Abstract
Objectives
We set out to investigate the benefit of distal main or side branch treatment with a DCB compared to POBA in coronary bifurcation lesions.
Background
The standard treatment of bifurcation lesions is application of a DES to the main branch with provisional side branch stenting. While this resulted in considerable improvement in overall MACE rate suboptimal side branch results remained a problem.
Methods
The study was performed from 2011 to 2013 in six German centers. Native bifurcation lesions were included if side branch vessel diameter was ≥2 and ≤3.5 mm and no proximal main branch lesions was found. After successful predilatation randomization was performed to either DCB application or no further treatment. Follow-up angiograms for QCA analysis were done after 9 months. Primary endpoint was late lumen loss (LLL).
Results
64 patients were successfully randomized. Minimal lumen diameter and grade of stenosis were equal in both groups. Only five stents were used as bail out. Angiographic follow-up was achieved in 75 % of patients. No patient died. There was one NSTEMI in the POBA group. Restenosis rate was 6 % in the DCB group vs 26 % in the POBA group (p = 0.045). TLR was necessary in one patient of the DCB group vs three patients of the POBA. The primary endpoint LLL was 0.13 mm in the DCB vs 0.51 mm in the POBA group (p = 0.013).
Conclusion
In bifurcation lesions that show only class A or B dissection and recoil not beyond 30 % the use of DCBs is a sound strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary bifurcation stenoses account for some 15–20 % of percutaneous treated coronary artery lesions and have been associated with lower success rates, a higher risk of restenosis and higher complication rates [1]. While in the past drug-eluting stents (DES) improved the rate of renarrowing compared to bare metal stents (BMS), restenosis especially of the side branch (SB) remained a problem [2–4]. Their use was also accompanied by the risk of thrombosis [3, 4]. Application of paclitaxel via a drug coated balloon (DCB) has been shown to be superior to PTCA and equivalent to DES treatment of in-stent restenosis (ISR) both after bare metal [5] and drug-eluting stenting [6, 7] in several randomized trials [8–10]. While the efficacy of DCBs compared to DES in de novo lesions is still controversial and seems to depend on the type of DCB used [11, 12], they show excellent results in small coronary vessels [13–17].
The gold standard of treatment of bifurcations today is the treatment of the main branch with a drug eluting stent and only provisional side branch stenting. While this resulted in considerable improvement in overall MACE rate, outcome of PCI of bifurcated lesions remained unpredictable [18], mainly because of suboptimal side branch results. The optimal technique for side branch only PCI is even less defined. Given the very good results of DCB in small vessels, we set out to explore the benefit of side branch and/or distal main branch PTCA with a drug coated balloon and compared this to regular balloon angioplasty of these branches.
Methods
The study has been approved by the appropriate ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. The study was performed from 2011 to 2013 in six German centers. All patients planned for elective or deferred treatment of lesions involving bifurcations were screened for participation in the trial.
Inclusion and exclusion criteria
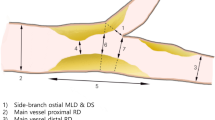
Bifurcation lesions to be included had to be within 5 mm of the branching point, had to have a side branch vessel diameter at the take off of ≥2 mm and to be Medina type 0, 0, 1/0, 1, 0 or 0, 1, 1 [19].
Patients were included if they were at least 18 years of age, had stable or unstable angina or documented silent ischemia and gave written informed consent. Patients with acute or recent myocardial infarction (<48 h) or with angina at rest were excluded. Also, patients with severe CHF, severe valvular heart disease or severely compromised life expectancy were excluded.
Only native coronary lesions were included. Side branch lesion had to be ≤10 mm in length and side branch diameters between 2.0 and 3.5 mm. Left main bifurcations and CTOs were not considered.
Randomization and blinding
Randomization was performed only after successful predilatation of the distal main (Medina 0, 1, 0) and/or side branch (Medina 0, 0, 1 or Medina 0, 1, 1) adjudicating the patient randomly to one of the treatment options, i.e., PTCA-balloon catheter angioplasty with additional paclitaxel coated balloon application (DCB treatment group) or plain old balloon angioplasty (POBA control group).
Block randomization was performed by center and by groups of four. A list of treatment assignments was generated per study center by the study statistician comprised of consecutive blocks with the order of assignments chosen at random (i.e., random permuted block of size 4). The lists were prepared prior to the initiation of the study. The allocation of the treatment was contained in sequentially numbered, sealed envelopes which were opened immediately before investigational product administration.
The POBA group was not further treated after predilatation, while in the DCB group the drug was applied with a balloon of similar size inflated to nominal or close to nominal pressure. Provisional stenting was discouraged but allowed in both groups.
Patients and evaluators of the quantitative coronary angiographies were blinded to assigned treatment.
Procedure
PCI technique required a two wire procedure for Medina 0, 0, 1 and 0, 1, 1 lesions, but not for Medina type 0, 1, 0. As first step the side branch (for Medina 0, 0, 1 and 0, 1, 1) or the distal main branch (for Medina 0, 1, 0) had to be dilated with an uncoated balloon with a balloon/artery ratio of 0.8–1.0. Second step was predilatation of the second branch where applicable. Randomization was initiated only following successful dilatation of the single or both lesions (see above).
For subjects randomized to the DCB treatment group the SeQuent® Please (B. Braun Melsungen AG, Berlin, Germany) balloon catheter was used for intervention. The drug-coated balloon had to be long enough to cover the entire lesion and to exceed the length of the predilation balloon for a minimum of 2–3 mm distally and proximally and to fully cover the main branch directly before the carina. The DCB diameter was again 0.8–1.0 of the vessel diameter. The inflation time had to be ≥30 s. Each drug coated balloon catheter was allowed for single use only. Principally the decision to stent or not to stent had to be made before randomization and after predilatation with the uncoated balloon, however, in case of severe recoil and/or dissection (>type B) after DCB application a provisional stenting using a bare metal stent was allowed. Application of the drug coated balloon was done according the recommendations of the German Swiss Consensus Group [20, 21] which in principle advice to prepare the lesions carefully with a balloon-to-artery ratio of 0.8–1.0 and apply drug coated balloons only if dissection grade is not beyond type B according to the NHLBI classification [22] and recoil is not compromising the lumen by more than 30 %.
For the subjects not randomized to the DCB treatment group, the procedure was completed with the dilatation with an uncoated balloon. Patients unless pretreated with aspirin and clopidogrel or prasugrel were given a loading dose of 500 mg aspirin and 600 mg clopidogrel in the cath lab. Aspirin was continued throughout the study, while clopidogrel was used for a minimum of 4 weeks. In case of additional stenting, a minimum duration of dual antiplatelet therapy (DAPT) of 12 months was requested.
All angiograms were performed according to standard projections and additional projections as individually needed to optimize the view of the bifurcation. Intracoronary use of 0.2 mg nitroglycerin was mandatory.
Follow up was scheduled after 9 months and angiograms were done in the same projections.
Quantitative coronary angiography
For quantitative coronary angiographic analysis, the computer-assisted CAAS II (Pie-Medical, 6227 AJ Maastricht, The Netherlands) was used. Great care was taken to optimize images and projections for later QCA. QCA was performed for the projection showing the highest degree of stenosis (lesion length according to the “shoulder-to-shoulder” criterion) and the orthogonal view if available. The maximum of both of the values determines the severity of the stenosis. This evaluation was carried out centrally by the Angiographic Core Lab of the Clinical Research Institute, Rotenburg a. d. Fulda, by a team of experienced and independent researchers. A difference of 3 % of the relative stenosis (%) between the two readings was prospectively determined to be accepted. If the discrepancy was to exceed this value, the team decided consensually after discussion upon the result of the assessment. In case of insufficient quality of the angiogram, the patient was rejected.
Measurements included the stenotic area with measurement from shoulder to shoulder (in-lesion), and the total treated area plus 5 mm of the edges (in-segment) but not beyond the take off of the side branch or distal main branch. Restenosis was defined as a diameter stenosis of at least 50 %.
For detailed evaluation purposes, parameters were calculated such as lesion length, minimal lumen diameter (MLD) as well as acute lumen gain, late lumen loss (LLL), and late loss index.
Endpoint
The primary endpoint was late lumen loss in the DCB vs POBA group.
Ethics and written informed consent
The study has been approved by the appropriate ethical committee and written informed consent was obtained from all patients prior to diagnostic cardiac catheterization or in cases of preplanned interventions prior to the interventional procedure. However, we did not keep track of informed consents that were not used because of non suitable lesions or inappropriate lesion preparation results.
Sample size calculation
The sample size estimate for the primary variable “late loss” was based on the following assumptions: predicted late loss DCB 0.21 ± 0.5 mm, late loss alternative POBA 0.57 ± 0.5 mm, alpha error 0.05, power 80 %, follow-up rate 80 %. Calculated samples size per treatment group was 32.
Statistical analysis
Data were analyzed according to the intention to treat. Following testing for normal distribution using the Kolmogorov–Smirnov test systematically, continuous variables were expressed as mean ± standard deviation. Categorical variables were compared with the Fisher’s exact test, continuous variables with the two-sided Student’s t test or the Welch’s test for unequal variances (BIAS 10.10 and PASW Statistics 18.0.0).
Results
Patients
64 patients have been successfully randomized. Patients age and risk factors are given in Table 1. One patient was randomized to POBA but received DCB. The data are presented as intention to treat. Lesions characteristics and procedural data are given in Table 2. There were no significant differences between the two groups. Most patients had distal main branch lesions. Only six of 64 patients needed stents after randomization. They were all part of the control group (Table 3). Indications were elastic recoil (n = 3), dissection (n = 2) and remaining stenosis >30 % after POBA (n = 1).
Angiographic results
Angiographic data are given in Table 4. Lesions were clearly small vessel lesions and were of rather short length. Initial stenoses were high grade and luminal gain with balloon angioplasty was sufficient (1.36 mm). Residual stenosis was 20 %.
Angiographic follow-up was achieved in 75 % of patients after 9.4 ± 2.9 months (DCB group 78.1 % and POBA group 71.9 %, p = 0.77). Mean time to angiographic follow-up was 9.34 months in the DCB group (median 9.32 months) and 8.67 months in the POBA group (median 8.9 months), p = n.s.. The remaining 25 % of patients had telephone follow-up and review of their medical records, no patient was lost to follow-up, no patient died.
Late luminal loss (the primary endpoint) was 0.13 mm in the DCB and 0.51 mm in the control POBA group [p = 0.013; 95 % CI (−0.66 to −0.08)]. Binary restenosis rate was 6 % in the DCB group and 26 % in the control group (p = 0.045). Diameter frequency distribution and MLD-frequency distribution is given in Figs. 1 and 2. Further angiographic follow up data see Table 5.
To exclude any influence of stenting on the results, we also made a sensitivity analysis excluding patients who were stented. The results were very similar and the difference between groups for LLL was 0.13 ± 0.31 vs 0.54 ± 0.67 p = 0.0131 for in lesion and 0.08 ± 0.31 vs 0.52 ± 0.612 p = 0.010 for in segment in favor of the DCB group.
There was one non ST-elevation myocardial infarction in the control group (no stent) after 2 months. TLR was necessary in one patient in the DCB and in three patients in the control group (p not significant).
Discussion
Bifurcation lesions are a complex entity with higher MACE, stent thrombosis and restenosis rates than non-bifurcated lesions [2–4]. While for so-called true bifurcations, the mostly accepted standard therapy is stenting of the main branch with a DES and provisional side branch stenting [18, 23], the side branch restenosis rates are still mostly over 10 % [24]. Lesions with ostial side branch or distal main branch lesions only, not involving the proximal main branch, await a clear recommendation.
A first feasibility study has been able to show that the application of DCBs to bifurcations can be successful and might reduce restenosis compared to other techniques [25]. The results of a first randomized trial were negative, but they were compromised by the use of a matrix free DCB [26]. Matrix free DCBs have been shown to be inferior to DCBs with matrix consisting of an excipient in addition to the drug, facilitating rapid absorption of paclitaxel into the vascular wall [27]. The PEPCAD V study reported a restenosis rate of 3.8 % in the MB and 7.7 % in the SB after DCB intervention [25]. This is considerably lower especially for the SB compared to restenosis after DES implantation which is reported to be between 4.6–6.7 % (MB) and 13.2–14.7 % (SB) in the CACTUS [28], 4.6–5.1 % (MB) and 11.5–19.2 % (SB) in the Nordic [23] and 2.5–3.1 % (MB) and 7.9–15.4 % (SB) in the Nordic III study [24]. Only the British Bifurcation Coronary Study showed lower rates of restenosis with 2.8–4 % (MB) and 2.8–3.6 % (SB) [18].
Another interesting finding of the PEPCAD V trial was that late lumen loss in the SB, where only few additional stents were used, was lower than in the MB. Though LLL is consistently lower without implantation of foreign bodies like stents, positive vascular remodeling after DCB intervention might have contributed to this finding [29]. While so far most bifurcation studies investigated the outcome after sequential application of DCBs to the MB and SB with stenting of the MB [30–32], including a recent randomized trial vs a DES strategy [33], there might be additional positive effects by applying the DCB only strategy to main and side branch: Especially the lack of carina shift and the maintenance of natural flow distribution seem to be attractive aims of a stentless bifurcation treatment. A first registry on using DCB only without stenting showed promising results [34].
Our results underscore the potential of DCB for bifurcation lesions. While the most challenging Medina 1, 1, 1 lesions have not been addressed in this first DCB only strategy randomized trial, the benefit vs POBA is readily apparent. Also, not using a stent was of no disadvantage in terms of restenosis rate and over the initial observation period there has been no thrombosis. Therefore, in bifurcation lesions that show only class A or B dissection according to the NHLBI classification [22] and recoil not beyond 30 % use of DCB is a sound strategy.
Limitations
Our study has several limitations. First of all the study size is small. Recruitment was stopped before the initial planned additional patients compensating for loss of follow-up had been reached. This was caused by the need to defer the PCI after the diagnostic angiogram for purposes of informed consent, but also by the lower rate of poor side branch and distal main branch lesions as compared to lesions involving also the proximal main branch. However, the trial included the statistically calculated sample size of 32 patients per group. No patient was lost to follow-up and the angiographic follow up rate was high.
With a drug coated balloon as stand alone procedure, so far no randomized trial has focused on bifurcation lesion. Indeed, the two randomized studies on treatment of bifurcation lesions with drug coated balloons used stents in the proximal and distal main branch and one of these two studies used a DCB without excipient. Therefore, even a rather small trial might add important new information in this field.
Furthermore, our results do apply only to patients that show an acceptable predilatation result according to the consensus recommendations.
Finally, the higher use of stents in the control group points to the disadvantage of a single blind randomized trial in this setting. However, the rate of stenting was low (see table) and the results are similar, if the stented lesions are excluded. The reasons given by the investigators in the POBA group to use stents point to less confidence of the interventionalists in the long-term result of the POBA patients (in three type A dissection was given as reason for stenting, the others were judged as “insufficient result”). This, however, favors the POBA group. Nevertheless, the DCB group had better results. Given the different surface and applicability of DCB vs regular balloons a double blind application is currently not possible.
For the homogeneity of the data we used the DCB of only one manufacturer using iopromide as excipient for paclitaxel. Since the excipients lead to different tissue concentrations, the results can not necessarily be extrapolated to other technologies.
Conclusion
In conclusion we found that PCI with DCB-only strategy in de-novo bifurcation lesions Medina type 0, X, X is a feasible and safe therapy with low rates of restenosis and TLR, not necessitating foreign body implantation or long-term DAPT. This trial proved the superiority of DCB over POBA for side branch or distal main branch bifurcated lesions demonstrating a lower in segment late loss at 9 months in the treated area.
References
Meier B, Gruentzig AR, King SB 3rd, Douglas JS Jr, Hollmann J, Ischinger T, Aueron F, Galan K (1984) Risk of side branch occlusion during coronary angioplasty. Am J Cardiol 53:10–14
Tanabe K, Hoye A, Lemos PA, Aoki J, Arampatzis CA, Saia F, Lee CH, Degertekin M, Hofma SH, Sianos G, McFadden E, Smits PC, van der Giessen WJ, de Feyter P, van Domburg RT, Serruys PW (2004) Restenosis rates following bifurcation stenting with sirolimus-eluting stents for de novo narrowings. Am J Cardiol 94:115–118
Colombo A, Moses JW, Morice MC, Ludwig J, Holmes DR Jr, Spanos V, Louvard Y, Desmedt B, Di Mario C, Leon MB (2004) Randomized study to evaluate sirolimus-eluting stents implanted at coronary bifurcation lesions. Circulation 109:1244–1249
Hoye A, Iakovou I, Ge L, van Mieghem CA, Ong AT, Cosgrave J, Sangiorgi GM, Airoldi F, Montorfano M, Michev I, Chieffo A, Carlino M, Corvaja N, Aoki J, Rodriguez Granillo GA, Valgimigli M, Sianos G, van der Giessen WJ, de Feyter PJ, van Domburg RT, Serruys PW, Colombo A (2006) Long-term outcomes after stenting of bifurcation lesions with the “crush” technique: predictors of an adverse outcome. J Am Coll Cardiol 47:1949–1958
Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W, Leschke M, Ackermann H, Boxberger M, Speck U, Degenhardt R, Scheller B (2009) Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation 119:2986–2994
Habara S, Mitsudo K, Kadota K, Goto T, Fujii S, Yamamoto H, Katoh H, Oka N, Fuku Y, Hosogi S, Hirono A, Maruo T, Tanaka H, Shigemoto Y, Hasegawa D, Tasaka H, Kusunose M, Otsuru S, Okamoto Y, Saito N, Tsujimoto Y, Eguchi H, Miyake K, Yoshino M (2011) Effectiveness of paclitaxel-eluting balloon catheter in patients with sirolimus-eluting stent restenosis. JACC Cardiovasc Interv 4:149–154
Rittger H, Brachmann J, Sinha AM, Waliszewski M, Ohlow M, Brugger A, Thiele H, Birkemeyer R, Kurowski V, Breithardt OA, Schmidt M, Zimmermann S, Lonke S, von Cranach M, Nguyen TV, Daniel WG, Wöhrle J (2012) A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: the PEPCAD-DES study. J Am Coll Cardiol 59:1377–1382
Indermuehle A, Bahl R, Lansky AJ, Froehlich GM, Knapp G, Timmis A, Meier P (2013) Drug-eluting balloon angioplasty for in-stent restenosis: a systematic review and meta-analysis of randomised controlled trials. Heart 99:327–333
Scheller B, Clever YP, Kelsch B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Speck U, Böhm M, Cremers B (2012) Long-term follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. JACC Cardiovasc Interv 5:323–330
Byrne RA, Neumann FJ, Mehilli J, Pinieck S, Wolff B, Tiroch K, Schulz S, Fusaro M, Ott I, Ibrahim T, Hausleiter J, Valina C, Pache J, Laugwitz KL, Massberg S, Kastrati A, ISAR-DESIRE 3 investigators (2013) Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet 381:461–467
Fröhlich GM, Lansky AJ, Ko DT, Archangelidi O, De Palma R, Timmis A, Meier P (2013) Drug eluting balloons for de novo coronary lesions—a systematic review and meta-analysis. BMC Med 11:123
Belkacemi A, Agostoni P, Voskuil M, Stella PR (2011) Coronary bifurcation lesions treated with the drug eluting balloon: a preliminary insight from the DEBIUT study. EuroIntervention 7 Suppl K:K66–K69
Latib A, Colombo A, Castriota F, Micari A, Cremonesi A, De Felice F, Marchese A, Tespili M, Presbitero P, Sgueglia GA, Buffoli F, Tamburino C, Varbella F, Menozzi A (2012) A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (balloon elution and late loss optimization) study. J Am Coll Cardiol 60:2473–2480
Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H, Boxberger M, Degenhardt R, Scheller B (2010) Treatment of small coronary arteries with a paclitaxelcoated balloon catheter. Clin Res Cardiol 99:165–174
Wöhrle J, Zadura M, Möbius-Winkler S, Leschke M, Opitz C, Ahmed W, Barragan P, Simon JP, Cassel G, Scheller B (2012) SeQuent Please World Wide Registry: clinical results of SeQuent please paclitaxel-coated balloon angioplasty in a large-scale, prospective registry study. J Am Coll Cardiol 60:1733–1738
Zeymer U, Waliszewski M, Spiecker M, Gastmann O, Faurie B, Ferrari M, Alidoosti M, Palmieri C, Heang TN, Ong PJ, Dietz U (2014) Prospective ‘real world’ registry for the use of the ‘PCB only’ strategy in small vessel de novo lesions. Heart 100:311–316
Ho HH, Ooi YW, Loh KK, Tan J, Aung TH, Jafary FH, Ong PJL (2013) Clinical efficacy and safety of SeQuent Please Paclitaxel-Eluting Balloon in a real-world single-center registry of south–east Asian patients. IJC Heart Vessels 1:37–41
Hildick-Smith D, de Belder AJ, Cooter N, Curzen NP, Clayton TC, Oldroyd KG, Bennett L, Holmberg S, Cotton JM, Glennon PE, Thomas MR, Maccarthy PA, Baumbach A, Mulvihill NT, Henderson RA, Redwood SR, Starkey IR, Stables RH (2010) Randomized trial of simple versus complex drug-eluting stenting for bifurcation lesions: the British Bifurcation Coronary Study: old, new, and evolving strategies. Circulation 121:1235–1243
Medina A, Suárez de Lezo J, Pan M (2006) A new classification of coronary bifurcation lesions. Rev Esp Cardiol 59:183
Kleber FX, Mathey DG, Rittger H, Scheller B, German Drug-eluting Balloon Consensus Group (2011) How to use the drug-eluting balloon: recommendations by the German Consensus Group. EuroIntervention 7 Suppl K:K125–K128
Kleber FX, Rittger H, Bonaventura K, Zeymer U, Wöhrle J, Jeger R, Levenson B, Möbius-Winkler S, Bruch L, Fischer D, Hengstenberg C, Pörner T, Mathey D, Scheller B (2013) Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol 102:785–797
Huber MS, Mooney JF, Madison J, Mooney MR (1991) Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol 68:467–471
Steigen TK, Maeng M, Wiseth R, Erglis A, Kumsars I, Narbute I, Gunnes P, Mannsverk J, Meyerdierks O, Rotevatn S, Niemelä M, Kervinen K, Jensen JS, Galløe A, Nikus K, Vikman S, Ravkilde J, James S, Aarøe J, Ylitalo A, Helqvist S, Sjögren I, Thayssen P, Virtanen K, Puhakka M, Airaksinen J, Lassen JF, Thuesen L, Nordic PCI Study Group (2006) Randomized study on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation 114:1955–1961
Niemelä M, Kervinen K, Erglis A, Holm NR, Maeng M, Christiansen EH, Kumsars I, Jegere S, Dombrovskis A, Gunnes P, Stavnes S, Steigen TK, Trovik T, Eskola M, Vikman S, Romppanen H, Mäkikallio T, Hansen KN, Thayssen P, Aberge L, Jensen LO, Hervold A, Airaksinen J, Pietilä M, Frobert O, Kellerth T, Ravkilde J, Aarøe J, Jensen JS, Helqvist S, Sjögren I, James S, Miettinen H, Lassen JF, Thuesen L, Nordic-Baltic PCI Study Group (2011) Randomized comparison of final kissing balloon dilatation versus no final kissing balloon dilatation in patients with coronary bifurcation lesions treated with main vessel stenting: the Nordic-Baltic Bifurcation Study III. Circulation 123:79–86
Mathey DG, Wendig I, Boxberger M, Bonaventura K, Kleber FX (2011) Treatment of bifurcation lesions with a drug-eluting balloon: the PEPCAD V (paclitaxel eluting PTCA balloon in coronary artery disease) trial. EuroIntervention 7 Suppl K:K61–K65
Stella PR, Belkacemi A, Dubois C, Nathoe H, Dens J, Naber C, Adriaenssens T, van Belle E, Doevendans P, Agostoni P (2012) A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in bifurcation lesions treated with a single-stenting technique: 6-month angiographic and 12-month clinical results of the drug-eluting balloon in bifurcations trial. Catheter Cardiovasc Interv 80:1138–1146
Bondesson P, Lagerqvist B, James SK, Olivecrona GK, Venetsanos D, Harnek J (2012) Comparison of two drug eluting balloons: a report from the SCAAR registry. EuroIntervention 8:444–449
Colombo A, Bramucci E, Saccà S, Violini R, Lettieri C, Zanini R, Sheiban I, Paloscia L, Grube E, Schofer J, Bolognese L, Orlandi M, Niccoli G, Latib A, Airoldi F (2009) Randomized study of the crush technique versus provisional side-branch stenting in true coronary bifurcations: the CACTUS (coronary bifurcations: application of the crushing technique using sirolimus-eluting stents) study. Circulation 119:71–78
Kleber FX, Schulz A, Waliszewski M, Hauschild T, Böhm M, Dietz U, Cremers B, Scheller B, Clever YP (2014) Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol 104:217–225
Herrador JA, Fernandez JC, Guzman M, Aragon V (2013) Drug-eluting vs. conventional balloon for side branch dilation in coronary bifurcations treated by provisional T stenting. J Interv Cardiol 26:454–462
Sgueglia GA, Todaro D, Bisciglia A, Conte M, Stipo A, Pucci E (2011) Kissing inflation is feasible with all second-generation drug-eluting balloons. Cardiovasc Revasc Med 12:280–285
Chin K, Rajagopal P (2014) The combined use of bioresorbable scaffold or DES and drug coated balloon in bifurcation stenting. EuroIntervention Euro14A-MA045 (Abstract)
López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ, Sandoval J, Romany S, Martínez Romero P, Fernández Díaz JA, Fernández Portales J, González Fernández R, Martínez Cáceres G, Merchán Herrera A, Alfonso Manterola F, Investigators BABILON (2014) A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention 10:50–57
Schulz A, Hauschild T, Kleber FX (2014) Treatment of de novo coronary bifurcation lesions with DCB only strategy. Clin Res Cardiol 103:451–456
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following conflicts of interest: Franz X. Kleber is a consultant to B.Braun and has received institutional grant support by Medtronic and B. Braun. Antonia Schulz received travel expenses from B. Braun. Bruno Scheller is a consultant to B. Braun, received lecture honoraria by Medtronic and B. Braun and is co-inventor on a patent application by Charité University Hospital. Ralf Degenhardt is employee of the Clinical Research Institute that is paid for conduct of the study. Michael Boxberger is employee as medical director at B. Braun. Harald Rittger, Josef Ludwig, Detlef G. Mathey and Ruth H. Strasser declare no conflict of interest.
Funding
The study was financially supported in part by B. Braun Melsungen AG.
Rights and permissions
About this article
Cite this article
Kleber, F.X., Rittger, H., Ludwig, J. et al. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: results of the randomized multicenter PEPCAD-BIF trial. Clin Res Cardiol 105, 613–621 (2016). https://doi.org/10.1007/s00392-015-0957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0957-6