Abstract
Background
To evaluate the efficacy of paclitaxel-coated balloons (SeQuent Please®) for large de novo coronary lesions with diameters greater than 2.8 mm.
Methods
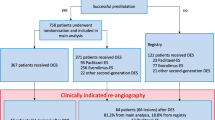
We performed a retrospective study of 527 consecutive patients with 595 de novo lesions (222 lesions) that comprised the large vessel disease (LVD) group, with a reference diameter (RD) ≥ 2.8 mm; the other 373 lesions comprised the small vessel disease (SVD) group, with a RD < 2.8 mm who received drug-coated balloon (DCB) angioplasty at the Beijing Hospital, Beijing, China. Sixty-eight patients with 91 lesions, including 45 LVD lesions, underwent coronary angiography at an average 10.7 months after DCB intervention. Clinical characteristics were recorded, and coronary angiograms were analysed with Quantitative Coronary Angiography (QCA) software.
Results
The patients in the LVD group were much younger than those in the small vessel group (61.7 ± 11.3 vs. 63.8 ± 11.7, P = 0.003), and fewer LVD patients had diabetes (27.0 vs. 57.8%, P = 0.001), three-vessel disease (37.5 vs. 52.6%, P = 0.003) and complex lesions (37.8 vs. 48.8%, P = 0.009) than those in the SVD group. Lesion preparations for LVD were more complicated than for SVD, such as 40.1% of lesions required the additional use of a cutting or scoring balloon (P = 0.004), and 21.2% lesions required non-compliant (NC) balloons (P < 0.001). Coronary dissections occurred in 63(28.3%) lesions in the LVD group but bail-out drug-eluting stent (DES) implantation was required only in one lesion (0.5%), which were both comparable with those in the SVD group. The success rate of DCB intervention was quite high and also similar in the LVD group and SVD group (99.5 vs. 99.7%, P > 0.05). QCA analysis showed that the follow-up minimal lumen diameter (MLD) was significantly increased compared with the MLD immediately post angioplasty both in the SVD group (1.75 ± 0.48 vs. 1.58 ± 0.31 mm, P = 0.008) and the LVD group (2.26 ± 0.66 vs. 2.09 ± 0.40 mm, P = 0.067). At an average of 10.1 months of clinical follow-up, the major adverse cardiovascular event (MACE) rate was 0% in the LVD group and 1.4% in the SVD group, with target lesion revascularization (TLR) rates of 0% and 1.1%, respectively. No death was observed in either group.
Conclusions
DCB for de novo coronary lesions with diameters greater than 2.8 mm was as safe and effective as for small vessel lesions, suggesting that the DCB-only strategy is also feasible in large de novo lesions intervention.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At present, percutaneous coronary intervention (PCI) with DES implantation is still the mainstay of non-pharmacological therapy for symptomatic coronary heart disease. However, complications following DES angioplasty, such as restenosis, late in-stent thrombosis [1], bleeding risk associated with long-term double anti-platelet therapy (DAPT) [2], stent fracture or allergic to metal or polymer, are of great concern. DCB may be an alternative intervention strategy to DES due to its relatively simple procedure, reduced use of contrast agents during surgery, shorter duration (1–3 months) of post-operation DAPT and absence of metal and polymer residue. In recent years, numerous trials regarding the use of DCB to treat de novo lesions have concluded that the immediate- and long-term outcomes post DCB intervention are non-inferior to those post DES [3,4,5,6]. However, most of these trials were limited to small vessel disease (SVD). No specific report regarding a DCB-only strategy for large de novo coronary lesions with diameters ≥ 2.8 mm is currently available. In the present study, we retrospectively observed patients who received DCB alone for de novo coronary lesions at our hospital and compared the clinical efficacy of DCB for LVD with that for SVD.
Methods
Study design
We performed a retrospective study of 527 consecutive patients with 595 de novo lesions who received paclitaxel DCB (SeQuent Please®, B. Bruan Melsungen AG, Berlin, Germany) angioplasty to treat de novo coronary lesions at the Beijing Hospital from May 1st 2014 to July 31st 2017. Lesions with a reference vessel diameter (RD) ≥ 2.8 mm were defined as LVD, and lesions with an RD of < 2.8 mm were defined as SVD. The LVD group included 200 patients with 222 lesions, and the SVD group included 327 patients with 373 lesions. All patients underwent clinical follow-up and a subset of 86 patients also underwent angiographic follow-up. The clinical data and coronary angiogram results of these patients were analysed.
Interventional procedure
Cardiac intervention was performed through the radial or femoral artery. Patients received a loading dose of 300 mg of aspirin and 300 mg of clopidogrel the day before the procedure. Heparin was given as an initial bolus of 70–100 IU/kg body weight with an additional dose of 1000 IU administered every hour. Glycoprotein IIb/IIIa antagonists were administered at the operator’s discretion. After intracoronary injection of nitroglycerin (100–200 μg), baseline angiography of the target vessel was performed with at least two near-orthogonal views showing the target lesion free of foreshortening and vessel overlap. According to the recommendations for treatment of small vessel de novo lesions from How to Use the Drug-coated Balloon: Recommendations by the German Consensus Group [7], written by Kleber et al., conventional balloon pre-dilation, NC balloon, cutting balloon, scoring balloon (also known as dual wire balloon) or non-slip element(NSE) balloon dilation with a balloon/vessel diameter ratio of 0.8-1.0 should be performed to reduce the occurrence of intimal dissection prior to the use of a paclitaxel-releasing balloon catheter. If the final outcome of pre-dilation was satisfactory, i.e., with ≤ 30% residual stenosis, TIMI grade 3 flow, and no dissection of the lesion or type A/B dissection based on NHLBI65 criteria [8], the patient was regarded eligible for DCB therapy. For DCB intervention, the balloon/vessel diameter ratio was still 0.8–1.0, and both ends of the balloon extended beyond both margins of the lesion by 2–3 mm under a pressure of 8–10 atm that lasted for at least 30 s. If re-dilation was necessary after the DCB was released, only a balloon without drug coating was allowed under all circumstances to avoid drug overdose. Each DCB catheter was used once. If the QCA determined that residual stenosis was ≤ 30% and the TIMI flow was grade 3, the procedure was considered successful. If an apparent dissection (i.e., of NHLBI65 type C or above) occurred and the TIMI flow was below grade 3, the DCB intervention was considered a failure, and bailout stenting with DES was performed.
Quantitative coronary angiography (QCA)
All targeted coronary lesions of enrolled patients were analysed by the built-in QCA software of the Allura Xper FD20 Angiography System (Philips Healthcare, Netherlands). Two blinded personnel measured and recorded the RD, minimal lumen diameter (MLD), length of lesion, percent diameter stenosis and percent area stenosis. For each lesion, measurements were performed in triplicate, and the mean value was obtained.
Statistical methods
Data were analysed with SPSS 22.0 statistical software (IBM, Munich, Germany). Quantitative data with a normal distribution were expressed as \(\bar {x} \pm s\). An unpaired t test was used to analyse the quantitative data in the LVD group and SVD group, and a paired t test was used to analyse pre-PCI, post-PCI and follow-up quantitative data. Qualitative data were expressed as rates or percentages. Fisher’s exact test was used for between-group comparisons. A two-sided test was applied, and a P value of less than 0.05 indicated a statistically significant difference.
Results
Patient and lesion characteristics
Compared with the patients in the SVD group, the patients in the LVD group had a lower mean age and a lower rate of concomitant diabetes. The other risk factors were similar for the two groups. There was no between-group difference in the percentage of subtypes of coronary heart disease (CHD), and unstable CHD was the predominant subtype in both groups. Three-vessel disease was less common in the LVD group than in the SVD group, as shown in Table 1.
Target lesions on the right coronary or its branches were more common in the LVD group, while in the SVD group, target lesions were more frequently located on the LCX or its branches. The percentage of calcium lesions or type B2/C lesions was higher in the SVD group than in the LVD group (P = 0.019 and P < 0.001, respectively). The degree of target vessel stenosis assessed by visual inspection was lower in the LVD group than in the SVD group, while when the QCA method was used, these parameters were similar in the two groups. The MLD in the LVD group was significantly higher than that in the SVD group (P < 0.001) (Table 2).
Procedural characteristics
During DCB intervention, the ratio of maximum pre-dilation balloon diameter/RD was significantly lower in the LVD group than in the SVD group (P = 0.025). The percentage of patients using plain old balloon alone for successful pre-dilation in the SVD group was significantly higher than the percentage in the LVD group (P < 0.001), whereas the percentage of patients using plain old balloon combined with NC balloon was significantly higher in the LVD group than in the SVD group (P < 0.001), as was use of the cutting/scoring/NSE balloon (P = 0.004). The DCB/pre-dilation balloon diameter ratio was similar in the two groups, but the DCB diameter/RD ratio and pre-dilation balloon diameter/RD ratio in the LVD group were both lower than those in the SVD group (P < 0.001 and P = 0.025). The DCB length and lesion length ratio in the LVD group was lower than that in the SVD group (P = 0.035). The DCB deployment pressure in the two groups was not significantly different, but the dilation time was shorter in the LVD group than in the SVD group (P < 0.001). Artery dissection of varying grades occurred in 32.9% (196/595) of all lesions receiving DCB intervention. The constituent ratio of dissection among the two groups was different (P = 0.04). In the SVD group, Type B dissection was more frequent than Type A dissection; furthermore, Type B dissection was more common in the SVD group than in the LVD group (P = 0.007). In the LVD group, the rates of the two types of dissections were similar. One patient in the SVD group experienced Type C dissection after DCB was deployed at the diagonal artery, and the dissection extended to the LAD causing the lumen stenosis increased from 50 to 75%. Consequently, bailout stenting with DES was implanted in the diagonal artery. The lesion in the LAD was treated successfully with another DCB. A spiral coronary artery dissection occurred in one patient in the LVD group after the DCB was released in the middle of the LAD. As a result, the flow decreased to TIMI grade 2, and a bailout DES was implanted. The device success rate of DCB treatment for de novo lesions was high (99.7%, 593/595) and was similar in both groups (Table 3).
In-hospital and mid-term follow-up after DCB intervention
Both pre-intervention and post-intervention MLDs in the LVD group were significantly higher than those in the SVD group (P < 0.001). The acute lumen gain (ALG) in the LVD group was obviously higher than that in the SVD group (P = 0.007), but when adjusted by Post-intervention MLD, the difference was not statistically significant (P = 0.092) (Table 4).
Clinical events are shown in Table 5. During hospitalization, one patient in the SVD group discontinued aspirin and clopidogrel 2 days after DCB intervention due to multiple myeloma and significant thrombocytopenia. On the 4th day after the intervention, acute ST-segment elevation myocardial infarction occurred in this patient, and emergency angiography showed thrombosis in the target lesion and TIMI flow grade 0. After thrombus aspiration and dilation with a plain balloon, the blood flow recovered to TIMI grade 2, and no stent was implanted. None of the patients in the LVD group experienced MACE, defined as all-cause death, myocardial infarction, target vessel revascularization and vessel thrombosis during hospitalization.
We followed 450 patients (57 were lost to follow-up) at clinic or by phone at least every 3 months, and the mean follow-up duration was 10.1 months. One patient in the SVD group was admitted to the hospital for recurrent angina 5 months after DCB intervention. Angiogram showed no restenosis in the target lesion, but a very tight new lesion appeared proximal to the previous lesion. Another DCB intervention was performed at the new lesion. Two patients were re-admitted to hospital due to non-ST-segment elevation myocardial infarction 5 months post-PCI. Both were still on DAPT. Coronary angiogram showed severe restenosis at the target lesions (both were LCX/OM), 90% and 100%, respectively. We performed a DCB intervention again at the OM for the first patient; the second patient was treated with plain old balloon angioplasty (POBA) at another centre. Since DCBs were not available there and the patient was allergic to metal, no stent was implanted.
No deaths occurred in either group. The incidences of MACE in the LVD group and SVD group were 0% (0/189) and 1.4% (4/281), respectively.
In this cohort, 68 patients with 91 lesions received coronary angiography follow-up 3 to 30 months (average 10.7 ± 5.7) after DCB intervention. There were 33 patients with 45 lesions in the LVD group and 35 patients with 46 lesions in the SVD group. The follow-up MLDs were significantly increased compared with the pre-intervention MLDs in both groups (P < 0.001). Compared with post-intervention, the follow-up MLDs in all lesions and the lesions in the SVD group increased significantly (P = 0.002 and 0.008, respectively), but in the LVD group, the difference did not reach statistical significance (P = 0.067) (Fig. 1). The late lumen losses (LLL), defined as follow-up MLD minus post-intervention immediate MLD, were negative in both groups but did not differ significantly (P = 0.993) (Table 6).
Discussion
We report a clinical study to evaluate the use of DCB alone to treat large de novo coronary lesions, i.e., those with a diameter ≥ 2.8 mm. To our knowledge, there are few exclusive studies aiming at LVD PCI using DCB-only PCI strategy. The percentage of LVD was 37.3% (222/595) in this study. The main findings of our study are as follows: (1) the patients in the LVD group tended to be younger and were less likely to have concomitant diabetes than those in the SVD group. Three-vessel disease was more common in the SVD group. (2) The pre-dilation procedure for LVD requires greater caution and is slightly more complicated than that for SVD, but the success rate of the DCB device was as high as that of the SVD group, and there were few complications during hospitalization in both groups. (3) As shown the mid-term follow-up angiogram shows, the outcome of large vessels treated with DCB alone was similar to that of small vessels [9], including the tendency toward lumen enlargement and the low incidence of MACE.
DCB have been successful in treating bare metal stent (BMS) in-stent restenosis (ISR) and DES ISR and is recommended by the ESC/EACT Coronary Intervention Guideline 2014 with an IA level of evidence [3]. Although the use of a local drug delivery system without a metallic stent or durable polymer sounds attractive in complex lesions where restenosis risk remains high, much less is understood of their applicability in large de novo coronary lesions. In the era of POBA, it is widely accepted that large coronary arteries have more smooth muscle fibres than small arteries and are more susceptible to recoil and dissection, which may lead to the acute occlusion or restenosis of blood vessels. Therefore, many interventional cardiologists had doubts about the safety of DCB alone for large vessel lesions. Expert consensus [7, 10] indicates that the success of DCB intervention for coronary de novo lesions relies greatly on optimal pre-dilation results and suggests a pre-dilation balloon/RD ratio of 0.8-1.0:1. To achieve a desirable pre-dilation outcome, the ratio of maximum pre-dilation balloon diameter/RD for large vessels was more conservative than that for small vessels (0.87 ± 0.12 vs. 0.96 ± 0.15, P < 0.001). If the lesion had a high plaque burden or significant calcification or fibrosis, stepwise dilation with a smaller-diameter balloon may be considered. In addition, the combined use of NC balloons, cutting balloons or scoring balloons was more common in the LVD group(40.1 vs. 28.7%,P = 0.004), while POBA alone was sufficient to achieve a good pre-dilation result in most small vessels(65.1 vs. 37.8%, P < 0.001). The pre-dilation procedure for LVD was more complex than that for SVD, and careful pre-dilation may reduce the occurrence of recoil and the dissection of large vessels. Our results showed a similar incidence of dissection in the two groups. We chose DCBs with a diameter similar to that of the maximum pre-dilation balloon for both groups, and the ratio of DCB diameter:RD was also more conservative in the LVD group than in the SVD group (0.90 ± 0.12 vs. 0.98 ± 0.15, P = 0.03). Because DCB was used to deliver the drug to the vessel wall rather than to dilate the blood vessel [10], the use of a DCB with an excessively large diameter or excessively high dilation pressure in an attempt to obtain stent-like imaging results should be avoided because it may cause procedure failure. In the expert consensus [7, 10], ≤ 30% residual stenosis after pre-dilation and DCB treatment is acceptable provided that the blood flow distal to the lesion can achieve TIMI flow grade III.
Previously published literature reported that the incidence of dissection in a successful POBA procedure with < 50% residual stenosis was 32% [11], similar to the results of the present study (32.9%, 196/595). This is probably because large vessels were always dilated more carefully than small vessels, and even less dissection occurred in large vessels than in small vessels during PCI (28.3% vs. 35.7%, P = 0.04), especially type B dissection (9.0 vs. 16.9%, P = 0.007). Hermans et al. [12] found that the restenosis rate after a POBA procedure with < 50% residual stenosis was 29% in the dissection group and 30% in the non-dissection group (RR = 0.97), and dissection was not associated with an increase in the incidence of MACEs, including non-fatal myocardial infarction and coronary revascularization. In our study, 6.7% of patients (40/595) experienced type C dissection without slowing of the distal blood flow. The dissection did not progress after 5–10 min of observation during the procedure. No bailout stent was implanted. Among the 91 angiographic follow-up lesions, 27 lesions that experienced type A to type C dissection were completely healed and showed no restenosis. Such dissection that is not associated with obvious compression of the coronary lumen has no effect on blood flow or clinical adverse events and does not prolong hospitalization is called the therapeutic type of dissection [12]. Studies have demonstrated that this type of dissection is associated with a low restenosis rate [13]. Our results suggested that type A and B dissections, even some type C dissections without limitation of blood flow appearing right after DCB PCI, usually do not require bailout stenting and have a favourable clinical outcome.
Currently, reports available on DCB treatment for de novo coronary lesions focus on SVD with a diameter of < 2.8 mm [4,5,6, 9, 14]. In Valentines II [15], a study similar to ours, a DIOR® paclitaxel-coated balloon alone was used to treat de novo coronary lesions, and a total of 103 patients with 109 lesions were enrolled; the diameter of the DCB (RVD was 2.40 ± 0.51 mm, less than the 2.73 ± 0.53 mm in our 91 follow-up lesions) was 2.7 ± 0.4 mm (2.0–3.5 mm), which included large vessels with a diameter of ≥ 2.8 mm, but the percentage of large vessels was not specified. Follow-up angiogram at 6 months showed that the LLL of target lesions was 0.38 ± 0.39 mm, significantly higher than the corresponding value in all follow-up lesions (− 0.17 ± 0.53 mm) of our study. Compared with the LLLs in the BELLO study [4] (IN.PACT Falcon DCB) and PEPCAD I study [5] (SeQuent Please® DCB) (0.08 ± 0.38 and 0.18 ± 0.38 mm, respectively), which used DCB alone to treat small vessel disease, the LLL in the SVD follow-up group of the present study was also significantly lower (− 0.17 ± 0.43 mm). Whether the difference in LLL was due to different designs of DCB or a relatively short period of follow-up (mean: 6 months) remains unclear. Compared with the post-intervention immediate MLD, the follow-up MLD in small vessels was significantly increased (P = 0.008) but not in the LVD follow-up group (P = 0.063), which may be due to the small sample size. However, the follow-up MLD of all lesions was significantly increased compared to the post-intervention MLD (P = 0.002). Angiogram showed a tendency of late positive remodelling or late catch-up of the lumen after DCB treatment, which was consistent with the results reported by Kleber et al. [16,17,18,19], and a “stent-like” effect was observed in the imaging of many patients (Fig. 2).
Angiograms of two study patients who underwent treatment of lesions in LVD group and SVD group underwent PCI. Patient 1 was treated with the drug-coated balloon in the left anterior descending artery: a the initial angiogram, b the post-procedural angiogram, c the 9-month follow-up angiogram free from restenosis. Patient 2 was treated with the drug-coated balloon in the circumflex coronary artery: d the initial angiogram, e the post-procedural angiogram, f the 12-month follow-up angiogram showing no restenosis
When stenosis greater than 50% [20] was used as the diagnosis criterion for restenosis, the restenosis rate among the 91 lesions was 8.8% (8/91), as shown by a mean angiography follow-up of 10.7 months; only 2 lesions required revascularization. The mean diameter stenosis of target lesions at follow-up was approximately 30%. This morphological basis was certainly associated with a low incidence of clinical MACE. A mean follow-up of 7.5 months in the Valentines II study showed that the incidences of TLR and MACE were 2.9 and 20.4%, respectively. In our study, a mean clinical follow-up of 10.1 months showed that the incidence of MACE was 0% in the LVD group and that the incidence of TLR and MACE was 1.1% (3/281) and 1.4% (4/281), respectively, in the SVD group, which was much lower than the values reported in previous papers [15, 21]. We look forward to determining the outcomes with longer-term follow-up.
Moreover, DCB-only therapy without stenting confers the additional advantage of safety without the need for prolonged DAPT. Here, DAPT was prescribed to all patients for at least 3 months. There were three episodes of target lesion thrombosis during follow-up. One occurred in the hospital due to cessation of DAPT, and the other two presented as NSTEMI 5 months after discharge. Studies using a DCB-only approach to revascularization show minimal risk of vessel thrombosis, even with only 1 month of DAPT [22, 23]. Vessel thrombosis after DCB therapy has been reported to always occur immediately post-PCI [4, 5, 24]. The reason for the latter two patients’ target lesion thrombosis was unclear. Both patients had optimal immediate results after DCB dilation, and the patients were still on DAPT. We assumed that prolonged time from entering the guiding catheter to reaching lesions caused more elution of the drug on the balloon, which may reduce the therapeutic effect of DCB. To our knowledge, this is the first report of late thrombosis events after DCB-only therapy for coronary de novo lesions.
Limitations
The study is retrospective, non-randomized and with limited number of angiography follow-up patients included. There may have been a learning curve effect influencing the DCB pre-dilation results which is believed relating to the instant or even LLL results. Our centre is one of the earliest centres in China that start to use DCB alone for coronary large de novo lesions and have accumulated rich experiences, that may explain the higher success rates and lower complication rates than other studies or other centres. A further prospective, randomized studies with longer follow-up duration periods are required to validate the results.
Conclusion
The mid-term follow-up results of this study showed that use of the paclitaxel DCB alone was as safe and effective for treating de novo coronary lesions involving blood vessels with a diameter of more than 2.8 mm as it was for treating SVD and it may be an alternative to stenting or even a first-choice treatment for patients ineligible for stenting.
References
Varenhorst C, Lindholm M, Sarno G, Olivecrona G, Jensen U, Nilsson J, Carlsson J, James S, Lagerqvist B (2018) Stent thrombosis rates the first year and beyond with new- and old-generation drug-eluting stents compared to bare metal stents. Clin Res Cardiol. https://doi.org/10.1007/s00392-018-1252-0
Lee SY, Hong MK, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Kim HS, Valgimigli M, Palmerini T, Stone GW (2017) Clinical outcomes of dual antiplatelet therapy after implantation of drug-eluting stents in patients with different cardiovascular risk factors. Clin Res Cardiol 106:165–173
Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V et al (2014) 2014 ESC/EACTS guidelines on myocardial revascularization: the task force on myocardial revascularization of the European society of cardiology (ESC) and the European association for cardio-thoracic surgery (EACTS) Developed with the special contribution of the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J 35:2541–2619
Latib A, Colombo A, Castriota F, Micari A, Cremonesi A, De Felice F et al (2012) A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol 60:2473–2480
Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, Boxberger M, Degenhardt R, Scheller B (2013) Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: are lesions clinically stable from 12 to 36 months? EuroIntervention 9:620–628
Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, Limbruno U (2010) Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO study. Heart 96:1291–1296
Kleber FX, Mathey DG, Rittger H, Scheller B (2011) How to use the drug-eluting balloon: recommendations by the German consensus group. EuroIntervention 7(Suppl K):K125–K128
Clinical use of drug-coated balloon: recommendations by the Chinese consensus group (2016). Chin J Intervent Cardiol 24:61–67 (in Chinese)
Funatsu A, Nakamura S, Inoue N, Nanto S, Nakamura M, Iwabuchi M, Ando K, Asano R, Habara S, Saito S, Kozuma K, Mitsudo K (2017) A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin Res Cardiol 106:824–832
Holmes DR, Holubkov R, Vlietstra RE, Kelsey SF, Reeder GS, Dorros G, Williams DO, Cowley MJ, Faxon DP, Kent KM (1988) Comparison of complications during percutaneous transluminal coronary angioplasty from 1977 to 1981 and from 1985 to 1986: the national heart, lung, and blood institute percutaneous transluminal coronary angioplasty registry. J Am Coll Cardiol 12:1149–1155
Huber MS, Mooney JF, Madison J, Mooney MR (1991) Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol 68:467–471
Hermans WR, Rensing BJ, Foley DP, Deckers JW, Rutsch W, Emanuelsson H, Danchin N, Wijns W, Chappuis F, Serruys PW (1992) Therapeutic dissection after successful coronary balloon angioplasty: no influence on restenosis or on clinical outcome in 693 patients. The MERCATOR Study Group (Multicenter European Research Trial with Cilazapril after Angioplasty to prevent Transluminal Coronary Obstruction and Restenosis). J Am Coll Cardiol 20:767–780
Bourassa MG, Lesperance J, Eastwood C, Schwartz L, Cote G, Kazim F, Hudon G (1991) Clinical, physiologic, anatomic and procedural factors predictive of restenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol 18:368–376
Sinaga DA, Ho HH, Zeymer U, Waliszewski M, Jafary FH, Ooi YW, Loh JK, Tan JK, Ong PJ (2015) Drug coated balloon angioplasty in elderly patients with small vessel coronary disease. Ther Adv Cardiovasc Dis 9:389–396
Waksman R, Serra A, Loh JP, Malik FT, Torguson R, Stahnke S, von Strandmann RP, Rodriguez AE (2013) Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention 9:613–619
Kleber FX, Rittger H, Ludwig J, Schulz A, Mathey DG, Boxberger M, Degenhardt R, Scheller B, Strasser RH (2016) Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol 105:613–621
Kleber FX, Schulz A, Waliszewski M, Hauschild T, Bohm M, Dietz U, Cremers B, Scheller B, Clever YP (2015) Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol 104:217–225
Xue Y, Xinyue W, Wenduo Z, Bing L, Dan L, Fucheng S, Fusui J (2016) Study of efficacy and safety of paclitaxel drug coated balloon in elderly patients with de novo coronary disease. Chin J Geriatr 35:834–838 (in Chinese)
Poerner TC, Duderstadt C, Goebel B et al (2017) Fractional flow reserve-guided coronary angioplasty using paclitaxel-coated balloons without stent implantation: feasibility, safety and 6-month results by angiography and optical coherence tomography. Clin Res Cardiol 106:18–27
Gould KL, Lipscomb K (1974) Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol 34:48–55
Pastormerlo LE, Ciardetti M, Trianni G, Ravani M, Shlueter M, Vaghetti M, Coceani M, Rizza A, Berti S, Palmieri C (2014) Drug eluting balloon: a multipurpose tool for coronary revascularization with optimal long-term follow-up results. J Interv Cardiol 27:574–579
Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Bohm M, Speck U (2006) Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med 355:2113–2124
Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U (2008) Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol 97:773–781
Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, Boxberger M, Degenhardt R, Scheller B (2010) Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol 99:165–174
Acknowledgements
Authors would like to acknowledge Tong Xu, Yin Lin and Bo Xia for patient data recording and QCA analysis. This work was supported by the Ministry of Science and Technology of the People’s Republic of China (No. 2017ZX09304026) and the Capital Characteristic Clinical Application Research and Achievement Promotion Funding Project (No. Z161100000516054).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yu, X., Ji, F., Xu, F. et al. Treatment of large de novo coronary lesions with paclitaxel-coated balloon only: results from a Chinese institute. Clin Res Cardiol 108, 234–243 (2019). https://doi.org/10.1007/s00392-018-1346-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-018-1346-8