changes, whereas later disease stages are characterized by tau-associated axonal damage, global cognitive decline and reduced connectivity. These results are consistent with axonal degeneration and disruption of synaptic physiology as central events in the pathogenesis of AD (Ahmad et al. 2021; Pereira et al. 2021).
It is essential to gain better understanding of the AD pathogenesis, subtype variety, and to develop several distinct therapeutic approaches tailored to address this diversity, as well as the common presence of mixed pathologies. Improving methods for disease detection and monitoring its progression may hopefully lead to the developmental refinement of tau-based therapies. Neuropathological studies should use a wide range of molecular methods and should evaluate multiple brain regions. Advances in digital pathology and technologies such as single cell sequencing and digital spatial profiling have opened novel ways for improving the neuropathological diagnosis and advancing our understanding of underlying molecular processes (Trejo-Lopez et al. 2021). An optimal and less cost-intensive strategy would be to screen specifically neurodegeneration-related proteins and to examine their cross reactions. A similar procedure was used to establish a new system, the Lewy pathology consensus criteria in postmortem brains, which showed good reproducibility and allowed the classification of all cases of LBD into distinct categories, irrespective of concomitant neurodegenerative diseases (Attems et al. 2021). Recent correlative studies on concomitant pathologies have provided insights into their interrelations with ADNC in causing various clinical symptoms. Interdisciplinary studies may further improve our knowledge about the pathogenesis of the heterogeneous manifestations of AD and promote methods for its early diagnosis as the basis for further preventive and successful disease-modifying therapeutic measures of this devastating disease.
Abbreviations
- AD:
-
Alzheimer’s disease
- ADNC:
-
Alzheimer’s disease neuropathological changes
- ADNI:
-
Alzheimer’s Disease Neuroimaging Initiative
- Aβ:
-
Amyloid-β
- CAA:
-
Cerebral amyloid angiopathy
- CSF:
-
Cerebrospinal fluid
- CVD:
-
Cerebrovascular disease
- EOAD:
-
Early-onset Alzheimer’s disease
- FAD:
-
Familial Alzheimer’s disease
- FDG-PET:
-
Fluorodeoxyglucose-positron emission tomography
- FTLD-TDP:
-
Frontotemporal lobar degeneration with TDP-43
- GVD:
-
Granulovacuolar degeneration
- HcSP-AD:
-
Hippocampal-sparing Alzheimer’s disease
- HS:
-
Hippocampal sclerosis
- LATE:
-
Limbic-predominant age-related TDP-43 encephalopathy
- LATE-NC:
-
LATE-neuropathological changes
- LB:
-
Lewy body
- LOAD:
-
Late-onset Alzheimer’s disease
- LP-AD:
-
Limbic-predominant Alzheimer’s disease
- LPPA:
-
Logopenic primary progressive aphasia
- MA-AD:
-
Minimal atrophy Alzheimer’s disease
- MAPT:
-
Microtubule-associated protein tau
- MCI:
-
Mild cognitive impairment
- MMSE:
-
Mini Mental State Examination
- MRI:
-
Magnetic resonance imaging
- MTL:
-
Medial temporal lobe
- NACC:
-
National Alzheimer’s Coordinating Center
- NFT:
-
Neurofibrillary tangle
- NIA/AA:
-
National Institute on Aging/Alzheimer’s Association
- NT:
-
Neuropil thread
- PART:
-
Primary age-related tauopathy
- PCA:
-
Posterior cortical atrophy
- PPA:
-
Primary progressive aphasia
- p-tau:
-
Phosphorylated tau protein
- SAD:
-
Sporadic Alzheimer’s disease
- tAD:
-
Typical Alzheimer’s disease
References
Abu-Rumeileh S, Capellari S, Parchi P (2018a) Rapidly progressive Alzheimer’s disease: contributions to clinical-pathological definition and diagnosis. J Alzheimers Dis 63:887–897
Abu-Rumeileh S, Capellari S, Stanzani-Maserati M, Polischi B, Martinelli P, Caroppo P, Ladogana A, Parchi P (2018b) The CSF neurofilament light signature in rapidly progressive neurodegenerative dementias. Alzheimers Res Ther 10:3
Agrawal S, Yu L, Nag S, Arfanakis K, Barnes LL, Bennett DA, Schneider JA (2021) The association of Lewy bodies with limbic-predominant age-related TDP-43 encephalopathy neuropathologic changes and their role in cognition and Alzheimer’s dementia in older persons. Acta Neuropathol Commun 9:156
Ahmad F, Haque S, Chavda V, Ashraf GM (2021) Recent advances in synaptosomal proteomics in Alzheimer’s disease. Curr Protein Pept Sci 22:479-492
Ahmed S, de Jager CA, Haigh AM, Garrard P (2012) Logopenic aphasia in Alzheimer’s disease: clinical variant or clinical feature? J Neurol Neurosurg Psychiatry 83:1056–1062
Alafuzoff I, Libard S (2020) Mixed brain pathology is the most common cause of cognitive impairment in the elderly. J Alzheimers Dis 78:453–465
Alafuzoff I, Arzberger T, Al-Sarraj S, Bodi I, Bogdanovic N, Braak H, Bugiani O, Del-Tredici K, Ferrer I, Gelpi E, Giaccone G, Graeber MB, Ince P, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Tagliavini F, Stadelmann C, Streichenberger N, Thal DR, Wharton SB, Kretzschmar H (2008) Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol 18:484–496
Arnold SE, Louneva N, Cao K, Wang LS, Han LY, Wolk DA, Negash S, Leurgans SE, Schneider JA, Buchman AS, Wilson RS, Bennett DA (2013) Cellular, synaptic, and biochemical features of resilient cognition in Alzheimer’s disease. Neurobiol Aging 34:157–168
Alzheimer's Association (2021) 2021 Alzheimer’s disease facts and figures. Alzheimers Dement 17:327–406
Attems J, Toledo JB, Walker L, Gelpi E, Gentleman S, Halliday G, Hortobagyi T, Jellinger K, Kovacs GG, Lee EB, Love S, McAleese KE, Nelson PT, Neumann M, Parkkinen L, Polvikoski T, Sikorska B, Smith C, Grinberg LT, Thal DR, Trojanowski JQ, McKeith IG (2021) Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol 141:159–172
Badhwar A, McFall GP, Sapkota S, Black SE, Chertkow H, Duchesne S, Masellis M, Li L, Dixon RA, Bellec P (2020) A multiomics approach to heterogeneity in Alzheimer’s disease: focused review and roadmap. Brain 143:1315–1331
Bancher C, Egensperger R, Kosel S, Jellinger K, Graeber MB (1997) Low prevalence of apolipoprotein E epsilon 4 allele in the neurofibrillary tangle predominant form of senile dementia. Acta Neuropathol 94:403–409
Barenholtz Levy H (2021) Accelerated approval of aducanumab: where do we stand now? Ann Pharmacother. https://doi.org/10.1177/10600280211050405
Bayram E, Shan G, Cummings JL (2019) Associations between comorbid TDP-43, Lewy body pathology, and neuropsychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis 69:953–961
Beach TG, Malek-Ahmadi M (2021) Alzheimer's disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis 79:389–400
Bergeron D, Sellami L, Poulin S, Verret L, Bouchard RW, Laforce R Jr (2020) The behavioral/dysexecutive variant of Alzheimer’s disease: a case series with clinical, neuropsychological, and FDG-PET characterization. Dement Geriatr Cogn Disord 49:518–525
Berron D, Vogel JW, Insel PS, Pereira JB, Xie L, Wisse LEM, Yushkevich PA, Palmqvist S, Mattsson-Carlgren N, Stomrud E, Smith R, Strandberg O, Hansson O (2021) Early stages of tau pathology and its associations with functional connectivity, atrophy and memory. Brain 144:2771–2783
Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, Montine TJ, Schneider JA, Nelson PT (2018) The revised National Alzheimer’s Coordinating Center’s Neuropathology form-available data and new analyses. J Neuropathol Exp Neurol 77:717–726
Besser LM, Teylan MA, Nelson PT (2020) Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J Neuropathol Exp Neurol 79:305–313
Birkenbihl C, Salimi Y, Fröhlich H (2021) Unraveling the heterogeneity in Alzheimer's disease progression across multiple cohorts and the implications for data-driven disease modeling. Alzheimers Dement. https://doi.org/10.1002/alz.12387
Boyle PA, Wang T, Yu L, Wilson RS, Dawe R, Arfanakis K, Schneider JA, Bennett DA (2021) To what degree is late life cognitive decline driven by age-related neuropathologies? Brain 144:2166–2175
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Byun MS, Kim SE, Park J, Yi D, Choe YM, Sohn BK, Choi HJ, Baek H, Han JY, Woo JI, Lee DY (2015) Heterogeneity of regional brain atrophy patterns associated with distinct progression rates in Alzheimer’s disease. PLoS ONE 10:e0142756
Casanova MF, Starkstein SE, Jellinger KA (2011) Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropathol 122:117–135
Cedres N, Ekman U, Poulakis K, Shams S, Cavallin L, Muehlboeck S, Granberg T, Wahlund LO, Ferreira D, Westman E (2020) Brain atrophy subtypes and the ATN classification scheme in Alzheimer’s disease. Neurodegener Dis 20:153–164
Cerejeira J, Lagarto L, Mukaetova-Ladinska EB (2012) Behavioral and psychological symptoms of dementia. Front Neurol 3:73
Charil A, Shcherbinin S, Southekal S, Devous MD, Mintun M, Murray ME, Miller BB, Schwarz AJ (2019) Tau subtypes of Alzheimer’s disease determined in vivo using flortaucipir PET imaging. J Alzheimers Dis 71:1037–1048
Chételat G, Arbizu J, Barthel H, Garibotto V, Law I, Morbelli S, van de Giessen E, Agosta F, Barkhof F, Brooks DJ, Carrillo MC, Dubois B, Fjell AM, Frisoni GB, Hansson O, Herholz K, Hutton BF, Jack CR Jr, Lammertsma AA, Landau SM, Minoshima S, Nobili F, Nordberg A, Ossenkoppele R, Oyen WJG, Perani D, Rabinovici GD, Scheltens P, Villemagne VL, Zetterberg H, Drzezga A (2020) Amyloid-PET and (18)F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol 19:951–962
Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky ST, Fillit H, Harrison JE, Schneider LS, Scheltens P, de Haan W, Grundman M, van Dyck CH, Izzo NJ, Catalano SM (2020) The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther 12:21
Confer MP, Holcombe BM, Foes AG, Holmquist JM, Walker SC, Deb S, Ghosh A (2021) Label-free infrared spectroscopic imaging reveals heterogeneity of beta-sheet aggregates in Alzheimer's disease. J Phys Chem Lett 12:9662–9671
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Gearing M, Grinberg LT, Hof PR, Hyman BT, Jellinger K, Jicha GA, Kovacs GG, Knopman DS, Kofler J, Kukull WA, Mackenzie IR, Masliah E, McKee A, Montine TJ, Murray ME, Neltner JH, Santa-Maria I, Seeley WW, Serrano-Pozo A, Shelanski ML, Stein T, Takao M, Thal DR, Toledo JB, Troncoso JC, Vonsattel JP, White CL 3rd, Wisniewski T, Woltjer RL, Yamada M, Nelson PT (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766
Crutch SJ, Schott JM, Rabinovici GD, Murray M, Snowden JS, van der Flier WM, Dickerson BC, Vandenberghe R, Ahmed S, Bak TH, Boeve BF, Butler C, Cappa SF, Ceccaldi M, de Souza LC, Dubois B, Felician O, Galasko D, Graff-Radford J, Graff-Radford NR, Hof PR, Krolak-Salmon P, Lehmann M, Magnin E, Mendez MF, Nestor PJ, Onyike CU, Pelak VS, Pijnenburg Y, Primativo S, Rossor MN, Ryan NS, Scheltens P, Shakespeare TJ, Suarez Gonzalez A, Tang-Wai DF, Yong KXX, Carrillo M, Fox NC (2017) Consensus classification of posterior cortical atrophy. Alzheimers Dement 13:870–884
Das SR, Lyu X, Duong MT, Xie L, McCollum L, de Flores R, DiCalogero M, Irwin DJ, Dickerson BC, Nasrallah IM, Yushkevich PA, Wolk DA (2021) Tau-atrophy variability reveals phenotypic heterogeneity in Alzheimer’s disease. Ann Neurol 90:751–762
DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14:32
Di Fede G, Catania M, Maderna E, Ghidoni R, Benussi L, Tonoli E, Giaccone G, Moda F, Paterlini A, Campagnani I, Sorrentino S, Colombo L, Kubis A, Bistaffa E, Ghetti B, Tagliavini F (2018) Molecular subtypes of Alzheimer’s disease. Sci Rep 8:3269
Di Stefano F, Kas A, Habert MO, Decazes P, Lamari F, Lista S, Hampel H, Teichmann M (2016) The phenotypical core of Alzheimer’s disease-related and nonrelated variants of the corticobasal syndrome: a systematic clinical, neuropsychological, imaging, and biomarker study. Alzheimers Dement 12:786–795
Dong A, Toledo JB, Honnorat N, Doshi J, Varol E, Sotiras A, Wolk D, Trojanowski JQ, Davatzikos C (2017) Heterogeneity of neuroanatomical patterns in prodromal Alzheimer’s disease: links to cognition, progression and biomarkers. Brain 140:735–747
Dronse J, Fliessbach K, Bischof GN, von Reutern B, Faber J, Hammes J, Kuhnert G, Neumaier B, Onur OA, Kukolja J, van Eimeren T, Jessen F, Fink GR, Klockgether T, Drzezga A (2017) In vivo patterns of tau pathology, amyloid-beta burden, and neuronal dysfunction in clinical variants of Alzheimer’s disease. J Alzheimers Dis 55:465–471
Drummond E, Nayak S, Faustin A, Pires G, Hickman RA, Askenazi M, Cohen M, Haldiman T, Kim C, Han X, Shao Y, Safar JG, Ueberheide B, Wisniewski T (2017) Proteomic differences in amyloid plaques in rapidly progressive and sporadic Alzheimer’s disease. Acta Neuropathol 133:933–954
Dubois B, Villain N, Frisoni GB, Rabinovici GD, Sabbagh M, Cappa S, Bejanin A, Bombois S, Epelbaum S, Teichmann M, Habert MO, Nordberg A, Blennow K, Galasko D, Stern Y, Rowe CC, Salloway S, Schneider LS, Cummings JL, Feldman HH (2021) Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. Lancet Neurol 20:484–496
Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, De Los Santos MB, Klickstein N, Corjuc DL, Corjuc BT, Dooley PM, Viode A, Oakley DH, Moore BD, Mullin K, Jean-Gilles D, Clark R, Atchison K, Moore R, Chibnik LB, Tanzi RE, Frosch MP, Serrano-Pozo A, Elwood F, Steen JA, Kennedy ME, Hyman BT (2020) Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med 26:1256–1263
Duran-Aniotz C, Moreno-Gonzalez I, Gamez N, Perez-Urrutia N, Vegas-Gomez L, Soto C, Morales R (2021) Amyloid pathology arrangements in Alzheimer’s disease brains modulate in vivo seeding capability. Acta Neuropathol Commun 9:56
Duyckaerts C, Braak H, Brion JP, Buée L, Del Tredici K, Goedert M, Halliday G, Neumann M, Spillantini MG, Tolnay M, Uchihara T (2015) PART is part of Alzheimer disease. Acta Neuropathol 129:749–756
Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, Teunissen CE, van Berckel BNM, Pijnenburg YAL, Scheltens P, van der Flier WM, Ossenkoppele R, Papma JM (2021) Neuropsychiatric and cognitive symptoms across the Alzheimer disease clinical spectrum: cross-sectional and longitudinal associations. Neurology 97:e1276–e1287
Ewers M, Luan Y, Frontzkowski L, Neitzel J, Rubinski A, Dichgans M, Hassenstab J, Gordon BA, Chhatwal JP, Levin J, Schofield P, Benzinger TLS, Morris JC, Goate A, Karch CM, Fagan AM, McDade E, Allegri R, Berman S, Chui H, Cruchaga C, Farlow M, Graff-Radford N, Jucker M, Lee JH, Martins RN, Mori H, Perrin R, Xiong C, Rossor M, Fox NC, O’Connor A, Salloway S, Danek A, Buerger K, Bateman RJ, Habeck C, Stern Y, Franzmeier N (2021) Segregation of functional networks is associated with cognitive resilience in Alzheimer’s disease. Brain 144:2176–2185
Ferrari C, Sorbi S (2021) The complexity of Alzheimer’s disease: an evolving puzzle. Physiol Rev 101:1047–1081
Ferreira D, Verhagen C, Hernandez-Cabrera JA, Cavallin L, Guo CJ, Ekman U, Muehlboeck JS, Simmons A, Barroso J, Wahlund LO, Westman E (2017) Distinct subtypes of Alzheimer’s disease based on patterns of brain atrophy: longitudinal trajectories and clinical applications. Sci Rep 7:46263
Ferreira D, Pereira JB, Volpe G, Westman E (2019) Subtypes of Alzheimer’s disease display distinct network abnormalities extending beyond their pattern of brain atrophy. Front Neurol 10:524
Ferreira D, Nordberg A, Westman E (2020) Biological subtypes of Alzheimer disease: a systematic review and meta-analysis. Neurology 94:436–448
Ferrer I, Andrés-Benito P (2020) White matter alterations in Alzheimer’s disease without concomitant pathologies. Neuropathol Appl Neurobiol 46:654–672
Fleisher AS, Pontecorvo MJ, Devous MD Sr, Lu M, Arora AK, Truocchio SP, Aldea P, Flitter M, Locascio T, Devine M, Siderowf A, Beach TG, Montine TJ, Serrano GE, Curtis C, Perrin A, Salloway S, Daniel M, Wellman C, Joshi AD, Irwin DJ, Lowe VJ, Seeley WW, Ikonomovic MD, Masdeu JC, Kennedy I, Harris T, Navitsky M, Southekal S, Mintun MA (2020) Positron emission tomography imaging with [18f]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol 77:829–839
Fornari S, Schäfer A, Kuhl E, Goriely A (2020) Spatially-extended nucleation-aggregation-fragmentation models for the dynamics of prion-like neurodegenerative protein-spreading in the brain and its connectome. J Theor Biol 486:110102
Galili T, Mitelpunkt A, Shachar N, Marcus-Kalish M, Benjamini Y (2014) Categorize, cluster, and classify: a 3-C strategy for scientific discovery in the medical informatics platform of the Human Brain Project. In: Džeroski S, Panov P, Kocev D, Todorovski L (eds) Discovery science. DS 2014. Lecture notes in computer science, vol 8777. Springer, Cham
Galton CJ, Patterson K, Xuereb JH, Hodges JR (2000) Atypical and typical presentations of Alzheimer’s disease: a clinical, neuropsychological, neuroimaging and pathological study of 13 cases. Brain 123(Pt 3):484–498
Gauthreaux K, Bonnett TA, Besser LM, Brenowitz WD, Teylan M, Mock C, Chen YC, Chan KCG, Keene CD, Zhou XH, Kukull WA (2020) Concordance of clinical Alzheimer diagnosis and neuropathological features at autopsy. J Neuropathol Exp Neurol 79:465–473
Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, Wieneke C, Bigio E, Geula C, Mesulam MM (2012) Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain 135:1554–1565
Giovacchini G, Giovannini E, Borso E, Lazzeri P, Riondato M, Leoncini R, Duce V, Mansi L, Ciarmiello A (2019) The brain cognitive reserve hypothesis: a review with emphasis on the contribution of nuclear medicine neuroimaging techniques. J Cell Physiol 234:14865–14872
Gomes LA, Hipp SA, Rijal Upadhaya A, Balakrishnan K, Ospitalieri S, Koper MJ, Largo-Barrientos P, Uytterhoeven V, Reichwald J, Rabe S, Vandenberghe R, von Arnim CAF, Tousseyn T, Feederle R, Giudici C, Willem M, Staufenbiel M, Thal DR (2019) Abeta-induced acceleration of Alzheimer-related tau-pathology spreading and its association with prion protein. Acta Neuropathol 138:913–941
Gottesman RT, Stern Y (2019) Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer’s disease. Front Pharmacol 10:1062
Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME (2021) New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol 20:222–234
Grinberg LT, Rüb U, Ferretti RE, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H (2009) The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol Appl Neurobiol 35:406–416
Groot C, Grothe MJ, Mukherjee S, Jelistratova I, Jansen I, van Loenhoud AC, Risacher SL, Saykin AJ, Mac Donald CL, Mez J, Trittschuh EH, Gryglewski G, Lanzenberger R, Pijnenburg YAL, Barkhof F, Scheltens P, van der Flier WM, Crane PK, Ossenkoppele R (2021b) Differential patterns of gray matter volumes and associated gene expression profiles in cognitively-defined Alzheimer's disease subgroups. Neuroimage Clin 30:102660
Groot C, Doré V, Robertson J, Burnham SC, Savage G, Ossenkoppele R, Rowe CC, Villemagne VL (2021) Mesial temporal tau is related to worse cognitive performance and greater neocortical tau load in amyloid-beta-negative cognitively normal individuals. Neurobiol Aging 97:41–48
Grothe MJ, Sepulcre J, Gonzalez-Escamilla G, Jelistratova I, Schöll M, Hansson O, Teipel SJ (2018) Molecular properties underlying regional vulnerability to Alzheimer’s disease pathology. Brain 141:2755–2771
Hampel H, Nisticò R, Seyfried NT, Levey AI, Modeste E, Lemercier P, Baldacci F, Toschi N, Garaci F, Perry G, Emanuele E, Valenzuela PL, Lucia A, Urbani A, Sancesario GM, Mapstone M, Corbo M, Vergallo A, Lista S (2021) Omics sciences for systems biology in Alzheimer’s disease: state-of-the-art of the evidence. Ageing Res Rev 69:101346
Hanna Al-Shaikh FS, Duara R, Crook JE, Lesser ER, Schaeverbeke J, Hinkle KM, Ross OA, Ertekin-Taner N, Pedraza O, Dickson DW, Graff-Radford NR, Murray ME (2020) Selective vulnerability of the nucleus basalis of Meynert among neuropathologic subtypes of Alzheimer disease. JAMA Neurol 77:225–233
Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M et al (1990) The Lewy body variant of Alzheimer’s disease: a clinical and pathologic entity. Neurology 40:1–8
Harrison WT, Lusk JB, Liu B, Ervin JF, Johnson KG, Green CL, Wang SJ (2021) Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is independently associated with dementia and strongly associated with arteriolosclerosis in the oldest-old. Acta Neuropathol 142:917–919
Hickman RA, Flowers XE, Wisniewski T (2020) Primary age-related tauopathy (PART): addressing the spectrum of neuronal tauopathic changes in the aging brain. Curr Neurol Neurosci Rep 20:39
Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, Schneider JA, Bennett DA (2012) Cognitive reserve, presynaptic proteins and dementia in the elderly. Transl Psychiatry 2:e114
Hou X, Fiesel FC, Truban D, Castanedes Casey M, Lin WL, Soto AI, Tacik P, Rousseau LG, Diehl NN, Heckman MG, Lorenzo-Betancor O, Ferrer I, Arbelo JM, Steele JC, Farrer MJ, Cornejo-Olivas M, Torres L, Mata IF, Graff-Radford NR, Wszolek ZK, Ross OA, Murray ME, Dickson DW, Springer W (2018) Age- and disease-dependent increase of the mitophagy marker phospho-ubiquitin in normal aging and Lewy body disease. Autophagy 14:1404–1418
Hwang J, Kim CM, Jeon S, Lee JM, Hong YJ, Roh JH, Lee JH, Koh JY, Na DL (2015) Prediction of Alzheimer’s disease pathophysiology based on cortical thickness patterns. Alzheimers Dement (amst) 2:58–67
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 8:1–13
Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL, Gorno-Tempini ML, Janabi M, Kramer JH, Lazaris A, Lockhart SN, Miller BL, Miller ZA, O’Neil JP, Ossenkoppele R, Rosen HJ, Schonhaut DR, Jagust WJ, Rabinovici GD (2017) Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer’s Disease. Neuroimage Clin 17:452–464
Iaccarino L, La Joie R, Edwards L, Strom A, Schonhaut DR, Ossenkoppele R, Pham J, Mellinger T, Janabi M, Baker SL, Soleimani-Meigooni D, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD (2021) Spatial relationships between molecular pathology and neurodegeneration in the Alzheimer’s disease continuum. Cereb Cortex 31:1–14
Ikeda M, Kodaira S, Kasahara H, Takai E, Nagashima K, Fujita Y, Makioka K, Hirayanagi K, Furuta N, Furuta M, Sanada E, Kobayashi A, Harigaya Y, Nagamine S, Hattori N, Tashiro Y, Kishi K, Shimada H, Suto T, Tanaka H, Sakai Y, Yamazaki T, Tanaka Y, Aihara Y, Amari M, Yamaguchi H, Okamoto K, Takatama M, Ishii K, Higuchi T, Tsushima Y, Ikeda Y (2021) Cerebral microbleeds, cerebrospinal fluid, and neuroimaging markers in clinical subtypes of Alzheimer's disease. Front Neurol 12:543866 https://doi.org/10.3389/fneur.2021.543866
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R (2018a) NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14:535–562
Jack CR Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, Weigand SD, Therneau TM, Knopman DS, Gunter JL, Jones DT, Graff-Radford J, Kantarci K, Roberts RO, Mielke MM, Machulda MM, Petersen RC (2018b) Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 141:1517–1528
Jack CR Jr, Therneau TM, Weigand SD, Wiste HJ, Knopman DS, Vemuri P, Lowe VJ, Mielke MM, Roberts RO, Machulda MM, Graff-Radford J, Jones DT, Schwarz CG, Gunter JL, Senjem ML, Rocca WA, Petersen RC (2019) Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol 76:1174–1183
Jang H, Kim HJ, Choe YS, Kim SJ, Park S, Kim Y, Kim KW, Lyoo CH, Cho H, Ryu YH, Choi JY, DeCarli C, Na DL, Seo SW (2020) The impact of amyloid-beta or tau on cognitive change in the presence of severe cerebrovascular disease. J Alzheimers Dis 78:573–585
Janocko NJ, Brodersen KA, Soto-Ortolaza AI, Ross OA, Liesinger AM, Duara R, Graff-Radford NR, Dickson DW, Murray ME (2012) Neuropathologically defined subtypes of Alzheimer’s disease differ significantly from neurofibrillary tangle-predominant dementia. Acta Neuropathol 124:681–692
Jellinger KA (2006) Clinicopathological analysis of dementia disorders in the elderly—an update. J Alzheimers Dis 9:61–70
Jellinger KA (2011) Criteria for the neuropathological diagnosis of dementing disorders: routes out of the swamp? In: Braissant O, Wakamatsu H, Liu IKK, Allegaert K, Lenbury Y, Wachholtz A (eds) Recent researches in modern medicine. Proceedings of the WSEAS international conferences in Cambridge, UK, Feb. 23–25, 2011. WSEAS Press, Cambridge, pp 71–97
Jellinger KA (2012) Neuropathological subtypes of Alzheimer’s disease (correspondence). Acta Neuropathol 123:153–154
Jellinger KA (2016) Commentary on the paper “PART, a distinct tauopathy, different from classical sporadic Alzheimer disease.” J Clin Cell Immunol 7:1000480
Jellinger KA (2018) Different patterns of hippocampal tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136:811–813
Jellinger KA (2020) Neuropathology of the Alzheimer’s continuum: an update. Free Neuropathol 1:32. https://doi.org/10.17879/freeneuropathology-12020-13050
Jellinger KA, Attems J (2007) Neuropathological evaluation of mixed dementia. J Neurol Sci 257:80–87
Jellinger KA, Attems J (2010) Prevalence of dementia disorders in the oldest-old: an autopsy study. Acta Neuropathol 119:421–433
Jellinger KA, Attems J (2015) Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm (vienna) 122:505–521
Jellinger KA, Grazer A, Petrovic K, Ropele S, Alpi G, Kapeller P, Strobel T, Schmidt R (2011) Four-repeat tauopathy clinically presenting as posterior cortical atrophy: atypical corticobasal degeneration? Acta Neuropathol 121:267–277
Jellinger KA, Alafuzoff I, Attems J, Beach TG, Cairns NJ, Crary JF, Dickson DW, Hof PR, Hyman BT, Jack CR Jr, Jicha GA, Knopman DS, Kovacs GG, Mackenzie IR, Masliah E, Montine TJ, Nelson PT, Schmitt F, Schneider JA, Serrano-Pozo A, Thal DR, Toledo JB, Trojanowski JQ, Troncoso JC, Vonsattel JP, Wisniewski T (2015) PART, a distinct tauopathy, different from classical sporadic Alzheimer disease. Acta Neuropathol 129:757–762
Jeon S, Kang JM, Seo S, Jeong HJ, Funck T, Lee SY, Park KH, Lee YB, Yeon BK, Ido T, Okamura N, Evans AC, Na DL, Noh Y (2019) Topographical heterogeneity of Alzheimer’s disease based on MR imaging, tau PET, and amyloid PET. Front Aging Neurosci 11:211
Jones DT, Graff-Radford J, Lowe VJ, Wiste HJ, Gunter JL, Senjem ML, Botha H, Kantarci K, Boeve BF, Knopman DS, Petersen RC, Jack CR Jr (2017) Tau, amyloid, and cascading network failure across the Alzheimer’s disease spectrum. Cortex 97:143–159
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127:441–450
Josephs KA, Whitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE, Petersen RC, Dickson DW (2015) TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol 78:697–709
Josephs KA, Murray ME, Whitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, Liesinger AM, Petersen RC, Parisi JE, Dickson DW (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131:571–585
Josephs KA, Murray ME, Tosakulwong N, Whitwell JL, Knopman DS, Machulda MM, Weigand SD, Boeve BF, Kantarci K, Petrucelli L, Lowe VJ, Jack CR Jr, Petersen RC, Parisi JE, Dickson DW (2017) Tau aggregation influences cognition and hippocampal atrophy in the absence of beta-amyloid: a clinico-imaging-pathological study of primary age-related tauopathy (PART). Acta Neuropathol 133:705–715
Jutten RJ, Sikkes SAM, Van der Flier WM, Scheltens P, Visser PJ, Tijms BM (2021) Finding treatment effects in Alzheimer trials in the face of disease progression heterogeneity. Neurology 96:e2673–e2684
Kapasi A, DeCarli C, Schneider JA (2017) Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol 134:171–186
Karlawish J (2021) Aducanumab and the business of Alzheimer disease—some choice. JAMA Neurol 78:1303–1304
Kaufman SK, Del Tredici K, Thomas TL, Braak H, Diamond MI (2018) Tau seeding activity begins in the transentorhinal/entorhinal regions and anticipates phospho-tau pathology in Alzheimer’s disease and PART. Acta Neuropathol 136:57–67
King A, Bodi I, Troakes C (2020) The neuropathological diagnosis of Alzheimer’s disease—the challenges of pathological mimics and concomitant pathology. Brain Sci 10:479
Knopman DS, Haeberlein SB, Carrillo MC, Hendrix JA, Kerchner G, Margolin R, Maruff P, Miller DS, Tong G, Tome MB, Murray ME, Nelson PT, Sano M, Mattsson N, Sultzer DL, Montine TJ, Jack CR Jr, Kolb H, Petersen RC, Vemuri P, Canniere MZ, Schneider JA, Resnick SM, Romano G, van Harten AC, Wolk DA, Bain LJ, Siemers E (2018) The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the research roundtable. Alzheimers Dement 14:563–575
Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, von Arnim CAF, Tousseyn T, Balusu S, De Strooper B, Thal DR (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 139:463–484
Koychev I, Hofer M, Friedman N (2020) Correlation of Alzheimer disease neuropathologic staging with amyloid and tau scintigraphic imaging biomarkers. J Nucl Med 61:1413–1418
La Joie R, Visani AV, Lesman-Segev OH, Baker SL, Edwards L, Iaccarino L, Soleimani-Meigooni DN, Mellinger T, Janabi M, Miller ZA, Perry DC, Pham J, Strom A, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD (2021) Association of APOE4 and clinical variability in Alzheimer disease with the pattern of tau- and amyloid-PET. Neurology 96:e650–e661
Lam B, Masellis M, Freedman M, Stuss DT, Black SE (2013) Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimers Res Ther 5:1
Lau HHC, Ingelsson M, Watts JC (2021) The existence of Abeta strains and their potential for driving phenotypic heterogeneity in Alzheimer’s disease. Acta Neuropathol 142:17–39
Lehmann M, Ghosh PM, Madison C, Laforce R Jr, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD (2013) Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain 136:844–858
Lesman-Segev OH, La Joie R, Iaccarino L, Lobach I, Rosen HJ, Seo SW, Janabi M, Baker SL, Edwards L, Pham J, Olichney J, Boxer A, Huang E, Gorno-Tempini M, DeCarli C, Hepker M, Hwang JL, Miller BL, Spina S, Grinberg LT, Seeley WW, Jagust WJ, Rabinovici GD (2021) Diagnostic accuracy of amyloid versus (18) F-fluorodeoxyglucose positron emission tomography in autopsy-confirmed dementia. Ann Neurol 89:389–401
Levin F, Ferreira D, Lange C, Dyrba M, Westman E, Buchert R, Teipel SJ, Grothe MJ (2021) Data-driven FDG-PET subtypes of Alzheimer’s disease-related neurodegeneration. Alzheimers Res Ther 13:49
Levine DN, Lee JM, Fisher CM (1993) The visual variant of Alzheimer’s disease: a clinicopathologic case study. Neurology 43:305–313
Li CH, Fan SP, Chen TF, Chiu MJ, Yen RF, Lin CH (2020) Frontal variant of Alzheimer’s disease with asymmetric presentation mimicking frontotemporal dementia: case report and literature review. Brain Behav 10:e01548
Liu KY, Howard R (2021) Can we learn lessons from the FDA’s approval of aducanumab? Nat Rev Neurol 17:715–722
Liu KY, Reeves S, McAleese KE, Attems J, Francis P, Thomas A, Howard R (2020) Neuropsychiatric symptoms in limbic-predominant age-related TDP-43 encephalopathy and Alzheimer’s disease. Brain 143:3842–3849
Liu H, Kim C, Haldiman T, Sigurdson CJ, Nyström S, Nilsson KPR, Cohen ML, Wisniewski T, Hammarström P, Safar JG (2021) Distinct conformers of amyloid beta accumulate in the neocortex of patients with rapidly progressive Alzheimer’s disease. J Biol Chem 297:101267
Long JM, Holtzman DM (2019) Alzheimer disease: an update on pathobiology and treatment strategies. Cell 179:312–339
Machado A, Ferreira D, Grothe MJ, Eyjolfsdottir H, Almqvist PM, Cavallin L, Lind G, Linderoth B, Seiger Ã, Teipel S, Wahlberg LU, Wahlund LO, Westman E, Eriksdotter M (2020) The cholinergic system in subtypes of Alzheimer’s disease: an in vivo longitudinal MRI study. Alzheimers Res Ther 12:51
Malek-Ahmadi M, Perez SE, Chen K, Mufson EJ (2020) Braak stage, cerebral amyloid angiopathy, and cognitive decline in early Alzheimer’s disease. J Alzheimers Dis 74:189–197
Matchett BJ, Grinberg LT, Theofilas P, Murray ME (2021) The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol 141:631–650
Matej R, Tesar A, Rusina R (2019) Alzheimer’s disease and other neurodegenerative dementias in comorbidity: a clinical and neuropathological overview. Clin Biochem 73:26–31
Mattsson N, Schott JM, Hardy J, Turner MR, Zetterberg H (2016) Selective vulnerability in neurodegeneration: insights from clinical variants of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 87:1000–1004
Mattsson N, Ossenkoppele R, Smith R, Strandberg O, Ohlsson T, Jogi J, Palmqvist S, Stomrud E, Hansson O (2018) Greater tau load and reduced cortical thickness in APOE epsilon4-negative Alzheimer’s disease: a cohort study. Alzheimers Res Ther 10:77
McAleese KE, Walker L, Erskine D, Attems J (2020) The impact of concomitant LATE-NC on hyperphosphorylated-s pathology and cognitive decline in Alzheimer’s disease (abstract). Neuropathol Appl Neurobiol 46(Suppl. 1):37
McAleese KE, Colloby SJ, Thomas AJ, Al-Sarraj S, Ansorge O, Neal J, Roncaroli F, Love S, Francis PT, Attems J (2021a) Concomitant neurodegenerative pathologies contribute to the transition from mild cognitive impairment to dementia. Alzheimers Dement 17:1121–1133
McAleese KE, Miah M, Graham S, Hadfield GM, Walker L, Johnson M, Colloby SJ, Thomas AJ, DeCarli C, Koss D, Attems J (2021b) Frontal white matter lesions in Alzheimer’s disease are associated with both small vessel disease and AD-associated cortical pathology. Acta Neuropathol 142:937–950
McMillan CT, Boyd C, Gross RG, Weinstein J, Firn K, Toledo JB, Rascovsky K, Shaw L, Wolk DA, Irwin DJ, Lee EB, Trojanowski JQ, Grossman M (2016) Multimodal imaging evidence of pathology-mediated disease distribution in corticobasal syndrome. Neurology 87:1227–1234
Mehta RI, Schneider JA (2021) What is ‘Alzheimer’s disease’? The neuropathological heterogeneity of clinically defined Alzheimer’s dementia. Curr Opin Neurol 34:237–245
Mesulam MM, Coventry C, Kuang A, Bigio EH, Mao Q, Flanagan ME, Gefen T, Sridhar J, Geula C, Zhang H, Weintraub S, Rogalski EJ (2021) Memory resilience in Alzheimer disease with primary progressive aphasia. Neurology 96:e916–e925
Mirra SS, Hart MN, Terry RD (1993) Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med 117:132–144
Mitelpunkt A, Galili T, Kozlovski T, Bregman N, Shachar N, Markus-Kalish M, Benjamini Y (2020) Novel Alzheimer’s disease subtypes identified using a data and knowledge driven strategy. Sci Rep 10:1327
Mock C, Teylan M, Beecham G, Besser L, Cairns NJ, Crary JF, Katsumata Y, Nelson PT, Kukull W (2020) The utility of the National Alzheimer’s Coordinating Center’s database for the rapid assessment of evolving neuropathologic conditions. Alzheimer Dis Assoc Disord 34:105–111
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123:1–11
Moscoso A, Silva-Rodríguez J, Aldrey JM, Cortés J, Pías-Peleteiro JM, Ruibal Á, Aguiar P (2021) (18)F-florbetapir PET as a marker of myelin integrity across the Alzheimer’s disease spectrum. Eur J Nucl Med Mol Imaging. https://doi.org/10.1007/s00259-00021-05493-y
Mrdjen D, Fox EJ, Bukhari SA, Montine KS, Bendall SC, Montine TJ (2019) The basis of cellular and regional vulnerability in Alzheimer’s disease. Acta Neuropathol 138:729–749
Mukherjee S, Mez J, Trittschuh EH, Saykin AJ, Gibbons LE, Fardo DW, Wessels M, Bauman J, Moore M, Choi SE, Gross AL, Rich J, Louden DKN, Sanders RE, Grabowski TJ, Bird TD, McCurry SM, Snitz BE, Kamboh MI, Lopez OL, De Jager PL, Bennett DA, Keene CD, Larson EB, Crane PK (2020) Genetic data and cognitively defined late-onset Alzheimer’s disease subgroups. Mol Psychiatry 25:2942–2951
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10:785–796
Murray ME, Cannon A, Graff-Radford NR, Liesinger AM, Rutherford NJ, Ross OA, Duara R, Carrasquillo MM, Rademakers R, Dickson DW (2014) Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol 128:411–421
Murray ME, Lowe VJ, Graff-Radford NR, Liesinger AM, Cannon A, Przybelski SA, Rawal B, Parisi JE, Petersen RC, Kantarci K, Ross OA, Duara R, Knopman DS, Jack CR Jr, Dickson DW (2015) Clinicopathologic and 11C-Pittsburgh compound B implications of Thal amyloid phase across the Alzheimer’s disease spectrum. Brain 138:1370–1381
Musiek ES, Morris JC (2021) Possible consequences of the approval of a disease-modifying therapy for Alzheimer disease. JAMA Neurol 78:141–142
Na HK, Kang DR, Kim S, Seo SW, Heilman KM, Noh Y, Na DL (2016) Malignant progression in parietal-dominant atrophy subtype of Alzheimer’s disease occurs independent of onset age. Neurobiol Aging 47:149–156
Nance C, Ritter A, Miller JB, Lapin B, Banks SJ (2019) The pathology of rapid cognitive decline in clinically diagnosed Alzheimer’s disease. J Alzheimers Dis 70:983–993
Neff RA, Wang M, Vatansever S, Guo L, Ming C, Wang Q, Wang E, Horgusluoglu-Moloch E, Song WM, Li A, Castranio EL, Tcw J, Ho L, Goate A, Fossati V, Noggle S, Gandy S, Ehrlich ME, Katsel P, Schadt E, Cai D, Brennand KJ, Haroutunian V, Zhang B (2021) Molecular subtyping of Alzheimer’s disease using RNA sequencing data reveals novel mechanisms and targets. Sci Adv 7. https://doi.org/10.1126/sciadv.abb5398
Nelson PT, Schmitt FA, Lin Y, Abner EL, Jicha GA, Patel E, Thomason PC, Neltner JH, Smith CD, Santacruz KS, Sonnen JA, Poon LW, Gearing M, Green RC, Woodard JL, Van Eldik LJ, Kryscio RJ (2011) Hippocampal sclerosis in advanced age: clinical and pathological features. Brain 134:1506–1518
Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kovari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71:362–381
Nelson PT, Trojanowski JQ, Abner EL, Al-Janabi OM, Jicha GA, Schmitt FA, Smith CD, Fardo DW, Wang WX, Kryscio RJ, Neltner JH, Kukull WA, Cykowski MD, Van Eldik LJ, Ighodaro ET (2016) “New Old Pathologies”: AD, PART, and cerebral age-related TDP-43 with sclerosis (CARTS). J Neuropathol Exp Neurol 75:482–498
Nelson PT, Dickson DW, Trojanowski JQ, Jack CR, Boyle PA, Arfanakis K, Rademakers R, Alafuzoff I, Attems J, Brayne C, Coyle-Gilchrist ITS, Chui HC, Fardo DW, Flanagan ME, Halliday G, Hokkanen SRK, Hunter S, Jicha GA, Katsumata Y, Kawas CH, Keene CD, Kovacs GG, Kukull WA, Levey AI, Makkinejad N, Montine TJ, Murayama S, Murray ME, Nag S, Rissman RA, Seeley WW, Sperling RA, White Iii CL, Yu L, Schneider JA (2019) Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain 142:1503–1527
Nisticò R, Borg JJ (2021) Aducanumab for Alzheimer’s disease: a regulatory perspective. Pharmacol Res 171:105754
Noh Y, Jeon S, Lee JM, Seo SW, Kim GH, Cho H, Ye BS, Yoon CW, Kim HJ, Chin J, Park KH, Heilman KM, Na DL (2014) Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83:1936–1944
Noor A, Zafar S, Shafiq M, Younas N, Siegert A, Mann FA, Kruss S, Schmitz M, Dihazi H, Ferrer I, Zerr I (2021) Molecular profiles of amyloid-beta proteoforms in typical and rapidly progressive Alzheimer’s disease. Mol Neurobiol. https://doi.org/10.1007/s12035-021-02566-9
Ohm DT, Fought AJ, Martersteck A, Coventry C, Sridhar J, Gefen T, Weintraub S, Bigio E, Mesulam MM, Rogalski E, Geula C (2021) Accumulation of neurofibrillary tangles and activated microglia is associated with lower neuron densities in the aphasic variant of Alzheimer's disease. Brain Pathol 31:189–204
Oppedal K, Ferreira D, Cavallin L, Lemstra AW, Ten Kate M, Padovani A, Rektorova I, Bonanni L, Wahlund LO, Engedal K, Nobili F, Kramberger M, Taylor JP, Hort J, Snaedal J, Blanc F, Walker Z, Antonini A, Westman E, Aarsland D (2019) A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from the European DLB consortium. Alzheimers Dement 15:400–409
Ossenkoppele R, Pijnenburg YA, Perry DC, Cohn-Sheehy BI, Scheltens NM, Vogel JW, Kramer JH, van der Vlies AE, La Joie R, Rosen HJ, van der Flier WM, Grinberg LT, Rozemuller AJ, Huang EJ, van Berckel BN, Miller BL, Barkhof F, Jagust WJ, Scheltens P, Seeley WW, Rabinovici GD (2015) The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain 138:2732–2749
Ossenkoppele R, Lyoo CH, Sudre CH, van Westen D, Cho H, Ryu YH, Choi JY, Smith R, Strandberg O, Palmqvist S, Westman E, Tsai R, Kramer J, Boxer AL, Gorno-Tempini ML, La Joie R, Miller BL, Rabinovici GD, Hansson O (2020) Distinct tau PET patterns in atrophy-defined subtypes of Alzheimer’s disease. Alzheimers Dement 16:335–344
Ossenkoppele R, Smith R, Mattsson-Carlgren N, Groot C, Leuzy A, Strandberg O, Palmqvist S, Olsson T, Jögi J, Stormrud E, Cho H, Ryu YH, Choi JY, Boxer AL, Gorno-Tempini ML, Miller BL, Soleimani-Meigooni D, Iaccarino L, La Joie R, Baker S, Borroni E, Klein G, Pontecorvo MJ, Devous MD Sr, Jagust WJ, Lyoo CH, Rabinovici GD, Hansson O (2021) Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol 78:961–971
Paquin V, Therriault J, Pascoal TA, Rosa-Neto P, Gauthier S (2020) Frontal variant of Alzheimer disease differentiated from frontotemporal dementia using in vivo amyloid and tau imaging. Cogn Behav Neurol 33:288–293
Park JY, Na HK, Kim S, Kim H, Kim HJ, Seo SW, Na DL, Han CE, Seong JK (2017) Robust identification of Alzheimer's disease subtypes based on cortical atrophy patterns. Sci Rep 7:43270 https://doi.org/10.1038/srep43270
Pelkmans W, Ossenkoppele R, Dicks E, Strandberg O, Barkhof F, Tijms BM, Pereira JB, Hansson O (2021) Tau-related grey matter network breakdown across the Alzheimer’s disease continuum. Alzheimers Res Ther 13:138
Pereira JB, Janelidze S, Ossenkoppele R, Kvartsberg H, Brinkmalm A, Mattsson-Carlgren N, Stomrud E, Smith R, Zetterberg H, Blennow K, Hansson O (2021) Untangling the association of amyloid-beta and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 144:310–324
Petersen C, Nolan AL, de Paula Franca Resende E, Miller Z, Ehrenberg AJ, Gorno-Tempini ML, Rosen HJ, Kramer JH, Spina S, Rabinovici GD, Miller BL, Seeley WW, Heinsen H, Grinberg LT (2019) Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol 138:597–612
Phillips ML, Stage EC Jr, Lane KA, Gao S, Risacher SL, Goukasian N, Saykin AJ, Carrillo MC, Dickerson BC, Rabinovici GD, Apostolova LG (2019) Neurodegenerative patterns of cognitive clusters of early-onset Alzheimer’s disease subjects: evidence for disease heterogeneity. Dement Geriatr Cogn Disord 48:131–142
Pillai JA, Appleby BS, Safar J, Leverenz JB (2018) Rapidly progressive Alzheimer’s disease in two distinct autopsy cohorts. J Alzheimers Dis 64:973–980
Pini L, Wennberg AM, Salvalaggio A, Vallesi A, Pievani M, Corbetta M (2021) Breakdown of specific functional brain networks in clinical variants of Alzheimer’s disease. Ageing Res Rev 72:101482
Planche V, Villain N (2021) US Food and Drug Administration approval of aducanumab—is amyloid load a valid surrogate end point for Alzheimer disease clinical trials? JAMA Neurol 78:1307-1308
Planche V, Coupe P, Helmer C, Le Goff M, Amieva H, Tison F, Dartigues JF, Catheline G (2019) Evolution of brain atrophy subtypes during aging predicts long-term cognitive decline and future Alzheimer’s clinical syndrome. Neurobiol Aging 79:22–29
Planche V, Bouteloup V, Mangin JF, Dubois B, Delrieu J, Pasquier F, Blanc F, Paquet C, Hanon O, Gabelle A, Ceccaldi M, Annweiler C, Krolak-Salmon P, Habert MO, Fischer C, Chupin M, Béjot Y, Godefroy O, Wallon D, Sauvée M, Bourdel-Marchasson I, Jalenques I, Tison F, Chêne G, Dufouil C (2021) Clinical relevance of brain atrophy subtypes categorization in memory clinics. Alzheimers Dement 17:641–652
Plassman BL, Khachaturian AS, Townsend JJ, Ball MJ, Steffens DC, Leslie CE, Tschanz JT, Norton MC, Burke JR, Welsh-Bohmer KA, Hulette CM, Nixon RR, Tyrey M, Breitner JC (2006) Comparison of clinical and neuropathologic diagnoses of Alzheimer’s disease in 3 epidemiologic samples. Alzheimers Dement 2:2–11
Pollet M, Skrobala E, Lopes R, Kuchcinski G, Bordier C, Rollin-Sillaire A, Bombois S, Pasquier F, Delbeuck X (2021) A multimodal, longitudinal study of cognitive heterogeneity in early-onset Alzheimer’s disease. Eur J Neurol 28:3990–3998
Poulakis K, Pereira JB, Mecocci P, Vellas B, Tsolaki M, Kloszewska I, Soininen H, Lovestone S, Simmons A, Wahlund LO, Westman E (2018) Heterogeneous patterns of brain atrophy in Alzheimer’s disease. Neurobiol Aging 65:98–108
Power MC, Mormino E, Soldan A, James BD, Yu L, Armstrong NM, Bangen KJ, Delano-Wood L, Lamar M, Lim YY, Nudelman K, Zahodne L, Gross AL, Mungas D, Widaman KF, Schneider J (2018) Combined neuropathological pathways account for age-related risk of dementia. Ann Neurol 84:10–22
Qian J, Betensky RA, Hyman BT, Serrano-Pozo A (2021) Association of APOE genotype with heterogeneity of cognitive decline rate in Alzheimer disease. Neurology 96:e2414–e2428
Qiang W, Yau WM, Lu JX, Collinge J, Tycko R (2017) Structural variation in amyloid-beta fibrils from Alzheimer's disease clinical subtypes. Nature 541:217–221
Rahimi J, Kovacs GG (2014) Prevalence of mixed pathologies in the aging brain. Alzheimers Res Ther 6:82
Ramanan VK, Lesnick TG, Przybelski SA, Heckman MG, Knopman DS, Graff-Radford J, Lowe VJ, Machulda MM, Mielke MM, Jack CR Jr, Petersen RC, Ross OA, Vemuri P (2021) Coping with brain amyloid: genetic heterogeneity and cognitive resilience to Alzheimer’s pathophysiology. Acta Neuropathol Commun 9:48
Rauchmann BS, Ersoezlue E, Stoecklein S, Keeser D, Brosseron F, Buerger K, Dechent P, Dobisch L, Ertl-Wagner B, Fliessbach K, Haynes JD, Heneka MT, Incesoy EI, Janowitz D, Kilimann I, Laske C, Metzger CD, Munk MH, Peters O, Priller J, Ramirez A, Roeske S, Roy N, Scheffler K, Schneider A, Spottke A, Spruth EJ, Teipel S, Tscheuschler M, Vukovich R, Wagner M, Wiltfang J, Yakupov R, Duezel E, Jessen F, Perneczky R (2021) Resting-state network alterations differ between Alzheimer’s disease atrophy subtypes. Cereb Cortex  31:4901-4915
Ricci M, Cimini A, Chiaravalloti A, Filippi L, Schillaci O (2020) Positron emission tomography (PET) and neuroimaging in the personalized approach to neurodegenerative causes of dementia. Int J Mol Sci 21:7481. https://doi.org/10.3390/ijms21207481
Risacher SL, Anderson WH, Charil A, Castelluccio PF, Shcherbinin S, Saykin AJ, Schwarz AJ (2017) Alzheimer disease brain atrophy subtypes are associated with cognition and rate of decline. Neurology 89:2176–2186
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141:2181–2193
Robinson JL, Porta S, Garrett FG, Zhang P, Xie SX, Suh E, Van Deerlin VM, Abner EL, Jicha GA, Barber JM, Lee VM, Lee EB, Trojanowski JQ, Nelson PT (2020) Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain 143:2844–2857
Robinson JL, Richardson H, Xie SX, Suh E, Van Deerlin VM, Alfaro B, Loh N, Porras-Paniagua M, Nirschl JJ, Wolk D, Lee VM, Lee EB, Trojanowski JQ (2021) The development and convergence of co-pathologies in Alzheimer’s disease. Brain 144:953–962
Rujeedawa T, Carrillo Félez E, Clare ICH, Fortea J, Strydom A, Rebillat AS, Coppus A, Levin J, Zaman SH (2021) The clinical and neuropathological features of sporadic (late-onset) and genetic forms of Alzheimer's disease. J Clin Med 10:4582
Sahoo A, Bejanin A, Murray ME, Tosakulwong N, Weigand SD, Serie AM, Senjem ML, Machulda MM, Parisi JE, Boeve BF, Knopman DS, Petersen RC, Dickson DW, Whitwell JL, Josephs KA (2018) TDP-43 and Alzheimer’s disease pathologic subtype in non-amnestic Alzheimer’s disease dementia. J Alzheimers Dis 64:1227–1233
Sakae N, Josephs KA, Litvan I, Murray ME, Duara R, Uitti RJ, Wszolek ZK, van Gerpen J, Graff-Radford NR, Dickson DW (2019) Clinicopathologic subtype of Alzheimer’s disease presenting as corticobasal syndrome. Alzheimers Dement 15:1218–1228
Schmidt C, Redyk K, Meissner B, Krack L, von Ahsen N, Roeber S, Kretzschmar H, Zerr I (2010) Clinical features of rapidly progressive Alzheimer’s disease. Dement Geriatr Cogn Disord 29:371–378
Schmidt C, Wolff M, Weitz M, Bartlau T, Korth C, Zerr I (2011) Rapidly progressive Alzheimer disease. Arch Neurol 68:1124–1130
Schmidt C, Haik S, Satoh K, Rabano A, Martinez-Martin P, Roeber S, Brandel JP, Calero-Lara M, de Pedro-Cuesta J, Laplanche JL, Hauw JJ, Kretzschmar H, Zerr I (2012) Rapidly progressive Alzheimer’s disease: a multicenter update. J Alzheimers Dis 30:751–756
Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69:2197–2204
Schulman KA, Greicius MD, Richman B (2021) Will CMS find aducanumab reasonable and necessary for Alzheimer disease after FDA approval? JAMA 326:383–384
Sepulveda-Falla D, Chavez-Gutierrez L, Portelius E, Vélez JI, Dujardin S, Barrera-Ocampo A, Dinkel F, Hagel C, Puig B, Mastronardi C, Lopera F, Hyman BT, Blennow K, Arcos-Burgos M, de Strooper B, Glatzel M (2021) A multifactorial model of pathology for age of onset heterogeneity in familial Alzheimer’s disease. Acta Neuropathol 141:217–233
Shafiq M, Zafar S, Younas N, Noor A, Puig B, Altmeppen HC, Schmitz M, Matschke J, Ferrer I, Glatzel M, Zerr I (2021) Prion protein oligomers cause neuronal cytoskeletal damage in rapidly progressive Alzheimer’s disease. Mol Neurodegener 16:11
Shiino A, Watanabe T, Maeda K, Kotani E, Akiguchi I, Matsuda M (2006) Four subgroups of Alzheimer’s disease based on patterns of atrophy using VBM and a unique pattern for early onset disease. Neuroimage 33:17–26
Shin WS, Di J, Cao Q, Li B, Seidler PM, Murray KA, Bitan G, Jiang L (2019) Amyloid beta-protein oligomers promote the uptake of tau fibril seeds potentiating intracellular tau aggregation. Alzheimers Res Ther 11:86
Simic G, Stanic G, Mladinov M, Jovanov-Milosevic N, Kostovic I, Hof PR (2009) Does Alzheimer’s disease begin in the brainstem? Neuropathol Appl Neurobiol 35:532–554
Singleton E, Hansson O, Pijnenburg YAL, La Joie R, Mantyh WG, Tideman P, Stomrud E, Leuzy A, Johansson M, Strandberg O, Smith R, Berendrecht E, Miller BL, Iaccarino L, Edwards L, Strom A, Wolters EE, Coomans E, Visser D, Golla SSV, Tuncel H, Bouwman F, Van Swieten JC, Papma JM, van Berckel B, Scheltens P, Dijkstra AA, Rabinovici GD, Ossenkoppele R (2021) Heterogeneous distribution of tau pathology in the behavioural variant of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 92:872–880
Sintini I, Graff-Radford J, Senjem ML, Schwarz CG, Machulda MM, Martin PR, Jones DT, Boeve BF, Knopman DS, Kantarci K, Petersen RC, Jack CR, Lowe VJ, Josephs KA, Whitwell JL (2020) Longitudinal neuroimaging biomarkers differ across Alzheimer’s disease phenotypes. Brain 143:2281–2294
Smirnov DS, Galasko D, Hiniker A, Edland SD, Salmon DP (2021) Age-at-onset and APOE-related heterogeneity in pathologically confirmed sporadic Alzheimer disease. Neurology 96:e2272–e2283
Sorrentino S, Ascari R, Maderna E, Catania M, Ghetti B, Tagliavini F, Giaccone G, Di Fede G (2021) Microglial heterogeneity and its potential role in driving phenotypic diversity of Alzheimer’s disease. Int J Mol Sci 22:2780
Spina S, La Joie R, Petersen C, Nolan AL, Cuevas D, Cosme C, Hepker M, Hwang JH, Miller ZA, Huang EJ, Karydas AM, Grant H, Boxer AL, Gorno-Tempini ML, Rosen HJ, Kramer JH, Miller BL, Seeley WW, Rabinovici GD, Grinberg LT (2021) Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain 144:2186–2198
Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, Grinberg LT, Huang EJ, Trojanowski JQ, Meyer M, Henry ML, Comi G, Rabinovici G, Rosen HJ, Filippi M, Miller BL, Seeley WW, Gorno-Tempini ML (2017) Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 81:430–443
Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11:1006–1012
Stopschinski BE, Del Tredici K, Estill-Terpack SJ, Ghebremdehin E, Yu FF, Braak H, Diamond MI (2021) Anatomic survey of seeding in Alzheimer’s disease brains reveals unexpected patterns. Acta Neuropathol Commun 9:164
Teipel SJ, Temp AGM, Levin F, Dyrba M, Grothe MJ (2021) Association of TDP-43 pathology with global and regional 18F-florbetapir PET signal in the Alzheimer’s disease spectrum. J Alzheimers Dis 79:663–670
Ten Kate M, Dicks E, Visser PJ, van der Flier WM, Teunissen CE, Barkhof F, Scheltens P, Tijms BM (2018) Atrophy subtypes in prodromal Alzheimer’s disease are associated with cognitive decline. Brain 141:3443–3456
Tetzloff KA, Graff-Radford J, Martin PR, Tosakulwong N, Machulda MM, Duffy JR, Clark HM, Senjem ML, Schwarz CG, Spychalla AJ, Drubach DA, Jack CR, Lowe VJ, Josephs KA, Whitwell JL (2018) Regional distribution, asymmetry, and clinical correlates of tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease. J Alzheimers Dis 62:1713–1724
Teylan M, Besser LM, Crary JF, Mock C, Gauthreaux K, Thomas NM, Chen YC, Kukull WA (2019) Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer’s neuropathology. Lab Investig 99:1049–1055
Thal DR, Rub U, Orantes M, Braak H (2002) Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thomas DX, Bajaj S, McRae-McKee K, Hadjichrysanthou C, Anderson RM, Collinge J (2020) Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and Lewy bodies with cognitive impairment in individuals with or without Alzheimer’s disease neuropathology. Sci Rep 10:14579
Tijms BM, Gobom J, Teunissen C, Dobricic V, Tsolaki M, Verhey F, Popp J, Martinez-Lage P, Vandenberghe R, Lleó A, Molinuévo JL, Engelborghs S, Freund-Levi Y, Froelich L, Bertram L, Lovestone S, Streffer J, Vos S, ADNI, Blennow K, Scheltens P, Zetterberg H, Visser PJ (2021) CSF proteomic Alzheimer’s disease-predictive subtypes in cognitively intact amyloid negative individuals. Proteomes 9:36
Tiraboschi P, Sabbagh MN, Hansen LA, Salmon DP, Merdes A, Gamst A, Masliah E, Alford M, Thal LJ, Corey-Bloom J (2004) Alzheimer disease without neocortical neurofibrillary tangles: “a second look.” Neurology 62:1141–1147
Togo T, Cookson N, Dickson DW (2002) Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 12:45–52
Tomé SO, Vandenberghe R, Ospitalieri S, Van Schoor E, Tousseyn T, Otto M, von Arnim CAF, Thal DR (2020) Distinct molecular patterns of TDP-43 pathology in Alzheimer’s disease: relationship with clinical phenotypes. Acta Neuropathol Commun 8:61
Tosto G, Gasparini M, Brickman AM, Letteri F, Renie R, Piscopo P, Talarico G, Canevelli M, Confaloni A, Bruno G (2015) Neuropsychological predictors of rapidly progressive Alzheimer’s disease. Acta Neurol Scand 132:417–422
Townley RA, Graff-Radford J, Mantyh WG, Botha H, Polsinelli AJ, Przybelski SA, Machulda MM, Makhlouf AT, Senjem ML, Murray ME, Reichard RR, Savica R, Boeve BF, Drubach DA, Josephs KA, Knopman DS, Lowe VJ, Jack CR Jr, Petersen RC, Jones DT (2020) Progressive dysexecutive syndrome due to Alzheimer’s disease: a description of 55 cases and comparison to other phenotypes. Brain Commun 2:fcaa068
Trejo-Lopez JA, Yachnis AT, Prokop S (2021) Neuropathology of Alzheimer's disease. Neurotherapeutics. https://doi.org/10.1007/s13311-021-01146-y
Ulugut H, Stek S, Wagemans LEE, Jutten RJ, Keulen MA, Bouwman FH, Prins ND, Lemstra AW, Krudop W, Teunissen CE, van Berckel BNM, Ossenkoppele R, Barkhof F, van der Flier WM, Scheltens P, Pijnenburg YAL (2021) The natural history of primary progressive aphasia: beyond aphasia. J Neurol. https://doi.org/10.1007/s00415-021-10689-1
Uretsky M, Gibbons LE, Mukherjee S, Trittschuh EH, Fardo DW, Boyle PA, Keene CD, Saykin AJ, Crane PK, Schneider JA, Mez J (2021) Longitudinal cognitive performance of Alzheimer’s disease neuropathological subtypes. Alzheimers Dement (n y) 7:e12201
van Loenhoud AC, van der Flier WM, Wink AM, Dicks E, Groot C, Twisk J, Barkhof F, Scheltens P, Ossenkoppele R (2019) Cognitive reserve and clinical progression in Alzheimer disease: a paradoxical relationship. Neurology 93:e334–e346
Vaquer-Alicea J, Diamond MI, Joachimiak LA (2021) Tau strains shape disease. Acta Neuropathol 142:57–71
Varol E, Sotiras A, Davatzikos C (2017) HYDRA: Revealing heterogeneity of imaging and genetic patterns through a multiple max-margin discriminative analysis framework. Neuroimage 145:346–364
Velásquez E, Szeitz B, Gil J, Rodriguez J, Palkovits M, Renner É, Hortobágyi T, Döme P, Nogueira FC, Marko-Varga G, Domont GB, Rezeli M (2021) Topological dissection of proteomic changes linked to the limbic stage of Alzheimer’s disease. Front Immunol 12:750665
Vergara C, Houben S, Suain V, Yilmaz Z, De Decker R, Vanden Dries V, Boom A, Mansour S, Leroy K, Ando K, Brion JP (2019) Amyloid-beta pathology enhances pathological fibrillary tau seeding induced by Alzheimer PHF in vivo. Acta Neuropathol 137:397–412
Vermunt L, Sikkes SAM, van den Hout A, Handels R, Bos I, van der Flier WM, Kern S, Ousset PJ, Maruff P, Skoog I, Verhey FRJ, Freund-Levi Y, Tsolaki M, Wallin ÅK, Olde Rikkert M, Soininen H, Spiru L, Zetterberg H, Blennow K, Scheltens P, Muniz-Terrera G, Visser PJ (2019) Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement 15:888–898
Villain N, Dubois B (2019) Alzheimer's disease including focal presentations. Semin Neurol 39:213–226
Vogel JW, Young AL, Oxtoby NP, Smith R, Ossenkoppele R, Strandberg OT, La Joie R, Aksman LM, Grothe MJ, Iturria-Medina Y, Pontecorvo MJ, Devous MD, Rabinovici GD, Alexander DC, Lyoo CH, Evans AC, Hansson O (2021) Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat Med 27:871–881
Walker LC (2020) Aß Plaques Free Neuropathol 1:31
Walker JM, Fudym Y, Farrell K, Iida MA, Bieniek KF, Seshadri S, White CL, Crary JF, Richardson TE (2021a) Asymmetry of hippocampal tau pathology in primary age-related tauopathy and Alzheimer disease. J Neuropathol Exp Neurol 80:436–445
Walker JM, Richardson TE, Farrell K, Iida MA, Foong C, Shang P, Attems J, Ayalon G, Beach TG, Bigio EH, Budson A, Cairns NJ, Corrada M, Cortes E, Dickson DW, Fischer P, Flanagan ME, Franklin E, Gearing M, Glass J, Hansen LA, Haroutunian V, Hof PR, Honig L, Kawas C, Keene CD, Kofler J, Kovacs GG, Lee EB, Lutz MI, Mao Q, Masliah E, McKee AC, McMillan CT, Mesulam MM, Murray M, Nelson PT, Perrin R, Pham T, Poon W, Purohit DP, Rissman RA, Sakai K, Sano M, Schneider JA, Stein TD, Teich AF, Trojanowski JQ, Troncoso JC, Vonsattel JP, Weintraub S, Wolk DA, Woltjer RL, Yamada M, Yu L, White CL, Crary JF (2021b) Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol 80:102–111
Wang BW, Lu E, Mackenzie IR, Assaly M, Jacova C, Lee PE, Beattie BL, Hsiung GY (2012) Multiple pathologies are common in Alzheimer patients in clinical trials. Can J Neurol Sci 39:592–599
Wang ZT, Zhang C, Wang YJ, Dong Q, Tan L, Yu JT (2020) Selective neuronal vulnerability in Alzheimer’s disease. Ageing Res Rev 62:101114
Wang Q, He C, Wang Z, Zhang Z, Xie C (2021) Dynamic connectivity alteration facilitates cognitive decline in Alzheimer’s disease spectrum. Brain Connect 11:213–224
Weigand AJ, Bangen KJ, Thomas KR, Delano-Wood L, Gilbert PE, Brickman AM, Bondi MW (2020) Is tau in the absence of amyloid on the Alzheimer’s continuum?: a study of discordant PET positivity. Brain Commun 2:fcz046
Weintraub S, Teylan M, Rader B, Chan KCG, Bollenbeck M, Kukull WA, Coventry C, Rogalski E, Bigio E, Mesulam MM (2020) APOE is a correlate of phenotypic heterogeneity in Alzheimer disease in a national cohort. Neurology 94:e607–e612
Wesenhagen KEJ, Teunissen CE, Visser PJ, Tijms BM (2020) Cerebrospinal fluid proteomics and biological heterogeneity in Alzheimer’s disease: a literature review. Crit Rev Clin Lab Sci 57:86–98
White LR, Edland SD, Hemmy LS, Montine KS, Zarow C, Sonnen JA, Uyehara-Lock JH, Gelber RP, Ross GW, Petrovitch H, Masaki KH, Lim KO, Launer LJ, Montine TJ (2016) Neuropathologic comorbidity and cognitive impairment in the Nun and Honolulu-Asia Aging Studies. Neurology 86:1000–1008
Whitwell JL, Dickson DW, Murray ME, Weigand SD, Tosakulwong N, Senjem ML, Knopman DS, Boeve BF, Parisi JE, Petersen RC, Jack CR Jr, Josephs KA (2012) Neuroimaging correlates of pathologically defined subtypes of Alzheimer’s disease: a case-control study. Lancet Neurol 11:868–877
Whitwell JL, Tosakulwong N, Weigand SD, Graff-Radford J, Ertekin-Taner N, Machulda MM, Duffy JR, Schwarz CG, Senjem ML, Jack CR, Lowe VJ, Josephs KA (2021) Relationship of APOE, age at onset, amyloid and clinical phenotype in Alzheimer disease. Neurobiol Aging 108:90–98
Wiels WA, Wittens MMJ, Zeeuws D, Baeken C, Engelborghs S (2021) Neuropsychiatric symptoms in mild cognitive impairment and dementia due to AD: relation with disease stage and cognitive deficits. Front Psychiatry 12:707580 https://doi.org/10.3389/fpsyt.2021.707580
Wiersma VI, van Ziel AM, Vazquez-Sanchez S, Nölle A, Berenjeno-Correa E, Bonaterra-Pastra A, Clavaguera F, Tolnay M, Musters RJP, van Weering JRT, Verhage M, Hoozemans JJM, Scheper W (2019) Granulovacuolar degeneration bodies are neuron-selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol 138:943–970
Wiersma VI, Hoozemans JJM, Scheper W (2020) Untangling the origin and function of granulovacuolar degeneration bodies in neurodegenerative proteinopathies. Acta Neuropathol Commun 8:153
Wisniewski T, Drummond E (2019) Future horizons in Alzheimer’s disease research. Prog Mol Biol Transl Sci 168:223–241
Wong B, Lucente DE, MacLean J, Padmanabhan J, Quimby M, Brandt KD, Putcha D, Sherman J, Frosch MP, McGinnis S, Dickerson BC (2019) Diagnostic evaluation and monitoring of patients with posterior cortical atrophy. Neurodegener Dis Manag 9:217–239
Wong BYX, Yong TT, Lim L, Tan JY, Ng ASL, Ting SKS, Hameed S, Ng KP, Zhou JH, Kandiah N (2019) Medial temporal atrophy in amyloid-negative amnestic type dementia is associated with high cerebral white matter hyperintensity. J Alzheimers Dis 70:99–106
Wurm R, Klotz S, Rahimi J, Katzenschlager R, Lindeck-Pozza E, Regelsberger G, Danics K, Kapas I, Bíró ZA, Stögmann E, Gelpi E, Kovacs GG (2020) Argyrophilic grain disease in individuals younger than 75 years: clinical variability in an underrecognized limbic tauopathy. Eur J Neurol 27:1856–1866
Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, Bennett DA, Schneider JA, De Jager PL (2018) Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE ε4 haplotype status: a community-based cohort study. Lancet Neurol 17:773–781
Younas N, Zafar S, Shafiq M, Noor A, Siegert A, Arora AS, Galkin A, Zafar A, Schmitz M, Stadelmann C, Andreoletti O, Ferrer I, Zerr I (2020) SFPQ and tau: critical factors contributing to rapid progression of Alzheimer’s disease. Acta Neuropathol 140:317–339
Zafar S, Shafiq M, Younas N, Schmitz M, Ferrer I, Zerr I (2017) Prion protein interactome: identifying novel targets in slowly and rapidly progressive forms of Alzheimer’s disease. J Alzheimers Dis 59:265–275
Zangrossi A, Montemurro S, Altoè G, Mondini S (2021) Heterogeneity and factorial structure in Alzheimer’s disease: a cognitive perspective. J Alzheimers Dis  83:1341-1351
Zhang X, Mormino EC, Sun N, Sperling RA, Sabuncu MR, Yeo BT (2016) Bayesian model reveals latent atrophy factors with dissociable cognitive trajectories in Alzheimer’s disease. Proc Natl Acad Sci USA 113:E6535–E6544
Zhang X, Sun B, Wang X, Lu H, Shao F, Rozemuller AJM, Liang H, Liu C, Chen J, Huang M, Zhu K (2019) Phosphorylated TDP-43 staging of primary age-related tauopathy. Neurosci Bull 35:183–192
Zhang L, Jiang Y, Zhu J, Liang H, He X, Qian J, Lin H, Tao Y, Zhu K (2020) Quantitative assessment of hippocampal tau pathology in AD and PART. J Mol Neurosci 70:1808–1811
Zhang B, Lin L, Wu S, Al-Masqari Z (2021a) Multiple subtypes of Alzheimer’s disease base on brain atrophy pattern. Brain Sci 11:278
Zhang Y, Aman Y, Ng CT, Chau WH, Zhang Z, Yue M, Bohm C, Jia Y, Li S, Yuan Q, Griffin J, Chiu K, Wong DSM, Wang B, Jin D, Rogaeva E, Fraser PE, Fang EF, St George-Hyslop P, Song YQ (2021b) Amyloid-beta toxicity modulates tau phosphorylation through the PAX6 signalling pathway. Brain 144:2759–2770
Zhang B, Lin L, Wu S (2021c) A review of brain atrophy subtypes definition and analysis for Alzheimer's disease heterogeneity studies. J Alzheimers Dis 80:1339–1352
Zheng C, Xu R (2021) Molecular subtyping of Alzheimer’s disease with consensus non-negative matrix factorization. PLoS ONE 16:e0250278
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl, PhD, for secretarial and editorial work.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J Neural Transm 129, 1–24 (2022). https://doi.org/10.1007/s00702-021-02449-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-021-02449-2