Abstract
In this work, using monodispersed sulfonated polystyrene (SPS) microspheres as carriers, FeCl3·6H2O and FeSO4·7H2O as precursors, NaOH as precipitant in the presence of graphene oxide (GO), SPS/Fe3O4/GO micro-nano composites were fabricated by a simple one-pot method employing an inverse coprecipitation in-situ compound technology. The SPS/Fe3O4/GO micro-nano composites were characterized by scanning electron microscopy, transmission electron microscopy, X-ray powder diffractometer, Fourier transform infrared spectroscopy, nitrogen adsorption/desorption isotherms and vibrating sample magnetometer. The results show that the SPS/Fe3O4/GO micro-nano composites were fabricated with SPS as core, GO and Fe3O4 nanoparticles as shell. The SPS/Fe3O4/GO micro-nano composites had larger BET specific surface area, average pore width and micropore volume than the pure SPS microspheres. Meanwhile, the SPS/Fe3O4/GO micro-nano composites had superparamagnetism and hydrophilic property. The saturation magnetization (Ms) of the SPS/Fe3O4/GO micro-nano composites was 10.86 emu/g, which was enough to ensure the convenient magnetic separation of solid and liquid phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fe3O4 nanoparticles have been widely used in many areas, such as electronic equipment [1], water treatment [2, 3], catalysis [4], bio-medicine [5], etc., owing to biggish specific surface area, excellent magnetic response, superparamagnetism, special affinity to heavy metal ion, environmentally friendly and lower production costs. However, Fe3O4 nanoparticles are easily agglomerated to big particles because of their surface effect, which reduces their effective specific surface area. Meanwhile, the application economic cost of Fe3O4 nanoparticles rises due to their small size and Brownian movement, hardly been separated from aqueous solution in low magnetic field [6, 7]. One of effective ways to solve the problem is to load Fe3O4 on a carrier which has certain strength and strong binding force with the nanoparticles.
Monodisperse polystyrene (PS) microspheres usually are used as carriers of some materials because they have large specific surface area, strong adsorption, excellent cohesion, high surface reactivity and good affinity to substances such as ligand [8], protein [9] and dye. At the same time, the particle sizes of PS microspheres have good controllability and have excellent efficiency in chromatographic adsorption. Moreover, PS microspheres have excellent hydrophobic and are not easy to dissolve or swelling in general solvent, which is conducive to application and recovery.
Recently, Yu et al. prepared PS/Fe3O4 composites with unsinkability, highly hydrophobic and super-oleophilic properties by emulsion polymerization. The absorption capacity of the composites for diesel oil up to 2.492 times of their own weigh [10]. Fang et al. synthesized PS/Fe3O4 magnetic beads with core–shell structure through reverse coprecipitation method. Comparing with pure Fe3O4, the density of PS/Fe3O4 reduced and the sedimentation stability had been improved [11]. However, PS/Fe3O4 composites prepared above are easily oxidized due to exposure of Fe3O4 in the air, which reduces the stability of the composites.
Graphene is one-atom thick, two-dimensional (2-D) sheet of a honeycomb structure [12] with many excellent properties, which has sparked tremendous scientific interest in many applications [13, 14]. As a kind of important derivative of graphene, Graphene oxide (GO) has ultra-large surface area and excellent water dispersibility. GO can be obtained from the low cost material natural flake graphite (NFG) by modified Hummers method. Futhermore, GO possesses abundant oxygen-containing functional groups such as epoxide groups, –COOH and –OH on the basal planes and at the edges. These functional groups can be used as active sites to immobilization of a large number of substances such as protein [15], dye [16], heavy metal ions [17], small molecules [18], and preparation of nanocomposites materials.
Introducing GO into PS/Fe3O4 fabricated PS/Fe3O4/GO tri-component micro-nano composites have better stability due to GO reducing the oxidation of Fe3O4 in the air as well as improving the dispersibility of Fe3O4.
Kassaee et al. synthesized a new kind of magnetic composite of GO and PS (NanoFe3O4/GO/PS) using one-pot co-precipitation and in situ emulsion polymerization. The product with Fe3O4 Nps spreading evenly on the GO nanosheets had anticipated thermal stabilities. Meanwhile, the properties of PS were improved for the load of magnetite-GO hybrid [19]. Wang et al. prepared PS/Fe3O4/GO nanoparticles with core–shell structure and better magnetic responsiveness to external magnetic field compared to previously prepared GO/Fe3O4 by successively depositing GO nanosheets and Fe3O4 nanoparticles onto PS functionalized by carboxyl through electrostatic interactions used for drug-delivery system [20]. Liu et al. fabricated Fe3O4/GO/PS tri-component composites with well dispersibility and better mechanical properties through one-pot in situ radical bulk polymerization method. The preparation could avoid the aggregation of the Fe3O4 nanoparticles by modifying the Fe3O4 with GO and then coating the Fe3O4/GO on the outer surface of PS [21].
In this work, we prepared hydrophilic, superparamagnetic and core–shell structure SPS/Fe3O4/GO micro-nano composites by a simple one-pot method employing an inverse coprecipitation in-situ compound technology. The composites have large surface area and contain more Fe3O4 nanoparticles, reducing the agglomeration and oxidization of Fe3O4, which could be used as carrier of protein, water treatment adsorbent and bio-medicine.
2 Experimental section
2.1 Materials
Natural flake graphite (NFG) 50 BS mesh, with the purity of 99 wt% was purchased from ShanDong Qingdao Tianhe Graphite Company (China). Polyvinylpyrrolidone (PVP), ferric chloride hexahydrate, ferrous sulfate heptahydrate, potassium permanganate, concentrated sulfuric acid, hydrochloric acid and sodium nitrate obtained from Tianjin Guangfu Fine Chemical Research Institute (China) are of analytical grade and were used without further purification. Styrene (St) was washed with 5% NaOH solution and deionized water until neutral, then dried by calcium chloride and was purified by distillation under reduced pressure. Benzoyl peroxide (BPO) was recrystallized in chloroform.
2.2 Preparation of graphene oxide (GO)
GO was prepared by a modified Hummers method. Flake graphite (2 g) was dissolved in concentrated H2SO4 with stirring in ice-water bath for 30 min. Then 1 g NaNO3 and 7 g KMnO4 was slowly added into the above mixture. After 48 h, the ice-water bath was removed and the mixture was heated to 35 °C and lasted for 2 h. 92 mL deionized water was added into the flask, the temperature rapidly increased to 90 °C and lasted for 15 min, then the color turned into brown. 150 mL deionized water was added into the flask to stop the reaction. 10 mL 30% H2O2 was successively added into the mixture and the color turned into bright yellow. Afterwards, the mixture added into 5 mL HCl was centrifuged and washed with deionized water several times until pH of the solution at 7.0. Finally, the products were obtained in the vacuum freeze-drying at minus 40 °C.
2.3 Sythesis of polystyrene (PS) microspheres
3.6 g PVP was dispersed in 100 mL of ethanol, which was added into a 250 mL four-neck bottle and heated to 70 °C. 30 g St dissolved 0.9 g BPO was dropwise added into the four-neck bottle under the bubbling of nitrogen gas permanent reaction for 24 h. The mixture were centrifuged and washed with ethanol and deionized water until decants clear. The products were dried in vacuum at 60 °C for 24 h.
2.4 Synthesis of sulfonated polystyrene (SPS) microspheres
2 g PS was dispersed into 40 mL concentrated H2SO4, and then the mixture was sonicated for 8 min. Subsequently, the suspension was heated to 40 °C for 6 h. The mixture were centrifuged and washed with deionized water until pH of the solution at 7.0. Lastly, the products were dried in vacuum at 60 °C for 24 h.
2.5 Preparation of SPS/Fe3O4/GO micro-nano composites
SPS/Fe3O4/GO nanocomposites were prepared by an inverse coprecipitation in-situ compound technology. 2 g SPS microspheres and 40 mL 0.2 M NaOH aqueous solution were added into a 250 mL four-neck bottle under N2 atmosphere at 80 °C for 30 min. And then, 0.0164 g GO, 1.08 g FeCl3·6H2O and 0.56 g FeSO4·7H2O were dispersed into 60 mL 1:1 (V/V) ethanol–water mixed solvents by an ultrasonic disperse method. The mixture was then dropwise added into the above four-neck bottle under vigorous mechanical stirring for 30 min at 80 °C. The color of the suspension turned into black immediately. The stirring was continued for 1 h at 50 °C when the dropping was finished. N2 was bubbled throughout the reaction. After being cooled to room temperature, the nanocomposites were separated by magnetic separation, washed with deionized water until the solution at neutral and dried in vacuum at 60 °C for 24 h.
2.6 Characterization
Fourier transform infrared (FTIR) spectra were obtained in transmission mode on a FTIR spectrometer (American Nicolet Corp. Model 170-SX) using the KBr pellet technique. Powder X-ray diffraction (XRD, Rigaku D/MAX-2400 X-ray diffractometer with Ni-filtered Cu Kα radiation (1.54056 Å)) was used to investigate the crystal structure of the nanoparticles. The morphology of the samples was characterized using a FEI Quanta 450 scanning electron microscope (SEM) at accelerating voltages of 10 kV. Transmission electron microscopy (TEM) images were obtained with TEM (FEI TECNAI G2 F30) to elucidate the dimensions and the structural details of the nanoparticles. TEM specimens were made by placing a drop of the nanoparticle suspension on a carbon-coated copper grid. The N2 adsorption-desorption isotherm was measured at liquid nitrogen temperature (77 K) using a Micromeritics ASAP 2010 analyzer. The specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. The pore size distribution was obtained from the Barret–Joner–Halenda (BJH) method. Magnetization measurements were performed on a Vibrating sample magnetometer (VSM, LAKESHORE-7304, USA) at room temperature.
3 Results and discussion
3.1 Fabrication of SPS/Fe3O4/GO micro-nano composites
Figure 1 is the scheme of the preparation of SPS/Fe3O4/GO micro-nano composites. GO was synthesized by modified Hummers method using NFG as raw material added concentrated H2SO4, KMnO4 and H2O2. PS homopolymer microspheres were prepared in ethanol system by dispersion polymerization method. Then, the monodisperse PS homopolymer microspheres were sulfonated by concentrated sulfuric acid for introducing hydrophilic sulfo group (SO3H) on the surface. In the presence of GO and SPS, SPS/Fe3O4/GO were synthesized based on the precursor of ferric chloride (FeCl3·6H2O) and ferrous sulfate (FeSO4·7H2O), the precipitator of sodium hydroxide (NaOH) by an inverse coprecipitation in-situ compound technology. The SPS/Fe3O4/GO micro-nano composites were fabricated with SPS as core, GO and Fe3O4 nanoparticles as shell.
3.2 Morphological and structural studies of SPS/Fe3O4/GO micro-nano composites
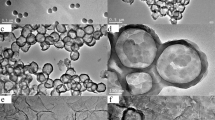
SEM and TEM were used to analyse the morphology and structure of the as-prepared composites. The SEM image shows that SPS are spherical, smooth and uniform size microspheres (Fig. 2a). In contrast to the surface of SPS, the SPS/Fe3O4/GO composites present rough surface and there are many nanoparticles which are loaded on the surface of SPS (Fig. 2b). From the partial magnification SEM image of circled pattern in Fig. 2b, GO film and Fe3O4 nanoparticles can be distinctly seen on the surface of sample (Fig. 2c). Particularly worth mentioning is that Fe3O4 nanoparticles can be clearly observed under gossamer GO, which indicated that the SPS/Fe3O4/GO micro-nano composites were fabricated with SPS as core, GO and Fe3O4 nanoparticles as shell. In our system, there are a number of hydrophilic sulfo groups on the surface of SPS microspheres and a many of hydrophilic oxygen-containing functional groups such as –COOH and –OH on the basal planes and at the edges of GO, iron(III) cations in the solution would like to attach to these hydrophilic functional groups particular positions of the SPS microspheres and GO and then in situ reduced into very fine magnetite particles during following an inverse coprecipitation method. GO and SPS provide a large contact surface for these tiny particles. The tiny nanoparticles will then serve as the nuclei for the growth of magnetite nanoparticles Fe3O4 [22].
These results are in accordance with the TEM image of SPS/Fe3O4/GO composites observations. In Fig. 2d, it can be seen that nanoparticles were loaded on the surface of spherical product. The TEM image of the edge of SPS/Fe3O4/GO composites further reveals that the obtained sample is fabricated by micron-sized spherical SPS, flexible and ultrathin GO film and Fe3O4 nanoparticles (Fig. 2e). The SPS/Fe3O4/GO composites have core–shell structure, the core is SPS microsphere which is encapsulated by ultrathin, flexible and pleated structure GO, and Fe3O4 nanoparticles. This complements well with the SAED pattern of SPS/Fe3O4/GO composites sample (Fig. 2f), which shows that there are diffraction ring of cubic Fe3O4 nanoparticles, diffraction spots of GO and halo ring of amorphous SPS microsphere, indicating that SPS microspheres are successfully covered by GO and Fe3O4 nanoparticles.
Figure 3 presents the XRD patterns of the SPS/Fe3O4/GO micro-nano composites. The diffraction peaks at 19.22°, 30.11°, 35.22°, 42.95°, 53.32°, 56.91°, 62.71° and 74.08° correspond to the diffraction planes of the (111), (220), (311), (400), (422), (511), (440) and (533) respectively [23], which match well with those of magnetite Fe3O4 according to JCPDS card (75-1610) [24]. Meanwhile, there is a weak diffraction peak appearing at 12.4°, corresponding to the diffraction plane of (001) of GO. The weak intensity diffraction peak is attribute to the low content of GO in the composites.
The FTIR spectra of SPS/Fe3O4/GO micro-nano composites are presented in Fig. 4. The wide and strong absorption band at 3434 cm−1 is attributed to the stretching vibration of O–H. The band at 1376 cm−1 corresponds to the bending vibration of O–H. The peak at 561 cm−1 is ascribed to stretching vibration of Fe-O bond in SPS/Fe3O4/GO, suggesting the existence of Fe3O4. The absorbance peak at 3028 and 907 cm−1 correspond to the stretching vibration and bending vibration of C–H of benzene ring, respectively. The peaks appearing at 1604, 1498 and 1454 cm−1 are all attributed to the benzene skeleton vibration. In addition, there are two absorbance peaks at 1028 and 1180 cm−1 which could be assigned to the symmetrical and asymmetrical adsorption of sulfonate, respectively. For all the characteristic peaks showed above, further indicating that the SPS/Fe3O4/GO micro-nano composites were successfully fabricated.
As shown in Fig. 5a, the N2 adsorption/desorption isotherms of SPS/Fe3O4/GO micro-nano composites belong to type III, demonstrating the presence of mesopores. The hysteresis loops appeared nearly at P/P0 = 0.7, indicating that the pore size was relative large. This was confirmed by the pore size distribution curve (Fig. 5b). The BET surface area, pore diameter and micropore volume of SPS/Fe3O4/GO micro-nano composites were 15.02 m2/g, 15.58 nm, 0.058 cm3/g, respectively, which were all much larger than those of pure SPS microspheres (1.27 m2/g, 0.077 nm, 0.024 × 10− 3 cm3/g) in Table 1. The results suggest that the SPS/Fe3O4/GO micro-nano composites have potential application values in immobilization of enzyme, separation of cells and protein, wastewater treatment and other fields.
3.3 Magnetic properties of SPS/Fe3O4/GO micro-nano composites
The magnetic property of the SPS/Fe3O4/GO micro-nano composites was measured at room temperature. As shown in Fig. 6, the SPS/Fe3O4/GO micro-nano composites have no remanence, indicating that the composites have superparamagnetism. The maximum saturation magnetization was 10.86 emµ/g, which was much higher than the value reported by M.Z. Kassaee (2.2 emµ/g) [19] and Jinfeng Wang (7.5 emµ/g) [20], respectively. This result revealed that the SPS/Fe3O4/GO micro-nano composites fabricated by in-situ compound technology have a higher content of Fe3O4 nanoparticles. Because hydrophilic sulfo groups on the surface of SPS microspheres and hydrophilic oxygen-containing functional groups on the basal planes and at the edges of GO provided a large number of nucleation sites for the formation of Fe3O4 nanoparticles, which improved the dispersibility of the GO nanosheets and avoided the aggregation of the Fe3O4 nanoparticles on the SPS and GO. The insets in Fig. 5 show that the composites could be homogeneously dispersed into ethanol to form a stable suspension (Fig. 5a) and could be separated from the suspension in external magnetic field (Fig. 5b), indicating that the SPS/Fe3O4/GO micro-nano composites with hydrophilic could meet the requirement of magnetic separation.
4 Conlusions
-
1.
The SPS/Fe3O4/GO micro-nano composites were successfully fabricated by an inverse coprecipitation in-situ compound technology. The composites were core–shell structure, with SPS polymer microspheres as core, GO and Fe3O4 nanoparticles as shell.
-
2.
The BET surface area, pore diameter and micropore volume of SPS/Fe3O4/GO micro-nano composites are all higher than those of pure SPS microspheres. The SPS/Fe3O4/GO micro-nano composites have hydrophilic property, superparamagnetism and a high content of Fe3O4 nanoparticles.
-
3.
The prepared SPS/Fe3O4/GO micro-nano composites have potential application values in immobilization of enzyme, separation of cells and protein, wastewater treatment and other fields.
References
Y.G. Zhang, Y. Li, H.P. Li, Y. Zhao, F.X. Yin, Z. Bakenov, Electrochemical performance of carbon-encapsulated Fe3O4 nanoparticles in lithium-ion batteries: morphology and particle size effects. Electrochim. Acta 216, 475–483 (2016)
F. Pinakidou, M. Katsikini, K. Simeonidis, E. Kaprara, E.C. Paloura, M. Mitrakas, On the passivation mechanism of Fe3O4 nanoparticles during Cr(VI) removal from water: A XAFS study. Appl. Surf. Sci 360, 1080–1086 (2016)
E. Ghasemi, A. Heydari, M. Sillanpää, Superparamagnetic Fe3O4@EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd(II) from water and soil environmental samples. Microchem. J. 131, 51–56 (2017)
L. Tan, S.Y. Lu, Z.Q. Fang, W. Cheng, E.P. Tsang, Enhanced reductive debromination and subsequent oxidative ring-opening of decabromodiphenyl ether by integrated catalyst of nZVI supported on magnetic Fe3O4 nanoparticles. Appl. Catal. B 200, 200–210 (2017)
A.H. Rezayan, M. Mousavi, S. Kheirjou, G. Amoabediny, M.S. Ardestani, J. Mohammadnejad, Monodisperse magnetite (Fe3O4) nanoparticles modified with water soluble polymers for the diagnosis of breast cancer by MRI method. J. Magn. Mater 420, 210–217 (2016)
N.J. Tang, W. Zhong, Y. Jiang, X.L. Wu, W. Liu, Y.W. Du, Nanostructured magnetite (Fe3O4) thin films prepared by sol–gel method. J. Magn. Mater 282, 92–95 (2004)
W. Jiang, X.B. Chen, Y.J. Niu, B.C. Pan, Spherical polystyrene-supported nano-Fe3O4 of high capacity and low-field separation for arsenate removal from water. J. Hazard. Mater 243, 319–325 (2012)
P.K.K.S. Heer, K.M. Khot, V.G. Gaikar, Development of polystyrene adsorbents functionalized with heterocyclic ligands for selective adsorption of CO2 from CH4 and N2. Sep. Purif. Technol. 158, 212–222 (2016)
M. Holmberg, T.S. Hansen, J.U. Lind, G.M. Hjortø, Increased adsorption of histidine-tagged proteins onto tissue culture polystyrene. Colloid Surf. B 92, 286–292 (2012)
L.H. Yu, G.Z. Hao, J.J. Gu, S. Zhou, N. Zhang, W. Jiang, Fe3O4/PS magnetic nanoparticles: synthesis, characterization and their application as sorbents of oil from wastewater. J. Magn. Mater 394, 14–21 (2015)
F.F. Fang, J.H. Kim, H.J. Choi, Synthesis of core–shell structured PS/Fe3O4 microbeads and their magnetorheology. Polymer 50, 2290–2293 (2009)
H. Kim, A.A. Abdala, C.W. Macosko, Graphene/Polymer Nanocomposites. Macromolecules 43, 6515–6530 (2010)
P. Avouris, Z.H. Chen, V. Perebeinos, Carbon-based electronics. Nat. Nanotechmol. 2, 605–615 (2007)
A.A. Balandin, S. Ghosh, W.Z. Bao, I. Calizo, D. Teweldebrhan, F. Miao, C.N. Lau, Superior thermal conductivity of single-layer graphene. Nano Lett. 8, 902–907 (2008)
M.A. Tabrizi, M. Shamsipur, R. Saber, S. Sarkar, Simultaneous determination of CYC and VEGF165 tumor markers based on immobilization of flavin adenine dinucleotide and thionine as probes on reduced graphene oxide-poly(amidoamine)/gold nanocomposite modified dual working screen-printed electrode. Sens. Actuator B 240, 1174–1181 (2017)
X.J. Bian, X.F. Lu, Y.P. Xue, C.C. Zhang, L.R. Kong, C. Wang, A facile one-pot hydrothermal method to produce SnS2/reduced graphene oxide with flake-on-sheet structures and their application in the removal of dyes from aqueous solution. J. Colloid Interf. Sci. 406, 37–43 (2013)
R. Sahraei, M. Ghaemy, Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removel and preparation of silver nanocomposite for antibacterial activity. Carbohydr. Polym. 157, 823–833 (2017)
H.B. Huang, Y.Y. Mao, Y.L. Ying, Y. Liu, L.W. Sun, X.S. Peng, Salt concentration, pH and pressure controlled separation of small molecules through lamellar graphene oxide membranes. Chem. Commun. 49, 5963–5965 (2013)
M.Z. Kassaee, E. Motamedi, M. Majdi, Magnetic Fe3O4-graphene oxide/polystyrene: Fabrication and characterization of a promising nanocomposite. Chem. Eng. J. 172, 540–549 (2011)
J.F. Wang, B. Tang, T. Tsuzuki, Q.T. Liu, X.L. Hou, L. Sun, Synthesis, characterization and adsorption properties of superparamagnetic polystyrene/Fe3O4/graphene oxide. Chem. Eng. J. 204–206, 258–263 (2012)
P. Liu, W. Zhong, X.L. Wu, J.H. Qiu, Facile synergetic dispersion approach for magnetic Fe3O4@graphene oxide/polystyrene tri-component nanocomposite via radical bulk polymerization. Chem. Eng. J. 219, 10–18 (2013)
Y.X. Ma, Y.F. Li, G.H. Zhao, L.Q. Yang, J.Z. Wang, X. Shan, X. Yan, Preparation and characterization of graphite nanosheets decorated with Fe3O4 nanoparticles used in the immobilization of glucoamylase. Carbon 50, 2973–2986 (2012)
X.Y. Guo, B. Du, Q. Wei, J. Yang, L.H. Hu, L.G. Yan, W.Y. Xu, Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI). Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 278, 211–220 (2014)
I.F. Nata, G.W. Salim, C.K. Lee, Facile preparation of magnetic carbonaceous nanoparticles for Pb2+ ions removal. J. Hazard. Mater. 183, 853–858 (2010)
Acknowledgements
The authors gratefully acknowledge financial supports from the National Natural Science Foundation of China (Grant Nos. 51403091 and 51663013) and the Postdoctoral Science Foundation of China (Grant No. 2015M572616).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Y., Jin, P., Lei, W. et al. One-pot method fabrication of superparamagnetic sulfonated polystyrene/Fe3O4/graphene oxide micro-nano composites. J Porous Mater 25, 1447–1453 (2018). https://doi.org/10.1007/s10934-018-0557-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0557-8