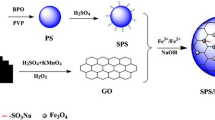
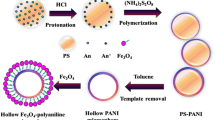
Abstract
Fe3O4/polyaniline (PANI) composite hollow spheres were prepared by using sulfonated polystyrene (SPS) microspheres as templates. The sulfonic acid groups were applied to induce absorbing Fe3O4 nanoparticle, and subsequently, conductive PANI was grown. Finally, the polystyrene cores were selectively dissolved to yield composite hollow microspheres with electromagnetic properties. The analysis results indicated that the adsorption of Fe3O4 on template core by electrostatic interaction resulted in magnetic composite microspheres. The conductivity of composite hollow spheres was remarkably increased after polyvinylpyrrolidone modification which favored the growth of PANI on SPS/Fe3O4 and enhanced the integrity of hollow microspheres. The saturated magnetization of the composite hollow microspheres was tuned from 2.7 to 9.1 emu/g, and the conductivity was in the range from 10−2 to 100 S/cm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hollow microspheres have attracted considerable academic and technological attention in chemistry and materials science because of their advantages of large specific area, low density, and high mechanical stability. The properties of hollow spheres depend on the integrated functionality of shell materials and the low effective density and high specific surface [1–5]. The shells can be made of polymeric, inorganic, or composite materials. Among the shell materials, the electromagnetic materials are proved to be an important class of functional materials for their superior performance as electromagnetic shielding and wave-absorbing materials [6–14]. Combining the electromagnetic properties with hollow geometry is an effective approach to promote their application in new fields [15]. For example, the electromagnetic hollow microspheres could be used as high-performance lightweight wave-absorbing materials [16].
Nowadays, several methods are employed to prepare hollow microspheres. Hollow spheres could be prepared by interfacial reaction [17], combination of sol–gel process with oil–water–oil microemulsion [18], self-assembly of block copolymers [19], aerosol pyrolysis [20], and Kirkendall diffusion [21]. Especially, template synthesis has been well-developed to prepare hollow spheres, in which solid or hollow colloids could be applied as cores [22–24]. The subsequent deposition of shell materials was usually assisted by a layer-by-layer process, and finally, the template cores were selectively removed to obtain the hollow microspheres [25]. Yang et al. proposed a new approach to generally prepare hollow microspheres, where the potential use of polymer colloid as general template was explored, addressing issues related to the derivation of functional groups on template, inducing adsorption of shell materials such as inorganic oxides, metal, and polymer, and the structure–properties relationship [26–30]. They pointed out that the derived functional groups by chemical transformation could induce a diversity of materials including inorganic, metals, and polymers to in situ growth on the template. Additionally, the cavity and shell sizes could be simultaneously tailored due to an inward growth of the shell materials.
The electromagnetic composite hollow microspheres were prepared by using such template [31, 32]. The sulfonated polystyrene (SPS) microspheres were used to adsorb aniline and as acid dopant, and then, the in situ polymerization led to a conductive layer of polyaniline (PANI). The magnetic oxides were deposited by coprecipitation of Fe2+ and Fe3+ on the conductive microspheres. To some extent, the conductive property was reduced due to de-doping during deposition of Fe3O4 by using alkali precipitant. On the other hand, the conductive network was interrupted by the exterior layer of Fe3O4.
In this paper, a favorable procedure is proposed to use Fe3O4 as interior and conductive PANI as exterior for preparing the electromagnetic composite microspheres against SPS colloid as template. The as-prepared Fe3O4 particles were firstly coated on the template via electrostatic adsorption to form SPS/Fe3O4, and subsequently, PANI was polymerized as exterior layer. After removing the template, the hollow structure could be achieved. The influences of sulfonation of the template and surface modification on the structure and properties of the electromagnetic hollow microspheres were investigated. The results showed that the magnetization of the electromagnetic hollow microspheres increased by increasing the sulfonation degree of PS. The conductivity was significantly enhanced after SPS/Fe3O4 was modified by polyvinylpyrrolidone (PVP) and then PANI was deposited because PVP favored the growth of PANI and formation of contiguous conductive network.
Experimental section
Synthesis
Raw materials
Aniline monomers were distilled under reduced pressure. Styrene (St) was washed with 10 % NaOH and then distilled water to remove the inhibitor. 2,2′-Azobis(2-methylpropionitrile) (AIBN) was recrystallized in absolute ethanol and dried. All other chemical reagents were used as received: PVP, concentrated sulfuric acid, ferrous chloride tetrahydrate (FeCl2·4H2O), ferric chloride hexahydrate (FeCl3·6H2O), sodium hydroxide (NaOH), hydrochloric acid (HCl), ammonium peroxydisulfate (APS), absolute ethanol, and distilled water.
Preparation of polystyrene microspheres
Polystyrene (PS) colloids were prepared by suspension polymerization. Certain amounts of AIBN and PVP were dissolved in ethanol and water under stirring for 30 min, and then, 20 g St was dropped into the mixture. The reaction system was mechanically stirred with 100 rpm and heated at 75 °C for 12 h with N2 protection. The product was centrifuged, washed with distilled water, and freeze-dried.
Sulfonation of PS microspheres
Sulfonation reaction was according to a typical procedure [27]: 2 g PS was dispersed in 40 ml concentrated sulfuric acid under stirring with 100 rpm. The reaction was conducted at 40 °C for 1, 2, and 4 h to obtain the SPS colloids denoted as SPS1h, SPS2h, and SPS4h, respectively. The SPS was centrifuged and washed with distilled water until the pH value of filtrate was near to 7.
Preparation of Fe3O4 nanoparticles
Fe3O4 nanoparticles were prepared by a modified coprecipitate method according to the literature [33]. The as-prepared Fe3O4 nanoparticles were dispersed in distilled water and filled with nitrogen to form the magnetic suspension.
Preparation of SPS/Fe3O4 composite microspheres
A typical procedure was to mix 1.2 g SPS with 0.22 g Fe3O4, and 65 ml HCl aqueous solution with pH in the range of 3–5 was added into the mixture. The suspension was stirred for 30 min and then allowed to separate into layers. The underlayer was isolated and then dried at 50 °C.
Preparation of Fe3O4/PANI composite hollow microspheres
SPS/Fe3O4 (0.5 g) and PVP (2.5 g) were added in 100 ml absolute ethanol at 30 °C and stirred for 5 h. The PVP-modified SPS/Fe3O4 was washed with absolute ethanol and then dried at 40 °C. The 0.5 g SPS/Fe3O4/PVP was dispersed in 22 ml acidic mixture containing 0.4 ml aniline monomer under constant mechanical stirring at 5 °C for 30 min. Then, 22 ml acidic solution including 0.98 g APS was dropped into the former mixture and the reaction was processed at 5 °C for 12 h. The pH value of the reaction system was 1.5. After reaction, the precipitates were collected and washed with absolute ethanol, distilled water, and then HCl aqueous solution until filtrate was transparent. Finally, the products were freeze-dried. For comparison, the aniline polymerization on SPS/Fe3O4 without PVP modification was conducted under the same reaction parameters. Fe3O4/PANI hollow spheres were obtained after dissolution of PS cores with N,N-dimethylformamide. The Fe3O4/PANI hollow spheres were designated as series of H-SPS/Fe3O4/PANI or H-SPS/Fe3O4/PVP/PANI.
Characterization
Scanning electron microscopy (SEM) measurement was performed with a SIRION200 instrument operated at an accelerating voltage of 20 kV. Particle size analysis was recorded by ZetaPALS of Brookhaven. Fourier transform infrared spectroscopy (FTIR) spectra were recorded on a Thermo Nicolet Nexus 670 spectrometer with samples pressed into KBr pellets. Wide-angle X-ray powder diffraction (D/Max-rB) was used to characterize the crystalline phase of these materials. Conductivity measurement was characterized by the four-point probes method (RTS-8). Vibrating-sample magnetometry (Lake Shore 7410 VSM) was used to characterize the magnetic property of the magnetic spheres. Conductivity measurement was characterized by the four-point probes method (KDY-1).
Results and discussion
The approach to prepare the electromagnetic hollow spheres was based on a template process. PS colloids were used as template to prepare the functional hollow microspheres. The sulfonation process produced the reactive sites for inducing adsorption of shell materials. Adsorption was formed by electrostatic interaction between positively charged Fe3O4 nanoparticles and negatively charged SPS. The subsequent growth of PANI on PVP-modified SPS/Fe3O4 was conducted, and finally, the template cores were dissolved to form the hollow spheres.
The synthesized PS template is shown in Fig. 1. The spherical structure with size concentrated in the range 1.3–1.6 μm was obtained. After sulfonation, the SPS adhered together due to the formation of the gel layer. The average particle size tended to increase with prolonging sulfonation time. FTIR results in Fig. 2 showed that the characteristic bands at 1,226 and 670 cm−1 was attributed to the derived sulfonic acid group (–SO3H) and the band at 1,124 cm−1 was due to the sulfone group –SO2−. The peak intensities of the functional groups were increased with prolonging sulfonation time, indicating that the contents of the sulfonic acid groups were correspondingly increased.
To obtain the composite hollow spheres with electromagnetic properties, Fe3O4 nanoparticles and PANI were applied as the functional shell materials. For ensuring the continuous conductive layer of PANI, the core–shell structure was designed with Fe3O4 as inner layer and conductive PANI as the outer layer. The adsorption of Fe3O4 particles instead of ferric ions was conducted by sulfonic acid groups via electrostatic interaction as driving force in order to maintain the properties of primitive Fe3O4 nanoparticles. Figure 3 exhibited that the synthesized Fe3O4 particles could be well-dispersed in water and quickly responded to the external magnetic field. The particle size distribution investigated by dynamic light scattering (DLS) was in the range from 40 to 65 nm.
The adsorption of Fe3O4 particles in the sulfonated gel layer could be carried out by electrostatic interaction between sulfonic acid groups and reaction precursor. In this article, the electrostatic adsorption of SPS with Fe3O4 was carried out according to the colloid adsorption theory. The pH value was controlled in the range of 3–5 so that sulfonic acid groups were deprotonated to form negatively charged surface, and at the same time, the Fe3O4 nanoparticles were positively charged since they have the isoelectric point (pI) of 6.6 [34]. The binding was carried out by the electrostatic interactions between negatively charged SPS spheres and positively charged Fe3O4. Therefore, sulfonic acid groups were dissociated to form negatively charged templates when pH values of the system were higher than pK a of the sulfonic acid groups. Fe3O4 nanoparticle will be positively charged when pH values were lower than its pI. The driving force for electrostatic interaction between SPS and Fe3O4 was constructed when pH values in the range between pK a of sulfonic acid groups and pI of Fe3O4. After the coating procedure, the SPS/Fe3O4 core–shell composites were obtained. Figure 4 showed the SEM images of SPS/Fe3O4 composite microspheres with different sulfonation degrees. Compared with SEM images of the corresponding SPS in Fig. 1, it was found that, after coating Fe3O4, the composites had coarse surface morphology and the particle size tended to increase. The enlarged SEM in the inset of Fig. 4a, b indicated that SPS were covered by nanoparticles which distributed throughout the whole shell. No free Fe3O4 particles were observed in the dispersion medium. FTIR spectra of SPS/Fe3O4 in Fig. S1 exhibited that the peak around 539 cm−1 of the template weakened and broadened due to the introduction of Fe3O4, which had a characteristic adsorption around 560 cm−1.
The in situ growth of PANI on SPS/Fe3O4 was conducted to introduce the conductive properties. From Fig. 5, it could be observed that SPS/Fe3O4/PANI had different surface structure from SPS/Fe3O4. The inset enlarged the SEM images exhibiting that the Fe3O4 nanoparticles were covered with polymer layer. After removing the PS cores, the corresponding Fe3O4/PANI hollow spheres hardly preserved the spherical morphology, although the integrity could be improved with prolonging sulfonation time. For low sulfonation degree, composite hollow spheres were bowl-shaped hemispheres. The perforation and rupture on the shell resulted from the osmotic pressure during dissolving of the cores. The polymerization of aniline was reacted in HCl aqueous solution, but the aniline monomer was slightly soluble in water, which led to the uneven adsorption on SPS/Fe3O4. For promoting the adsorption of aniline and polymerization on SPS/Fe3O4, PVP was used to modify SPS/Fe3O4 firstly. Aniline polymerization on PVP-modified SPS/Fe3O4 composites was carried out under the same condition. After removing the PS cores, the composite hollow spheres with well-defined shell structure were achieved (shown in Fig. 6), indicating the high coverage of PANI because PVP acted both as a site for adsorption of oligoaniline initiation centers and as steric stabilizer of the formed PANI particles [35].
SPS/Fe3O4/PANI before and after removing PS cores (hollow spheres). a SPS1h/Fe3O4/PANI and b the corresponding hollow spheres (H-SPS1h/Fe3O4/PANI); c SPS2h/Fe3O4/PANI and d the corresponding hollow spheres (H-SPS2h/Fe3O4/PANI); e SPS4h/Fe3O4/PANI and f the corresponding hollow spheres (H-SPS4h/Fe3O4/PANI) (insets are the enlarged SEM images)
FTIR spectra in Fig. S2 of PANI showed the characteristic adsorption bands at 1,558 and 1,471 cm−1 which were due to the C = C stretching vibration of quinoid ring and benzenoid ring of PANI, respectively. The peak at 1,294 cm−1 was attributed to the C–N stretching modes of the benzenoid unit. The broad band at 1,114 cm−1 was ascribed to the C–H structure of the aromatic ring. These results were in good agreement with previous reports [9]. For SPS4h/Fe3O4/PVP/PANI, almost same spectra with PANI were observed.
The crystalline structures of Fe3O4, SPS/Fe3O4, and Fe3O4/PANI hollow spheres were analyzed by X-ray diffraction (XRD) in Fig. 7. From pattern a, it was found that the synthesized Fe3O4 could be well-indexed as JCPDS-19-0629. The diffraction peaks at 2θ of 30.1°, 35.4°, 43.0°, 53.4°, 57.0°, 62.5°, and 74.0° were attributed to crystalline planes of (2,2,0), (3,1,1), (4,0,0), (4,2,2), (5,1,1), (4,4,0), and (5,3,3), respectively. The broad diffraction peaks revealed that the nanosized Fe3O4 crystals were obtained. It was observed from patterns b and c that the core–shell SPS/Fe3O4 with different sulfonation degrees both included the characteristic diffraction peaks of Fe3O4 which were not changed after coating on SPS, indicating that the method to directly absorb Fe3O4 could well reserve the structure of Fe3O4. A broad peak around 20° in curves b and c was attributed to SPS. The XRD pattern of the typical Fe3O4/PANI hollow spheres was shown in curve d. Apart from Fe3O4 diffraction peaks, the broad diffraction peaks at 2θ = 20.8° and 25.2° were ascribed to the periodicity parallel and perpendicular to the PANI chains, respectively [36].
The electromagnetic properties of hollow microspheres prepared under different parameters were studied by VSM and conductivity. Figure 8 displays the magnetic hysteresis curves of Fe3O4 and the hollow spheres by VSM at room temperature. The prepared Fe3O4 nanoparticles indicated a superparamagnetic behavior because the remnant magnetization was zero. The saturated magnetization was 86.8 emu/g. The hysteresis curves of hollow spheres also showed that the remnant magnetization was zero, indicating that the superparamagnetic behavior remained after forming magnetic composite hollow spheres. The saturation magnetizations of the hollow spheres were less than that of Fe3O4 since it depended on the content of Fe3O4 in the composites. By comparing the magnetizations, it was shown that the saturated magnetizations increased with increasing sulfonation degree of the PS templates. The highest value of about 9.1 emu/g was obtained for H-SPS4h/Fe3O4/PANI, revealing the highest content of Fe3O4 in H-SPS4h/Fe3O4/PANI. The two samples of SPS2h/Fe3O4/PVP/PANI and SPS4h/Fe3O4/PVP/PANI also indicated the same principle, but they had decreased magnetization compared with the sample without PVP modification. The decreased magnetization of the samples modified by PVP resulted from the increased content of PANI because the only difference in the two series was PVP modification. Further supporting the analyses, the conductivities of the hollow spheres were listed in Table 1. The values showed that PVP modification favored the enhancement of conductivity. The increase in conductivity should be attributed to the increase of the loading amount of PANI and the integrity of the conductivity layer, which was consistent with the result of SEM.
Conclusions
In this work, the composite hollow spheres with electromagnetic properties were prepared by applying SPS microspheres as template and Fe3O4 and PANI as functional shell. The procedures were conducted to ensure the hollow structure and good properties. The results indicated that the integrity of the hollow spheres was increased by increasing sulfonation degree. The adsorption of Fe3O4 nanoparticles on the template instead of their salt reactants ensured better preservation of magnetic properties. PVP modification favored forming continuous layer of conductive PANI and finally increased the conductivity of the sample. The magnetization was tuned by changing the sulfonation degree, and the highest value was 9.1 emu/g and the highest conductivity was 1.27 emu/g.
References
Caruso F, Caruso RA, Möhwald H (1998) Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science 282:1111–1114
Wang D, Zeng H (2009) Multifunctional roles of TiO2 nanoparticles for architecture of complex core–shells and hollow spheres of SiO2–TiO2–polyaniline system. Chem Mater 21:4811–4823
Hu J, Chen M, Fang X, Wu L (2011) Fabrication and application of inorganic hollow spheres. Chem Soc Rev 40:5472–5491
Ding SJ, Zhang CL, Yang M, Qu XZ, Lu YF, Yang ZZ (2006) Template synthesis of composite hollow spheres using sulfonated polystyrene hollow spheres. Polymer 47:8360–8366
Han SH, Hou WG, Xu J, Li ZM (2004) Synthesis of hollow spherical silica with MCM-41 mesoporous structure. Colloid Polym Sci 282:1286–1291
Saini P, Choudhary V, Vijayan N, Kotnala RK (2012) Improved electromagnetic interference shielding response of poly(aniline)-coated fabrics containing dielectric and magnetic nanoparticles. J Phys Chem C 116:13403–13412
Zhang YJ, Lin YW, Chang CC, Wu TM (2010) Magnetic properties of hydrophilic iron oxide/polyaniline nanocomposites synthesized by in situ chemical oxidative polymerization. Synth Met 160:1086–1091
He ZF, Fang Y, Wang XJ, Pang H (2011) Microwave absorption properties of PANI/CIP/Fe3O4 composites. Synth Met 161:420–425
Gu HB, Huang YD, Zhang X, Wang Q, Zhu JH, Shao L, Haldolaarachchige H, Young DP, Wei SY, Guo ZH (2012) Magnetoresistive polyaniline–magnetite nanocomposites with negative dielectrical properties. Polymer 53:801–809
Saini P, Choudhary V, Singh BP, Mathur RB, Dhawan SK (2009) Polyaniline–MWCNT nanocomposites for microwave absorption and EMI shielding. Mat Chem Phys 13:919–926
Saini P, Arora M Microwave absorption and EMI shielding behavior of nanocomposites based on intrinsically conducting polymers, graphene and carbon nanotubes. In: Gomes AD (ed) New polymers for special applications. InTech, Croatia. doi:10.5772/48779
Chung DDL (2001) Electromagnetic interference shielding effectiveness of carbon materials. Carbon 39:279–285
Yang YL, Gupta MC (2005) Novel carbon nanotube–polystyrene foam composites for electromagnetic interference shielding. Nano Lett 5:2131–2134
Li N, Huang Y, Du F, He XB, Lin X, Gao HJ, Ma YF, Li FF, Chen YS, Eklund PC (2006) Electromagnetic interference (EMI) shielding of single-walled carbon nanotubes epoxy composites. Nano Lett 6:1141–1145
Wang J, Xu HF, Song JW, Zhang HJ, Gao BL, Huang YD (2011) Lightweight glass/Fe3O4–polyaniline composite hollow spheres with conductive and magnetic properties. J Mater Sci 46:2955–2962
Yang CM, Li HY, Xiong DB, Cao ZY (2009) Hollow polyaniline/Fe3O4 microsphere composites: preparation, characterization, and applications in microwave absorption. React Funct Polym 69:137–144
Nakashima T, Kimizuka N (2003) Interfacial synthesis of hollow TiO2 microspheres in ionic liquids. J Am Chem Soc 125:6386–6387
Jiang YQ, Yang SF, Ding XF, Guo YP, Bala H, Zhao JZ, Yu KF, Wang ZC (2005) Synthesis and catalytic activity of stable hollow ZrO2–SiO2 spheres with mesopores in the shell wall. J Mater Chem 15:2041–2046
Discher DE, Eisenberg A (2002) Polymer vesicles. Science 297:967–973
Tartaj P, Gonzάlez-Carreño SCJ (2001) Single-step nanoengineering of silica coated maghemite hollow spheres with tunable magnetic properties. Adv Mater 13:1620–1624
Yin YD, Rioux RM, Erdonmez CK, Hughes S, Somorjai GA, Alivisatos AP (2004) Formation of hollow nanocrystals through the nanoscale Kirkendall effect. Science 304:711–714
Ding SJ, Wei W, Yang ZZ (2009) Composite colloids and patterning. Polymer 50:1609–1615
Yang M, Ma J, Niu ZW, Dong X, Xu HF, Meng ZK, Jin ZG, Lu YF, Hu ZB, Yang ZZ (2005) Synthesis of spheres with complex structures using hollow latex cages as templates. Adv Funct Mater 15:1523–1528
Arnal PM, Schüth F, Kleitz F (2006) A versatile method for the production of monodisperse spherical particles and hollow particles: templating from binary core–shell structures. Chem Comm 11:1203–1205
Wang YJ, Angelatos AS, Caruso F (2008) Template synthesis of nanostructured materials via layer-by-layer assembly. Chem Mater 20:848–858
Zhang CL, Ding SJ, Li JJ, Xu HF, Sun LL, Wei W, Li CP, Liu JG, Qu XZ, Lu YF, Yang ZZ (2008) Interpenetration network (IPN) assisted transcription of polymeric hollow spheres: a general approach towards composite hollow spheres. Polymer 49:3098–3102
Yang ZZ, Niu ZW, Lu YF, Hu ZB, Han CC (2003) Templated synthesis of inorganic hollow spheres with tunable cavity size onto core/shell gel particles. Angew Chem Int Ed 42:1943–1945
Yang M, Ma J, Zhang CL, Lu YF, Yang ZZ (2005) General synthetic route toward functional hollow spheres with double-shelled structures. Angew Chem Int Ed 44:6727–6730
Xu HF, Wei W, Zhang CL, Ding SJ, Qu XZ, Liu JG, Lu YF, Yang ZZ (2007) Low-temperature facile template synthesis of crystalline inorganic composite hollow spheres. Chem Asian J 2:828–836
Xu HF, Ding SJ, Wei W, Zhang CL, Qu XZ, Liu JG, Yang ZZ (2007) Template synthesis of tin-doped indium oxide (ITO)/polymer and the corresponding carbon composite hollow colloids. Colloid Polym Sci 285:1101–1107
Wang XC, Tang SD, Liu J, He ZQ, An LJ, Zhang CX, Hao JM, Feng W (2009) Uniform Fe3O4–PANi/PS composite spheres with conductive and magnetic properties and their hollow spheres. J Nanopart Res 11:923–929
Wang XC, Liu J, Feng XF, Guo MJ, Sun DL (2008) Fabrication of hollow Fe3O4–polyaniline spheres with sulfonated polystyrene templates. Mat Chem Phys 112:319–321
Chen L, Xu Z, Dai H, Zhang S (2010) Facile synthesis and magnetic properties of monodisperse Fe3O4/silica nanocomposite microspheres with embedded structures via a direct solution-based route. J Alloy Compd 497:221–227
Regazzoni AE, Urrutia GA, Blesa MA, Maroto AJG (1981) Some observations on the composition and morphology of synthetic magnetites obtained by different routes. J Inorg Nucl Chem 43:1489–1493
Lei ZB, Zhao MY, Dang LQ, An LZ, Lu M, Lo An-Ya YNY, Liu SB (2009) Structural evolution and electrocatalytic application of nitrogen-doped carbon shells synthesized by pyrolysis of near-monodisperse polyaniline nanospheres. J Mater Chem 19:5985–5995
Pouget JP, Józefowicz ME, Epstein AJ, Tang X, MacDiarmid AG (1991) X-ray structure of polyaniline. Macromolecules 24:779–789
Acknowledgments
The authors gratefully acknowledge the financial supports from the National Natural Science Foundation of China (grant nos. 51003020 and 51102084) and the postdoctoral initial funding of Heilongjiang Province.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Xu, H., Zhang, H., Lv, T. et al. Study on Fe3O4/polyaniline electromagnetic composite hollow spheres prepared against sulfonated polystyrene colloid template. Colloid Polym Sci 291, 1713–1720 (2013). https://doi.org/10.1007/s00396-013-2906-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-013-2906-0