Abstract
Core–shell multifunctional composite spheres consisting of Fe3O4–polyaniline (PANi) shell and polystyrene (PS) core were fabricated using core–shell-structured sulfonated PS spheres (with uniform diameter of 250 nm) as templates. PANi was doped in situ by sulfonic acid resulting the composite spheres are well conductive. Dissolved with solvent, PS cores were removed from the core–shell composite spheres and hollow Fe3O4–PANi spheres were obtained. Removing the PANi and PS components by calcinations produced hollow Fe3O4 spheres. The cavity size of the hollow spheres was uniformly approximate to 190 nm and the shell thickness was 30 nm. The cavity size and the shell thickness can be synchronously controlled by varying the sulfonation time of the PS templates. The shell thickness in size range was of 20–86 nm when the sulfonation time was changed from 1 to 4 h. These resulting spheres could be arranged in order by self-assembly of the templates. Both the Fe3O4–PANi/PS composite spheres and the hollow Fe3O4 spheres exhibit a super-paramagnetic behavior. Scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectroscopy, and X-ray powder scattering were used to characterize these as-prepared spheres.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nano-engineering of particle surfaces and hollow spheres has received considerable scientific and technological interest in recent years (Caruso 2000, 2001, 2003). The fabricated core–shell particles and hollow spheres could find many useful applications in catalysis, optics, electronics, drug delivery, and bio-sensing. Among all of the synthesis protocols, the LbL-colloidal templating approach has been demonstrated to be extremely effective in the fabrication of various uniform core–shell particles and hollow spheres (Caruso 2002). More and more interests are focused on the preparation and study of core–shell colloids mainly because these particles have the novel and enhanced properties, such as electrical, magnetic, mechanical, catalytic, and optical.
At the same time, multifunctionality of micro- or nanospheres of conducting polymers is an important subject in the field of material science. Conducting polymers have received especial attention because of their excellent electronic properties, with tunable conductivities covering the whole range from insulator to metal, mechanical properties, and the processing advantages of polymers (Hohnholz et al. 2005). Various approaches have been designed to realize the multifunctionality of micro- or nanostructured conducting polymers in order to match the requirements of applications in optics, electronics, mechanics, membranes, protective coatings, catalysis, sensors, biology, and others (e.g., Arshady et al. 1999; Nadian and Lindblom 2002; Xu et al. 2005; Smith et al. 2003; Cao et al. 2001; Porter et al. 1997; Zhang et al. 2006). Moreover, magnetic nanoparticles also have attracted an increasing interest in the fields of basic science and technological applications in electronic, optoelectronic, and spintronic devices (Fannin et al. 2002; Soeya et al. 2002; Sorenson et al. 2002). With this increased interest comes the realization that the shape of the particles takes a center stage in determining their magnetic properties. Much attention has been paid to the fabrication of polymer microspheres coated with magnetic nanoparticles, which may find application in diverse areas, ranging from photonics to fillers and pigments to microencapsulation (Caruso 2001; Tartaj et al. 2003). However, composite materials made from magnetic nanosize particles and conducting polymers are still the most interesting and challenging areas of research in recent times. The fabrication of uniform composite spheres and hollow spheres with both conductive and magnetic properties has rarely been reported so far.
Very recently, sulfonated polystyrene microspheres have been used as template to produce hollow polyaniline and hollow polypyrrole microspheres. The template particles were prepared by simply sulfonation of PS microspheres. Aniline or pyrrole was oxidatively polymerized on the surface of sulfonated PS (Yang et al. 2005). In this article, we report an extension of this chemical modification method to prepare conductively magnetic Fe3O4–PANi/PS composite core–shell spheres and their corresponding hollow spheres. The shell of the as-prepared composite magnetic sphere is Fe3O4–PANi, and the core consists of polymer PS. The super-paramagnetic properties of these nanoparticles are of great interest for biomedical applications. And the conducting polymers have good tunable conductivity and chemically active surface. Nanocomposites made from magnetic nanoparticles and conducting polymers possess the good properties of both of them. Such multifunctionality spheres may find theoretic and practical application in electronics and magnetism. Furthermore, the resulting spheres could be arranged in order by self-assembly of the templates. The hollow magnetic sphere arrays have uniform cavity size and shell thickness, which were difficultly reachable by other methods.
Experimental section
Preparation of sulfonated PS template spheres
Monodisperse polystyrene particles with uniform diameter of approximately 250 nm were prepared by seed-emulsion polymerization. The PS spheres were immersed in concentrated sulfuric acid and stirred at 45 °C for 2 h. After the products of sulfonated PS spheres were repeatedly washed with ethanol and water, they were separated by centrifugation at 8,000 r/min. To control the shell thickness of sulfonated PS spheres, the reaction time was varied from 1 to 4 h.
Preparation of PANi/PS composite spheres
After 1.0 g of sulfonated PS template spheres was dispersed in water, 0.279 g of aniline (Beijing Chem. Co., distilled under reduced pressure before using) was added with stirring for 30 min; then 0.03 mol of ammonium persulfate ((NH4)2S2O8, APS, A.R., Beijing 3rd Chemical Reagents Factory) was added, followed by an oxidative polymerization at room temperature for 24 h. The green products of PANi/PS composite spheres were purified by centrifugation and dried under vacuum at ambient temperature.
Preparation of Fe3O4–PANi/PS composite spheres
Of freeze-dried PANi/PS composite spheres, 0.5 g was immersed in 20 mL ammonium hydroxide (2 M) for 12 h. It was observed that the color of the PANi/PS spheres turned to dark blue from green. After saturating with ammonium hydroxide, the PANi/PS spheres were re-dispersed in 50 mL water; 3 mL aqueous mixture of ferrous chloride (0.5 M)/ferric chloride (1 M) (vol:vol = 1:1) were added with stirring under a nitrogen atmosphere for another 2 h. The product was kept for another 24 h and then isolated by centrifugation (6000 r/min).
Preparation of hollow spheres
Two common methods were used to prepare hollow spheres: (1) the PS cores were dissolved by solvent extraction using N,N-dimethylformamide (DMF) to produce hollow PANi/PS spheres and (2) the Fe3O4/PANi/PS composite spheres were calcinated at 450 °C for 3 h to yield hollow Fe3O4 spheres.
Characterization
Very dilute dispersion of the products in ethanol was spread on carbon-coated copper grids for transmission electron microscopy (TEM) characterization (JEOL 100CX operating at 100 kV). Scanning electron microscopy (SEM) measurements were performed with a HITACHI S-4300 instrument operated at an accelerating voltage of 15 kV. The samples were ambient dried and vacuum sputtered with Pt about average size 3 nm. The infrared spectra were recorded by a BRUKER EQUINOX 55 FT-IR using KBr disks. A Wide-angle X-ray powder scattering (Rigaku D/max-2500) was used to characterize the crystalline phase of these materials. A Perkin-Elmer TGA 7 was used to determine the inorganic content of the composite spheres. Elemental analysis was carried out using a Flash EA-1112 apparatus. A vibrating-sample magnetometer (VSM JDM-13) was used to characterize the magnetic property of the magnetite spheres. A Keithley 196 System DMM digital multimeter was used to measure the electrical conductivity of the compressed pieces of dry powders using a standard four-probe method at room temperature.
Results and discussion
As shown in Scheme 1, the core–shell sulfonated PS spheres were prepared by sulfonation of the PS spheres with concentrated sulfuric acid. The sulfonation process occurred synchronously through the whole PS spheres’ surfaces, ensuring the sulfonation thickness was uniform. The thickness of the sulfonated PS spheres is tunable by varying the reaction time and temperature. In this article, the reaction temperature was at 45 °C and the sulfonation time was 2 h, ensuring a suitable sulfonation thickness. The hydrophility of the PS spheres’ surfaces increasing as the sulfonation processing, and thus the sulfonated spheres were easily dispersed in water. As the sulfonic acid is a good dopant for aniline polymerization, aniline monomer can be easily absorbed in the sulfonated shell and in situ polymerize therein, resulting in the formation of PANi/PS composite spheres. The sulfonic acid group and the PANi (Zhang and Wan 2003) in the shell can be favorably bind metal ions by ion exchange facilitating formation of magnetite nanocrystallite, resulting in the formation of Fe3O4–PANi/PS composite spheres. After removing the PS cores by solvent extraction using DMF, composite hollow Fe3O4–PANi spheres were produced. On the condition that the Fe3O4–PANi/PS composite spheres were calcinated at high temperature, all the organic components were removed, resulting in the formation of inorganic hollow Fe3O4 spheres.
Figure 1a shows a typical SEM image of the sulfonated PS spheres. The diameters of these spheres were determined to be uniformly approximate to 250 nm from the microphotograph. The corresponding TEM image of the sulfonated PS spheres (Fig. 1b), with distinct difference from that of the mother template PS spheres (Fig. s1), reveals these spheres were core–shell structure. The thickness of the shell and the diameter of the core are approximately 30 and 190 nm, respectively. The existence of sulfonic group was confirmed by FTIR spectra. As shown in Fig. s2, the characteristic peaks of 1008, 1042, and 1183 cm−1 are attributed to the vibration of sulfonic group. Anline monomer can be preferentially absorbed in the shell because of its electric charge. Furthermore, the absorbed aniline is prior to polymerizing in the shell since the sulfonic acid is a good dopant. After the aniline monomer was absorbed and polymerized in the gel shell, PANi/PS composite capsules formed.
The chemical structure of PANi was confirmed by FTIR spectra (Fig. s2). The characteristic peaks around 1,304 and 1,585 cm−1 are attributed to the stretching modes of C–N and C=N bonds, respectively. This indicates the presence of leucoemeraldine and pernigraniline state. As shown in Fig. 2a, PANi was clearly found on the surface of the spheres. The spheres were connected to each other forming coalescent necks, and they were arranged in order by self-assembly. Elemental analysis indicated the content of PANi was 21%. The dry powder of the PANi/PS capsules posses a conductivity of 9.1 × 10−2 S cm−1 at room temperature, indicating the PANi was continuous in the gel shell.
The PANi/PS composite spheres were then used for preparation of Fe3O4–PANi/PS multifunctional spheres. The PANi/PS spheres turned blue from black green when immersed in ammonium hydroxide. This observed phenomenon indicates the PANi was de-doped by ammonium hydroxide. Along with the adding of aqueous mixture of ferrous chloride (0.5 M)/ferric chloride (1 M) (vol:vol = 1:1), the target precursor is being absorbed in the spheres’ shell and forming iron oxide therein. As shown in Fig. 2b, the resulting composite spheres have rough surface consisting of 10–20 nm nanoparticles. The conductivity of Fe3O4–PANi/PS composite spheres is 3.61 × 10−4 S cm−1, very lower than that of the PANi/PS spheres (0.091 S cm−1), which was mainly caused by the de-doping process of ammonium hydroxide and the potential blocking of conductive path by the Fe3O4 nanoparticles (Zhang and Wan 2003).
In order to resume the conductivity of the composite spheres, these spheres were immersed in sulfonic acid with low concentration (1 × 10−3 mol L−1) and treated for 10 min. Following the re-doping process of protonic acid, the conductivity of Fe3O4–PANi/PS composite capsules enhanced about 10 times. The morphology and the magnetism of the composite spheres have no distinct influence in that the treat time was short and the acid concentration was very low.
When dissolved the PS core from the composite spheres by DMF, composite hollow Fe3O4–PANi spheres formed. Mono-disperse hollow sphere could be obtained by ultrasonic dispersion. The typical TEM image of a mono-disperse hollow Fe3O4–PANi sphere (Fig. 3a) shows its shell thickness was about 30 nm; the PANi and Fe3O4 was interconnected. However, if both the PS and PANi were removed from the composite spheres by calcinations, hollow inorganic Fe3O4 spheres were prepared. Figure 3b is a corresponding typical TEM image of mono-disperse hollow Fe3O4 sphere. The thickness of the shell has no obvious change from that of Fe3O4–PANi indicated Fe3O4 was overall in the shell of the composite Fe3O4–PANi/PS spheres. Mesopores were clearly observed due to the removing of organic components. These mesopores are very important for application of such mesoporous hollow sphere materials in combining other components into magnetic composite materials.
Furthermore, it is very attractive that the shell thickness of the spheres could be tuned by control the sulfonation time of PS. For example, when the sulfonation time of PS was reduced to 1 h, the shell thickness of the hollow Fe3O4 decreased to about 20 nm (Fig. 3c). However, if prolonged the sulfonation time of PS to 4 h, the shell thickness was increased to about 82 nm, and the cavity diameter was 86 nm (Fig. 3d). As a result, the conductivity of the Fe3O4–PANi/PS composite spheres deriving from 4 h sulfonated templates increased to 8.23 × 10−4 S cm−1. The series of experiments by varying the sulfonation time demonstrate that we can obtain monodisperse core–shell gel particles with tunable gel thickness and controllable core size. This provided a way to adjust the conductivity of these spheres through varying the PANi content.
As the colloidal crystals could be formed by the self-assembly of mono-disperse particles (Bartlett et al. 2001), PANi/PS spheres could be arranged in ordered arrays (Fig. 2a). Ordered arrays of these spheres were prepared by drying the capsule dispersion in an ethanol/water mixture. After the removal of the polystyrene cores, either by dissolution or by calcination, the ordered structure was retained. Figure 4 shows the ordered arrays of the hollow Fe3O4 spheres. The ordered structure possesses promising applications in information storage and photonic crystals (Vickreva et al. 2000; Göltner 1999).
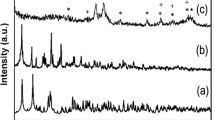
Powder X-ray diffraction patterns of the hollow Fe3O4 spheres and the hollow Fe3O4/PANi are shown in Fig. 5. The diffraction peaks of the magnetic particles synthesized in this study were measured to be 2θ = 30.19, 35.50, 43.14, 53.58, 57.08, 62.83, 74.41. These data suggested the magnetic particles are pure Fe3O4 crystalline without any impurity phases (Wang et al. 2004). Obviously, the broad peak is due to PANi and the sharp peaks are attributed to Fe3O4. No distinct peak shift or additional peak is found. That suggests there is no strong interaction between PANi and Fe3O4 in the composite hollow Fe3O4/PANi spheres.
Figure 6 shows the dependence of the magnetization on the applied magnetic fields for hollow Fe3O4 and PANi–Fe3O4 (20 wt%) spheres at room temperature. In case of hollow Fe3O4 spheres, saturated magnetization (Ms) were measured to be 46.2 emu/g. No hysteresis loop (i.e., remnant magnetization, Mr = 0 and coercive force, Hc = 0) was observed, indicating a super-paramagnetic behavior. As for hollow PANi/Fe3O4 spheres, Ms = 10, Mr = 0 and Hc = 0, respectively, indicating a super-paramagnetic behavior, too. And its super-paramagnetic behavior is similar to that of hollow Fe3O4 spheres. So, it is reasonable to believe that Fe3O4 in the Fe3O4/PANi/PS composite spheres contribute to the super-paramagnetic behavior. Furthermore, the Ms of the hollow PANi/Fe3O4 spheres is much lower than that of the Fe3O4 hollow spheres. It is because the content of the Fe3O4 in the composite spheres is wt 20%; nevertheless, there is no other component in the hollow Fe3O4 spheres. It is rational to believe that we can adjust the Ms by changing the content of Fe3O4 in the hollow PANi/Fe3O4 spheres, just as to change the conductivity of these spheres by varying the content of PANi.
Conclusions
Fe3O4–PANi/PS multifunctional composite spheres and their corresponding hollow spheres with uniform cavity size (190 nm) and shell thickness (30 nm) are prepared using core–shell-structured sulfonated PS spheres as templates. The cavity size and the shell thickness can be synchronously controlled by varying the sulfonation time of the PS templates. The shell thickness in size was of 20 nm when decreasing the sulfonation time to 1 h, and it was 86 nm when the sulfonation time was increased to 4 h. Conducting PANi was in situ doped without external additives, and thus the composite spheres show good conductivity. The composite and hollow spheres exhibit a super-paramagnetic behavior (i.e., no hysteresis loop). Ordered arrays of these spheres were successfully prepared by self-assembly. The template method is simple and effective. It can be extended to prepare other composite spheres and hollow spheres with varied composition. The multifunctionality spheres and hollow spheres may find many applications such as in electronics, controlled delivery, and microwave absorption.
References
Arshady R, Margel S, Pichot C et al (1999) Functionalization of performed microcapsules [A]. Microspheres, microcapsules and liposomes [C]. Citus Books, London
Bartlett PN, Birkin PR, Ghanem MA et al (2001) Electrochemical syntheses of highly ordered macroporous conducting polymers grown around self-assembled colloidal templates. J Mater Chem 11:849–852. doi:10.1039/b006992m
Cao H, Xu Z, Sang H et al (2001) An array of iron nanowires encapsulated in polyaniline nanotubules and its magnetic behavior. J Mater Chem 11(3):958–960. doi:10.1039/b006474m
Caruso F (2000) Hollow capsule processing through colloidal templating and self-assembly. Chem Eur J 6:413–419. doi :10.1002/(SICI)1521-3765(20000204)6:3<413::AID-CHEM413>3.0.CO;2-9
Caruso F (2001) Nanoengineering of particle surfaces. Adv Mater 13:11–22. doi :10.1002/1521-4095(200101)13:1<11::AID-ADMA11>3.0.CO;2-N
Caruso F (2002) Engineering of core-shell particles and hollow capsules. In: Rosoff M (ed) Nano-surface chemistry. Marcel Dekker, NewYork
Caruso F (2003) Hollow inorganic capsules via colloid-templated layer-by-layer electrostatic assembly. Top Curr Chem 226 & 227:145–168
Fannin PC, Charles SW, Vincent D et al (2002) Measurement of the high-frequency complex permittivity and conductivity of magnetic fluids. J Magn Magn Mater 252:80–82. doi:10.1016/S0304-8853(02)00604-2
Göltner CG (1999) Porous solids from rigid colloidal templates: morphogenesis. Angew Chem Int Ed Engl 38:3155–3156. doi :10.1002/(SICI)1521-3773(19991102)38:21<3155::AID-ANIE3155>3.0.CO;2-Q
Hohnholz D, Okuzaki H, MacDiarmid AG (2005) Plastic electronic devices through line patterning of conducting polymers. Adv Funct Mater 15:51–56. doi:10.1002/adfm.200400241
Nadian A, Lindblom L (2002) Studies on the development of a microencapsulated delivery system for norbormide, a species specific acute rodenticide. Int J Pharm 242:63–68. doi:10.1016/S0378-5173(02)00142-4
Porter TL, Eastman MP, Zhang DY et al (1997) Surface polymerization of organic monomers on Cu(II)-exchanged hectorite. J Phys Chem B 101(51):11106–11111. doi:10.1021/jp9703521
Smith JA, Josowicz M, Janata J (2003) Polyaniline-gold nanocomposite system. J Electrochem Soc 150:E384–E388. doi:10.1149/1.1589762
Soeya S, Hayakawa J, Takahashi H et al (2002) Development of half-metallic ultrathin Fe3O4 films for spin-transport devices. Appl Phys Lett 80:823–825. doi:10.1063/1.1446995
Sorenson TA, Morton SA, Waddill GD et al (2002) Epitaxial electrodeposition of Fe3O4 thin films on the low-index planes of gold. J Am Chem Soc 124:7604–7609. doi:10.1021/ja0201101
Tartaj P, Morales MD, Veintemillas-Verdaguer S et al (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D 36:R182–R197. doi:10.1088/0022-3727/36/13/202
Vickreva O, Kalinina O, Kumacheva E (2000) Colloid crystal growth under oscillatory shear. Adv Mater 12:110–112. doi :10.1002/(SICI)1521-4095(200001)12:2<110::AID-ADMA110>3.0.CO;2-X
Wang J, Chen QW, Zeng C et al (2004) Magnetic-field-induced growth of single-crystalline Fe3O4 nanowires. Adv Mater 16:137–140. doi:10.1002/adma.200306136
Xu J, Li X, Liu J et al (2005) Solution route to inorganic nanobelt-conducting organic polymer core-shell nanocomposites. J Polym Sci Part Polym Chem 43:2892–2900. doi:10.1002/pola.20769
Yang Y, Chu Y, Yang FY et al (2005) Uniform hollow conductive polymer microspheres synthesized with the sulfonated polystyrene template. Mater Chem Phys 92(1):164–171. doi:10.1016/j.matchemphys.2005.01.007
Zhang ZM, Wan MX (2003) Nanostructures of polyaniline composites containing nano-magnet. Synth Met 132:205–212. doi:10.1016/S0379-6779(02)00447-2
Zhang L, Wan M, Wei Y (2006) Nanoscaled polyaniline fibers prepared by ferric chloride as an oxidant. Macromol Rapid Commun 27:366–371. doi:10.1002/marc.200500760
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 20771081), the Science Developing Foundation of Tianjin Education Committee (No. 20060514), Tianjin Municipal Science and Technology Commission (No. 06YFJMJC14900), and Tianjin University of Science and Technology (No. 20050416 and No. 20070415).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, X., Tang, S., Liu, J. et al. Uniform Fe3O4–PANi/PS composite spheres with conductive and magnetic properties and their hollow spheres. J Nanopart Res 11, 923–929 (2009). https://doi.org/10.1007/s11051-008-9486-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11051-008-9486-9