Abstract
Hollow spherical mesoporous silica was synthesized by using sodium silicate as a precursor and a low concentration of cetyltrimethylammonium bromide (CTAB) (0.154 mol dm−3). The resulting hollow spherical particles were characterized with scanning electron microcopy (SEM), small-angle X-ray diffraction (SXRD), transmission electron microscopy (TEM), and N2 gas adsorption and desorption techniques. The results showed that regular spherical mesoporous silica could be obtained only if the molar ratio of propanol to CTAB was in the range of approximately 8:1–9:1. The spherical particles were hollow (inside), and the shell consisted of smaller particles with a pore structure of hexagonal symmetry. With an increase of the molar ratio of propanol to CTAB, the distance (a value) between centers of two adjacent pores increased, and the pore structure of mesoporous silica became less ordered. N2 adsorption–desorption curves revealed type IV isotherms and H1 hysteresis loops; with an increase of the molar ratio of propanol to CTAB, the pore size with Barrett–Joyner–Halenda (BJH) diameter of the most probable distribution decreased, but the half peak width of the pore size distribution peak increased
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hollow spheres have potential applications as delivery vehicles, for example, they can be used for the controlled release of substances such as drugs, cosmetics, dyes, and ink; for the protection of biologically active macromolecules; and for the removal of waste materials [1]. Several chemical and physicochemical methods have been used to synthesize hollow spheres, including emulsion-phase separation techniques, emulsion interfacial polymerization, and templating colloidal particle techniques [2, 3, 4, 5].
Particle shape control is a complex problem, since it relates to solid-state chemistry, interfacial reaction kinetics, and solution chemistry. Template techniques are effective methods to synthesize hollow spheres, such as colloidal polystyrene (PS) beads through layer-by-layer techniques [5], and vesicular solutions form surfactants [6]. Recently, hollow spheres with special shell structures are attracting great attention. Zhu et al.[7] reported that hollow spheres of microporous silica were obtained by using polystyrene beads as templates through a layer-by-layer technique. Wu et al. [8] obtained hollow spheres of mesostructure lead titanate based on oil-in-water emulsion. Lin et al. [9] reported a liquid crystalline/sol-gel approach to synthesize vesicular mesoporous aluminosilicates MCM-41. To the best of my knowledge, few reports on hollow spheres of silica with MCM-41 mesoporous structure have been published. In an earlier report [10], we synthesized fiber-like and spherical mesoporous silica by using worm-like micelles of cetyltrimethylammonium bromide (CTAB), HNO3, and pentanol as templating agents. In this paper, hollow spherical mesoporous silica was synthesized from a sodium silicate precursor by controlling the pH value of the mixed micellar solution of CTAB and propanol during hydrolysis of ethyl acetate. Compared with the earlier methods, this method is simple and convenient, and the concentration of CTAB is low (0.154 mol dm−3). It is predicted that hollow spherical mesoporous silica will be very useful in the field of adsorption, separation, and catalysis owing to its ordered pore structure and higher surface area.
Experimental
Reagents
Crystalline sodium silicate was obtained from Shanghai Fourth Reagent Plant. It contained 21.05% (w/w) Na2O, and the molar ratio of Na2O to SiO2 was 1.03. Solid CTAB, ethyl acetate, and propanol were analytical-grade reagents obtained from the Institute of Chemical Technology of Jining, Zhangzhou Second Reagent Plant, and Shanghai Chemical Regent Co., respectively.
Characterization
Powder X-ray diffraction data were obtained on a D/max-rB model with a Cu target (λ=0.154 nm) at 40 kV and 120 mA, with a speed of 0.2 deg min−1 and step of 0.01 deg.
Dried samples for scanning electron microscopy (SEM) were placed on glass-slice surfaces and then sputter-coated with gold. The SEM images were obtained on a H-7000 transmission electron microscope with a H-7110 SEM accessory. To gain an ultra-thin TEM picture, mesoporous silica was embedded in Spurr resin, heated at 80°C for 1 day to form a block, and cut with an M-6000 ultra-thin slicer from DuPont Co.
Nitrogen adsorption–desorption measurements were carried out on an Omnisorp 100 CX gas adsorption analyzer from Coulter Co. after samples had been degassed at 350°C for 12 h until a pressure of 10−5 Pa or below was reached. Calculations of surface area and total pore volume were supplied directly by commercial software accompanying the system according to the IUPAC recommendations [11].
Synthesis
Mesoporous silica was prepared by using aggregates of CTAB as template and propanol as cosurfactant. A general procedure was as follows: a mixture of CTAB (10.76 mmol) and propanol (the molar ratio of propanol to CTAB was varied) was dissolved in water, Na2SiO3·9 H2O (4 g) was then added (the total volume of the mixed solution was kept constant at 140 mL). After sonication, a clear solution was obtained. Ethyl acetate (10 mL) was added to the solution, which was then sonicated for 45 s. After hydrolysis for 5 h at 30°C, the mixture was kept at 80°C for 72 h in a heating oven. The resulting solid was recovered by filtration, then washed with distilled water and ethanol, and finally dried at room temperature. The mixed surfactants were removed by calcinations at 540°C for 6 h.
Results and discussion
Morphologies of mesoporous silica
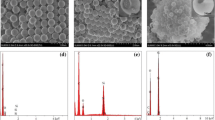
When the molar ratio of propanol to CTAB was below 8:1, a mixture of spherical and rod-like silica particles was obtained; moreover, the rod-like silica particles were crimped and oblate (Fig. 1). When the molar ratio of propanol to CTAB was above 9:1, irregular spherical particles with rough surfaces were obtained. Only when the molar ratio of propanol to CTAB was in the range of 8:1–9:1 could regular spherical particles be obtained (Fig. 2); the percentage of spherical silica was above 95% with a mean diameter of approximately 3 μm. Also it could be seen that some smaller particles adhered on the surface of particles, making the surface of particles become coarse. Figure 3a is a SEM photograph of a broken sample. As we can see, spherical particles were hollow inside, and the wall was about 200 nm thick. An ultra-thin section TEM picture (Fig. 3b) indicated that the center of the sphere was white, but the sphere shell was dark, that is, the spheres were hollow.
Structure of mesoporous silica
Small-angle X-ray diffraction patterns are shown in Fig. 4. There were three diffraction peaks, indexed as (100), (110), and (200), indicating a pore structure of hexagonal symmetry in hollow spherical particles (i.e., an MCM-41 mesoporous structure). The diffraction intensity of the (100) peak decreased with increasing molar ratio of propanol to CTAB (Table 1). When the molar ratio reached 9:1 (propanol to CTAB), the two diffraction peaks, (110) and (200), disappeared (Fig. 4). These results indicated that orderliness in hollow spheres became poorer on increasing the molar ratio. The center-to-center pore distance (a) of two adjacent pores was calculated according to the relationship a=2/31/2 d 100 (Table 1). The a value decreased with increasing the molar ratio of propanol to CTAB, that is, an excess of propanol made the pore size decrease.
N2 adsorption–desorption of mesoporous silica
N2 adsorption–desorption curves are given in Fig. 5 and show type IV isotherms and H1 hysteresis loops [11]. The smaller and larger step-like curves in the approximate ranges of 0.2–0.3 and 0.7–1P/P 0 may be caused by capillary condensation in the pore channel of mesoporous structure and the voids among particles of resulting samples [12]. Figure 6 is a pore size distribution curve of the resulting sample: the two peaks located at 1.83 nm and 45.0 nm, corresponded to the pore size of mesoporous structure and the voids among particles of resulting samples, respectively; the peak distribution of the former was narrow, whereas the latter was very broad. Brunauer–Emmet–Teller (BET) surface area, pore size with Barrett–Joyner–Halenda (BJH) diameter of the most probable distribution, and total pore volume are listed in Table 2. As we can see, an increase of the molar ratio of propanol to CTAB leads to a decrease in the pore size with BJH diameter of the most probable distribution, but an increase in the half peak width of the pore size distribution peak. These results indicated that excess propanol destroyed the ordering of the mesoporous structure, and this conclusion was in agreement with that of SXRD.
Reaction mechanism
The critical micelle concentration (CMC) of CTAB was approximately 0.9–1.0 mmol dm−3 (ca. 0.03%). Above the CMC, the size and shapes of micelles changed accordingly with the increase of CTAB concentration. When CTAB concentration was approximately 0.12–0.15 mol dm−3 [13], the shapes of the micelle changed from spheres to rods. If electrolytes, such as NaBr, NaNO3, and sodium salicylates, were added to solution, a long, flexible, worm-like micelle was formed. In our system the CTAB concentration was 5.3%(w/w) (0.154 mol dm−3). Under these concentrations the CTAB molecules could easily form rod-like micelles owing to the electrostatic shielding effect of sodium silicate as electrolyte and precursor. When an excess of propanol was added, however, the flexible, worm-like micelles were destroyed and transformed to spherical micelles [14].
In order to control the pH value of the solution, ethyl acetate was added. Ethyl acetate is insoluble in water; however, it was only solubilized in the palisade layer of the CTAB micelles. The cationic micelles catalyzed the following hydrolysis reaction of ethyl acetate:
The resulting acetic acid could be neutralized by sodium silicate on the surface of the micelles of CTAB. Therefore, the pH value of the solution decreased gradually from basic to neutral conditions, and finally resulting in the formation of silica. The change of pH values with time in the synthetic process of mesoporous silica is shown in Fig. 7. In the initial stage, the pH values fell rapidly (from 12.60 to 10.97 in 1.5 h), and then decreased gradually (from 10.97 to 9 in 23 h). A slow change in pH values was helpful for the formation of particles with regular shapes.
Propanol was very important in controlling morphologies and pore structures of the mesoporous silica. First, propanol molecules were mostly soluble in water: its partition constant between the micellar phase and water in the solution of CTAB and NaBr was 50 [15], which was smaller than that of butanol (190), pentanol (1,050), or hexanol (2,190) at the same concentration of alcohols. Thus, addition of propanol caused the polarity of the water solution to decrease (the dielectric constants of H2O and propanol at 25°C were 78.5 and 20.1 [16], respectively). Second, propanol had some surface activity in the water solution [17], and it could be adsorbed onto the interface of silicate particles. In our system both propanol and CTAB could be competitively adsorbed onto the surface of colloidal seeds. With an increase of the molar ratio of propanol to CTAB, more propanol was adsorbed. The hydrophobic interactions between the molecules of adsorbed propanol and CTAB on the surface of colloidal seeds weakened with increasing molar ratio of propanol to CTAB. The structure of mesoporous silica was mainly dependent on the hydrophobic interactions of surfactants, which were self-assembled into mesoporous silica; thus, the pore structure of the resulting mesoporous silica became less ordered (see Fig. 4 and Table 1).
The shapes of mesoporous silica were determined by intermolecular forces [18]: intermolecular forces between the critical size nuclei and their subsequent growth, such as van der Waals attractions and electrical double layer repulsions. The electrical double layer repulsion between particles depends on the dielectric and ionic strengths of the solution and the electric charge density of particles according to Derjaguin–Landau–Verwey–Overbeek (DLVO) theory. The decrease of the electrical double layer repulsion favored growth of gyroids and spheres particles. In our system, the dielectric constant of the mixed solution of propanol and water was adjusted by the amount of propanol, and it decreased with increasing propanol, which made the electrical double layer repulsion decrease; therefore, the total interaction forces, including van der Waals attraction and electrical double layer repulsion, decreased also. The result that the morphologies of the resulting mesoporous silica changed from rod-like, to crimped, then to hollow spheres with increasing molar ratio of propanol to CTAB was due to the decrease in the total interaction energy (favored formation of spherical particles owing to their having the smallest surface area in spherical geometry).
From the above analysis, a formation mechanism was proposed: firstly, smaller spherical particles were synthesized, templated by mixed micelles of CTAB and propanol; the smaller spherical particles had hexagonal array pore, but excess propanol made the pore structure of mesoporous silica become less ordered. Secondly, propanol and CTAB were adsorbed on the outside surface of smaller spherical particles; the smaller particles were then organized into bigger hollow spherical particles under control of hydrophobic interaction of carbon chains of mixed surfactants, resulting in hollow spheres with hierarchical pore structures. The above result is very instructive for studying biomineralization, since the above self-organization processes are similar to those of biomineralization, in which inorganic–organic hybrid composites with controlled hierarchical structures are formed [19, 20, 21].
Conclusions
The molar ratio of propanol to CTAB affected the morphologies of mesoporous silica. Hollow spherical particles of mesoporous silica could only be obtained if the molar ratio of propanol to CTAB was in the range of approximately 8:1–9:1.
The shell structures of the hollow spherical particles had MCM-41 hexagonal structure. With increasing molar ratio of propanol to CTAB, the distances (a values) between centers of two adjacent pores increased, and the pore structures of the mesoporous silica became less ordered
Type IV isotherms and H1 hysteresis loops occurred in N2 adsorption–desorption curves; with increasing molar ratio of propanol to CTAB, the pore size with BJH diameter of the most probable distribution decreased, and half peak width of the pore size distribution peak increased.
References
Cochran JK (1998) Curr Opin Solid State Mater Sci 3:474
Schacht S, Huo Q, Voigt-Martin IG, Stucky GD, Schuth F (1996) Science 273:768
Hotz J, Meier W (1998) Langmuir 14:1031
Caruso F, Shi X, Caruso RA, Susha A (2001) Adv Mater 13:740
Caruso F, Lichtenfeld H, Giersig M, Möhwald H (1998) J Am Chem Soc 120:8523
Hentze H-P, Raghavan SR, McKelvey CA, Kaler EW (2003) Langmuir 19:1069
Zhu G, Qiu Sh, Terasaki O, Wei Y (2001) J Am Chem Soc 123:7723
Wu M, Wang G, Xu H, Long J, Shek Fanny LY, Lo Samuel M-F, Williams ID, Feng Sh, Xu R (2003) Langmuir 19:1362
Lin H-P, Mou C-Y, Liu S-B, Tang C-Y (2001) Chem Commun 1970
Han S, Hou W, Yan X, Li Z, Zhang P, Li D (2003) Langmuir 19:4269
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquérol J, Siemieniewska T (1985) Pure Appl Chem 57:603
Bagshaw SA, Prouzet E, Pinnavaia TJ (1995) Science 269:1242
Quirion F, Magid LJ (1986) J Phys Chem 90:5435
Hoffmann H (1994) Adv Mater 6:116
Gamboa C, Olea A, Henriquez M (1992) Langmuir 8:23
CRC handbook of chemistry and physics, 69th edn (1988–1989). CRC Press, PE-49 and PE-51
Zana R (1995) Adv Colloid Interface Sci 57:1
Ozin GA, Kresge CT, Yang H (1998) Nucleation, growth and form of mesoporous silica: role of defects and a language of shape. In: Bonneviot L, Beland F, Danumah C, Giasson S, Kaliaguine S (eds) Mesoporous molecular sieves, vol 117. Elsiever Science, Amsterdam, p 119
Bowden NB, Weck M, Choi IS, Whitesides GM (2001) Acc Chem Res 34:231
Dujardin E, Mann S (2002) Adv Mater 14:775
Kato T, Sugawara A, Hosoda N (2002) Adv Mater 14:869
Acknowledgements
This research is supported by the State Key Laboratory of New Ceramic and Fine Processes of Tsinghua University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, S., Hou, W., Xu, J. et al. Synthesis of hollow spherical silica with MCM-41 mesoporous structure. Colloid Polym Sci 282, 1286–1291 (2004). https://doi.org/10.1007/s00396-004-1120-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1120-5