Abstract
Limited data exist regarding drug-coated balloon (DCB) treatment in de novo large coronary arteries. We sought to demonstrate procedural characteristics, residual stenosis, and clinical outcomes following DCB angioplasty for de novo lesions in large versus small coronary arteries. The study included 184 consecutive patients with 223 de novo coronary lesions undergoing paclitaxel DCB angioplasty between January 2019 and August 2020, who were divided according to whether the DCB diameter was ≥ 3.0 mm (large group, n = 58) or < 3.0 mm (small group, n = 125). The large group had a higher proportion of acute coronary syndrome more commonly with ostial, bifurcation, and calcified lesions in large vessels and received lesion preparation with more frequent use of scoring or cutting balloons and atherectomy devices compared to the small group. Postprocedural angiographic diameter stenosis was smaller in the large group compared to the small group (31% [22–37] vs. 35% [26–42], p = 0.032), and intravascular ultrasound revealed no significant difference in postprocedural area stenosis between the groups (66.2 ± 7.7% vs. 67.9 ± 7.8%; p = 0.26). The median follow-up duration was 995 days. The incidence of a composite of all-cause death, myocardial infarction, stroke, or target lesion revascularization was similar between the groups (log-rank p = 0.41) and was influenced by the presence of acute coronary syndrome and anemia but not by DCB diameter. The rate of cardiovascular outcomes after DCB treatment was comparable in de novo large and small coronary arteries. Notably, well-planned lesion preparation with intravascular imaging guidance was prevalent in large vessels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While drug-eluting stents have made remarkable advancement in the field of percutaneous coronary intervention (PCI) during these decades, drug-coated balloons (DCB) have been used in specific types of coronary lesions, including in-stent restenosis, de novo lesions in small vessels, and bifurcation lesions [1]. DCB treatment involves the property of local antiproliferative drug delivery while leaving no metal behind, offering the potential to prevent restenosis without an elevated thrombotic risk [2]. Thus, DCB angioplasty is an encouraging alternative therapy for coronary artery disease.
However, there are concerns regarding elastic recoil, flow-limiting dissection, and hematoma linked to acute vessel closure immediately after DCB angioplasty, unlike drug-eluting or bare-metal stent implantation. While previous trials showed the clinical feasibility of DCB treatment in de novo small coronary vessels [3], there are limited data on DCB use in de novo large arteries. Therefore, we sought to demonstrate procedural characteristics, residual stenosis, and clinical outcomes following DCB angioplasty for de novo lesions in large versus small coronary arteries.
Methods
Patients
This observational study was conducted using data from the clinical and intravascular imaging database of the Koseikai Takai Hospital cardiac catheterization lab. Between January 2019 and August 2020, 314 patients with 445 coronary lesions underwent PCI with DCB catheters. The study comprised 184 consecutive patients with 223 coronary de novo lesions who underwent PCI using DCBs. The baseline characteristics and procedures were obtained from the database. PCI procedures were guided by intravascular imaging according to current practice guidelines [4, 5]. The lesions to treat were selected at the operator’s discretion after the diagnostic angiogram, and careful lesion preparation, with scoring balloons or atherectomy devices if needed, was highly encouraged during PCI. The study patients underwent angioplasty using the paclitaxel DCB SeQuent Please (B. Braun, Melsungen, Germany). The patients were divided into two groups based on whether the DCB diameter was ≥ 3.0 mm (large group) or < 3.0 mm (small group). All patients received treatment with aspirin and a P2Y12 inhibitor, unless oral anticoagulants had been prescribed previously.
The study complied with the Declaration of Helsinki, and written informed consent was obtained from all patients before PCI.
Angiography and intravascular ultrasound
After intracoronary administration of nitroglycerine, angiography was performed during the procedure to evaluate the coronary arteries. The reference vessel diameter, minimal lumen diameter, percent diameter stenosis, and angiographic lesion length were measured based on diastolic frames in a single view showing the smallest minimal lumen diameter without foreshortening the target lesion. Quantitative coronary angiography analysis at pre- and post- procedures was performed offline by two independent operators. Moderate calcification was defined as radiopacities noted only during the cardiac cycle before contrast injection, and severe calcification was defined as radiopaque densities noted without cardiac motion before contrast injection and generally involving both sides of the arterial wall.
Intravascular ultrasound (IVUS) or other imaging modalities were used at the discretion of the operators after the diagnostic angiography. Two commercially available IVUS catheters with 60 MHz transducers were used in the present study: OptiCross HD (Boston Scientific, Natick, MA, USA) and AltaView (Terumo, Tokyo, Japan). IVUS examinations were conducted before PCI, after lesion preparation, and after DCB angioplasty. IVUS was utilized to determine the indication of a debulking device and scoring or cutting balloon, and to select balloon size and length using a motorized transducer pullback. Quantitative grayscale IVUS analysis was performed using QIvus 3.0 (Medis, Leiden, The Netherlands) to measure cross-sectional lumen and external elastic membrane (EEM) areas at proximal and distal references and in the target lesions, according to the American College of Cardiology consensus statement [6]. Plaque plus media area was calculated as EEM area minus lumen area. Plaque burden (%) was calculated as (plaque plus media area) divided by the EEM area. The remodeling index was expressed as the EEM area at the minimal lumen area (MLA) site divided by the average EEM area of the proximal and distal reference segments. To evaluate lumen stenosis, the percent area stenosis (%) was measured as (mean reference lumen area minus MLA) divided by the mean reference lumen area. To assess the circularity of the cross section, the lumen eccentricity index was calculated as the minimal lumen diameter divided by the maximal lumen diameter derived from the same cross section [7]. The lumen asymmetry index was calculated as one minus the minimal lumen diameter divided by the maximal lumen diameter throughout an entire lesion. Volumetric quantification of atheroma in the target lesion, including total atheroma volume and percent atheroma volume, was assessed according to the expert consensus documents [8]. The presence of dissection was defined as a linear rim of tissue with a width ≥ 0.20 mm and a clear separation. The intramural hematoma was characterized by an accumulation of blood within the medial space, displacing the internal elastic membrane inward and EEM outward by echolucent material in the media.
Clinical endpoints
The primary endpoint was a composite endpoint, including all-cause death, non-fatal myocardial infarction (MI), stroke, or target lesion revascularization. MI was defined by the fourth universal definition of myocardial infarction [9]. Periprocedural MI was defined as target vessel MI within 48 h after the index procedure. Stroke was defined as symptomatic stroke with the rapid onset of a focal or global neurological deficit lasting ≥ 24 h. Target lesion revascularization was defined as any revascularization for a stenosis within the target lesion. The clinical outcome data were obtained by reviewing the patient records.
Statistical analysis
Categorical variables were presented as counts (percentages), while continuous variables were presented as mean ± standard deviation or medians and interquartile (interquartile range [first quartile–third quartile]). Categorical variables were compared between the two groups with the chi-square test or Fisher’s exact test, as appropriate. Continuous variables were compared between the groups using the unpaired t-test or Mann–Whitney U test, based on the data distribution. The Shapiro–Wilk test was used to assess the normality of continuous data. Kaplan–Meier survival curves with time-to-event data after the index procedure were generated and compared using the long-rank test (Mantel-Cox). The difference in the clinical event rate between the two groups was compared using Cox proportional hazard models, with a report of the hazard ratio, 95% confidence interval (CI), and p value. A multivariate Cox proportional hazards model was used to determine the independent determinants of the primary endpoint. The candidate predictor variables included acute coronary syndrome (unstable angina or acute MI), hemodialysis, C-reactive protein, and hemoglobin of < 11.0 g/dL, which were significant predictors in univariate analysis. In addition, the patients in the large group were divided into calcified and noncalcified subgroups based on the absence or presence of angiographically moderate or severe calcification. Baseline characteristics, procedures, and the incidence of the primary endpoint were examined in calcified versus noncalcified subgroups.
Statistical analysis was performed using SPSS statistical software (version 24.0, SPSS Inc., Chicago, IL, USA). A p value of < 0.05 was considered statistically significant.
Results
Baseline characteristics and procedures
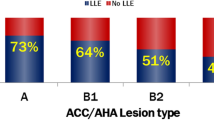
The median age was 73.0 years (66.5–79.0) and 77.0% were male. The median period of clinical follow-up was 995 days (765–1155). Out of 184 patients with 223 lesions, we excluded one patient with one lesion in whom a DCB catheter did not completely cross the target lesion. Then, the patients undergoing DCB treatment in de novo coronary lesions were classified as the large group (n = 58) or the small group (n = 125). Baseline patient and lesion characteristics are provided in Table 1 and Fig. 1. The large group, compared to the small group, had a higher proportion of acute coronary syndrome and a lower prevalence of diabetes and was more commonly treated for ostial, bifurcation, and calcified lesions in large vessels. The large group included more type B2 or C lesions according to the American College of Cardiology/American Heart Association classification than the small group (62.1% vs. 39.2%; p = 0.004).
As shown in Table 2 and Fig. 1, the median DCB diameter was 3.0 mm (3.0–3.5) in the large group and 2.5 mm (2.0–2.5) in the small group. The large group underwent lesion preparation with more frequent use of scoring or cutting balloons (77.6% vs. 54.4%; p = 0.003) and atherectomy devices (43.1% vs. 4.0%; p < 0.001) compared to the small group.
Angiography
As shown in Table 3, quantitative coronary angiography analysis showed reference vessel diameter was 2.81 mm (2.41–3.13) in the large group and 1.89 mm (1.66–2.12) in the small group. Postprocedural percent diameter stenosis was smaller in the large group than in the small group (31% [22–37] vs. 35% [26–42]; p = 0.032). The incidence of angiographic dissection was not different between the groups (20.7% vs. 16.0%; p = 0.53).
Intravascular ultrasound
As shown in Table 4, preprocedural IVUS revealed that the large group had larger plaque burden in the proximal reference segment and at the MLA site and greater percent area stenosis in the target lesion with more frequent attenuated plaques than the small group. Postprocedural IVUS analysis with volumetric quantification showed no significant differences in percent area stenosis (66.2 ± 7.7% vs. 67.9 ± 7.8%; p = 0.26) and percent atheroma volume (54.2 ± 7.6% vs. 55.3 ± 8.2%; p = 0.48) between the large and small groups. The incidence of dissection (61.7% vs. 70.1%; p = 0.42) and hematoma (17.0% vs. 32.8%; p = 0.083) after PCI did not differ significantly between the two groups. In terms of lumen circularity, IVUS analysis showed a similar postprocedural eccentricity and asymmetry index between the groups, but the mean increase in the asymmetry index was lower in the large group compared to the small group (Supplementary Table 1).
Clinical data
In our cohort, the large group underwent follow-up computed tomography or invasive angiography more frequently (Supplementary Fig. 1) and discontinued dual antiplatelet therapy earlier than the small group (Supplementary Fig. 2). As shown in Fig. 2 and Supplementary Table 2, there was no difference in the rate of the primary endpoint between the large group and the small group (log-rank p = 0.41). In the patient-level cohort, univariate analysis revealed that acute coronary syndrome, hemodialysis, C-reactive protein, and anemia (hemoglobin of < 11.0 g/dL) were associated with the primary endpoint. However, the DCB diameter did not show an association with the primary endpoint. In the study patients, multivariate analysis identified acute coronary syndrome (hazard ratio 4.06; 95% CI 1.75–9.44; p = 0.001) and anemia (hazard ratio 5.25; 95% CI 1.87–14.75; p = 0.002) as predictors of the primary endpoint (Table 5). Notably, the incidence of the primary endpoint in the large group was not different between in patients with and without angiographically moderate or severe calcification (Supplementary Table 3, Supplementary Table 4, Supplementary Fig. 3).
Discussion
The main findings of the present study were as follows: (1) in this observational study, the rate of cardiovascular outcomes after DCB treatment was comparable in de novo large and small coronary arteries, although the large group exhibited more acute coronary syndrome and more often involved ostial, bifurcation, and calcified lesions in large vessels; (2) well-planned lesion preparation followed by DCB use with intravascular imaging guidance was prevalent in de novo large vessels; and (3) the incidence of cardiovascular outcomes was not associated with DCB diameter but was influenced by the presence of acute coronary syndrome and anemia in our cohort.
While there are limited data on DCB treatment for de novo lesions in large vessels, previous studies demonstrated the safety and efficacy of DCB strategies in specific patient populations or types of coronary lesions. The randomized PEPCAD NSTEMI trial showed that treatment of de novo coronary lesions with DCBs that were 2.81 ± 0.49 mm in diameter was non-inferior to treatment with drug-eluting or bare-metal stents regarding 9-month target lesion failure in patients with non-ST-segment elevation MI [10]. In the PEPCAD NSTEMI trial, all-cause death occurred in 4.7% and target lesion reintervention occurred in 1.2% of patients undergoing DCB treatment at 9 months, which were comparable to the clinical performance observed in the large group of our study.
Another study, the DEBUT trial, demonstrated that PCI with DCB only for de novo lesions was superior to bare-metal stent implantation in terms of major adverse cardiac events at 9 months in patients with high bleeding risk, and two-thirds of the DCB-only group were treated using DCBs that were ≥ 3.0 mm in diameter [11]. Moreover, Uskela et al. reported that the 12-month rates of major adverse cardiovascular events were 7.1% in patients with stable coronary artery disease and 12% in patients with acute coronary syndrome [12]. These trials support the feasibility of DCB strategies in the treatment of de novo lesions in large coronary arteries.
Despite the absence of scaffolding material like drug-eluting stents or bioresorbable vascular scaffolds, we observed favorable angiographic findings in the large group. Previous studies also showed the efficacy of DCB treatment without coronary stents in hemodialysis patients [13] and patients with bifurcation lesions [14]. Our study demonstrated that angiographic percent diameter stenosis after PCI was approximately 30% in the large group. According to the current international DCB consensus [5], our study demonstrated that acceptable angiographic findings, defined as residual stenosis ≤ 30%, were more frequently achieved in the large group compared to the small group. Obviously, intravascular imaging guidance is considered to be indisputable in DCB treatment, as it provides an assessment of lesion morphology and can be utilized in adequate lesion preparation leading to optimal DCB angioplasty. According to expert consensus documents, intracoronary imaging modalities have a key role in the minimizing risk of complications [15]. Our study also provides preprocedural and postprocedural intravascular imaging data with volumetric IVUS quantification in patients undergoing DCB treatment for de novo lesions. The large group, compared to the small group, exhibited larger postprocedural MLA (4.5 mm2 [3.5–6.1] vs. 2.4 mm2 [1.8–2.7]; p < 0.001) and a greater initial lumen gain, as measured by the postprocedural MLA minus the preprocedural MLA (2.1 mm2 [1.2–3.6] vs. 1.0 mm2 [0.5–1.3]; p < 0.001). Although changes in atheroma volume during PCI were nearly imperceptible in both the large and small groups (0.3 mm3 [-7.8–4.3] and −0.6 mm3 [−4.0 to 2.9], respectively), the volumetric IVUS analysis revealed that balloon angioplasty in the large group had a greater effect on enlargement in lumen volume (20.8 mm3 [11.0–33.6] vs. 8.6 mm3 [4.1–15.7]; p < 0.001).
This study has limitations. First, the sample size is small. Larger prospective studies are needed to further investigate the efficacy and safety of DCB treatment in de novo large coronary lesions. Nonetheless, the present study provides unprecedented data on angiography and volumetric quantification by intravascular imaging as well as clinical outcomes following DCB treatment for de novo coronary lesions. Second, the study did not include patients in whom operators dismissed DCB angioplasty for suboptimal results after lesion preparation, because the study included patients undergoing PCI using DCB catheters. Finally, the selection of patients and lesions is biased. The large group more commonly had ostial, bifurcation, and calcified lesions, which were possibly unsuitable for drug-eluting stents. Thus, DCB angioplasty may be an available alternative even for complex lesions in de novo large arteries under lesion preparation using scoring or cutting balloons and atherectomy devices with intravascular ultrasound guidance.
Conclusions
In our cohort, the rate of cardiovascular outcomes after DCB angioplasty was comparable in de novo large and small coronary arteries. Particularly, DCB treatment in large vessels was characterized by well-planned lesion preparation with intravascular imaging guidance. Prospective studies with larger sample sizes are needed to further evaluate the effectiveness of DCB angioplasty in de novo large coronary arteries.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- CI:
-
Confidence interval
- DCB:
-
Drug-coated balloon
- EEM:
-
External elastic membrane
- IVUS:
-
Intravascular ultrasound
- MI:
-
Myocardial infarction
- MLA:
-
Minimal lumen area
- PCI:
-
Percutaneous coronary intervention
References
Yerasi C, Case BC, Forrestal BJ, Torguson R, Weintraub WS, Garcia-Garcia HM, Waksman R (2020) Drug-coated balloon for de novo coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 75:1061–1073
Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U (2006) Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med 355:2113–2124
Jeger RV, Farah A, Ohlow MA, Mangner N, Möbius-Winkler S, Leibundgut G, Weilenmann D, Wöhrle J, Richter S, Schreiber M, Mahfoud F, Linke A, Stephan FP, Mueller C, Rickenbacher P, Coslovsky M, Gilgen N, Osswald S, Kaiser C, Scheller B, BASKET-SMALL 2 Investigators (2018) Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet 392:849–856
Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, ESC Scientific Document Group (2019) 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 40:87–165
Jeger RV, Eccleshall S, Wan Ahmad WA, Ge J, Poerner TC, Shin ES, Alfonso F, Latib A, Ong PJ, Rissanen TT, Saucedo J, Scheller B, Kleber FX, International DCB Consensus Group (2020) Drug-coated balloons for coronary artery disease: third report of the international DCB consensus group. JACC Cardiovasc Interv 13:1391–1402
Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG (2001) American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 37:1478–1492
Suwannasom P, Sotomi Y, Ishibashi Y, Cavalcante R, Albuquerque FN, Macaya C, Ormiston JA, Hill J, Lang IM, Egred M, Fajadet J, Lesiak M, Tijssen JG, Wykrzykowska JJ, de Winter RJ, Chevalier B, Serruys PW, Onuma Y (2016) The impact of post-procedural asymmetry, expansion, and eccentricity of bioresorbable everolimus-eluting scaffold and metallic everolimus-eluting stent on clinical outcomes in the ABSORB II trial. JACC Cardiovasc Interv 9:1231–1242
Mintz GS, Garcia-Garcia HM, Nicholls SJ, Weissman NJ, Bruining N, Crowe T, Tardif JC, Serruys PW (2011) Clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound regression/progression studies. EuroIntervention 6:1123–1130
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction (2018) Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 72:2231–2264
Scheller B, Ohlow MA, Ewen S, Kische S, Rudolph TK, Clever YP, Wagner A, Richter S, El-Garhy M, Böhm M, Degenhardt R, Mahfoud F, Lauer B (2020) Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: the randomised PEPCAD NSTEMI trial. EuroIntervention 15:1527–1533
Rissanen TT, Uskela S, Eränen J, Mäntylä P, Olli A, Romppanen H, Siljander A, Pietilä M, Minkkinen MJ, Tervo J, Kärkkäinen JM, DEBUT trial investigators (2019) Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): a single-blind, randomised, non-inferiority trial. Lancet 394:230–239
Uskela S, Kärkkäinen JM, Eränen J, Siljander A, Mäntylä P, Mustonen J, Rissanen TT (2019) Percutaneous coronary intervention with drug-coated balloon-only strategy in stable coronary artery disease and in acute coronary syndromes: an all-comers registry study. Catheter Cardiovasc Interv 93:893–900
Funayama N, Muratsubaki S, Ito R, Tobisawa T, Konishi T (2023) Drug-coated balloons versus drug-eluting stents for coronary de novo lesions in dialysis patients. Heart Vessels 38:300–308
Okutsu M, Mitomo S, Ouchi T, Yuki H, Ueno T, Onish H, Yabushita H, Matsuoka S, Kawamoto H, Watanabe Y, Tanaka K, Naganuma T, Sato T, Tahara S, Kurita N, Nakamura S, Nakamura S (2022) Impact of directional coronary atherectomy followed by drug-coated balloon strategy to avoid the complex stenting for bifurcation lesions. Heart Vessels 37:919–930
Muramatsu T, Kozuma K, Tanabe K, Morino Y, Ako J, Nakamura S, Yamaji K, Kohsaka S, Amano T, Kobayashi Y, Ikari Y, Kadota K, Nakamura M, Task Force of the Japanese Association of Cardiovascular Intervention, Therapeutics (CVIT) (2023) Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and Therapeutics. Cardiovasc Interv Ther 38:166–176
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ueda, H., Fujiwara, Y., Nishida, Y. et al. Procedural characteristics and cardiovascular outcomes in patients undergoing drug-coated balloon angioplasty for de novo lesions in large coronary arteries: an observational study. Heart Vessels 39, 496–504 (2024). https://doi.org/10.1007/s00380-024-02368-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-024-02368-8