Abstract
Background
Patients with frequent premature ventricular contractions (PVCs) are often highly symptomatic with significantly reduced quality-of-life. We evaluated the outcome and success of PVC ablation in patients in the German Ablation Registry.
Methods
The German Ablation Registry is a nationwide prospective multicenter database of patients who underwent an ablation procedure, initiated by the “Stiftung Institut für Herzinfarktforschung” (IHF), Ludwigshafen, Germany. Data were acquired from March 2007 to May 2011. Patients underwent PVC ablation in the enrolling ablation centers.
Results
A total of 408 patients (age 53.5 ± 15 years, 55 % female) undergoing ablation for PVCs were included. 32 % of patients showed a co-existing structural heart disease. Acute ablation success of the procedure was 82 % in the overall patient group. In patients without structural heart disease, acute success was significantly higher compared with patients with structural heart disease (86 vs. 74 %, p = 0.002). All patients were discharged alive after a median of 3 days. No patient suffered an acute myocardial infarction, stroke, or major bleeding. After 12 months’ follow-up, 99 % of patients were still alive showing a significant different mortality between patients with structural heart disease compared with those without (2.3 vs. 0 %, p = 0.012). In addition, 76 % of patients showed significantly improved symptoms after 12 months of follow-up.
Conclusion
Based on the data from this registry, ablation of PVCs is a safe and efficient procedure with an excellent outcome and improved symptoms after 12 months.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Frequent premature ventricular contractions (PVCs) may lead to congestive heart failure and increased mortality [1]. Some smaller studies could demonstrate a significant improvement in left ventricular function (LVEF) after successful ablation of PVCs compared with patients without ablation [2, 3, 4]. In addition, significantly, a better improvement in PVC burden could be reached with ablation compared with antiarrhythmic drug therapy [5, 6]. In the EHRA/HRS Expert Consensus on catheter ablation of ventricular arrhythmia from 2009 [7], PVC ablation is mainly recommended in patients with highly symptomatic PVCs and reduced LVEF. Besides a reduced ejection fraction, frequent PVCs may be highly symptomatic leading to a significantly reduced quality-of-life. Consequently, many centers performed ablation of PVCs when patients were highly symptomatic and a significant amount of PVCs on Holter-ECG was found irrespective of LVEF. Until now, multicenter data evaluating ablation success, safety, and outcome are sparse.
We, therefore, evaluated the outcome and success of patients undergoing PVC ablation included in the German Ablation Registry.
Methods
Data and recruitment
The German Ablation Registry is a nationwide prospective multicenter database of patients who underwent an ablation procedure. Data were acquired from 40 German ablation centers from March 2007 to May 2011. Written informed consent was obtained from all patients and the registry was approved by the local ethic committees.
The “Stiftung Institut für Herzinfarktforschung (IHF, Ludwigshafen, Germany)” was responsible for project development, project management, data management, and clinical monitoring, and was the central contract research organization for the study. Documentation and data acquisition were voluntary, paperless, and were carried out on an Internet-based case report form system. All site information was confidential, and transmitted data were encrypted with a secure socket layer [8].
Patients underwent PVC ablation procedure in the enrolling ablation centers. If PVC burden during the procedure was low, orciprenaline was used to increase PVC occurrence. Necessary diagnostic workup to rule out structural heart disease was at the discretion of the enrolling hospital.
Demographic baseline characteristics, such as age, sex, cardiovascular co-morbidities, indication for ablation, procedural data, and complications, were gathered. All data were analyzed according to the presence or absence of structural heart disease.
Follow-up
Registry patients were contacted by IHF via telephone 1 year after their ablation procedure. The telephone interview was comprised of questions about major adverse events (MACE) (death and myocardial infarction during follow-up), severe non-fatal adverse events (myocardial infarction, stroke, and major bleeding), moderate non-fatal adverse events (syncope, TIA, systemic embolism, pulmonary embolism, deep vein thrombosis, and moderate bleeding), and ablation induced adverse events.
In addition, patients were asked if symptoms had improved and if they were satisfied with the ablation procedure. Repeat hospitalization, use of antiarrhythmic medication, recurrence of arrhythmia, and repeat ablation procedures were registered.
Statistical analysis
Categorical data are presented as percentages, metric data as median with 25th and 75th percentiles (interquartile range, IQR). The non-parametric Mann–Whitney test was used to compare continuous or ordinal variables between patient groups. For comparison of binary variables, the Chi-square test was used, or Fisher’s exact test in the case of infrequent events. One-year mortality and the cumulative incidence of MACCE (death/MI/stroke) were estimated by the Kaplan–Meier method and compared by the log-rank test. The statistics shown should be regarded as descriptive and were based on the available cases. We considered p values ≤0.05 statistically significant without adjustment for multiple testing. All p values were results of two-sided tests. Statistical analysis was performed at the biometrics department of the IHF using the SAS software release 9.3 (SAS Institute Inc., Cary, NC).
Results
Patients’ characteristics
A total of 408 patients underwent an ablation for PVCs and were included in the analysis. Patient characteristics are summarized in Table 1. The median age was 54 (43–67) years and 55 % of patients were female. Only 4 % of patients were older than 75 years. 32 % of patients had a co-existing structural heart disease, such as coronary artery disease (16 %), or cardiomyopathy (9 %). In this cohort, 80 % of patients had a normal LV function. In 13 %, LVEF was slightly reduced (41–50 %), in 4 % moderately reduced (31–40 %), and in 3 % severely reduced (<30 %). Patients with structural heart disease showed significantly more often reduced LV function compared with patients without structural heart disease (p < 0.001).
Patients’ symptoms
Before the procedure, 91 % of patients presented with palpitations; 9 % suffered from presyncope, and 5 % from syncope. In the patient group with structural heart disease 12 % of patients presented with heart failure symptoms NYHA III and IV. There were no significant differences in patients who had a structural heart disease compared with patients without regarding syncope or presyncope. No patient underwent previous cardiac resuscitation. 92 % of patients were symptomatic at least once a month, and in 70 % of patients, previous medical therapy was inefficient. Failed attempts of antiarrhythmic medical therapy were higher in patients with structural heart disease compared with patients without (77 vs. 66 %, p = 0.028) (Table 2).
Ablation procedure: Localization of PVCs in patients with structural heart disease compared with patients without structural heart disease
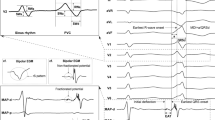
All patients underwent their first ablation procedure, because of PVCs. The prevalence of structural heart disease differed significantly across locations of PVC (p < 0.0001). In the majority (64 %) of all patients, the right ventricular outflow tract (RVOT) was the origin of the PVCs. This location was associated with the lowest prevalence of structural heart disease (25 %). The second group of patients (17 %) had PVCs originating from the left ventricle (LV); however, here was a higher rate of patients with structural heart disease (59 %) than patients without (41 %). The third group of patients (12 %) had PVCs from the left ventricular outflow tract (LVOT); again, this location was relatively frequent in the group with structural heart disease. The aorta was the origin in 7 % of patients and the right ventricle (RV) was the origin in 9 % of patients, both without a significant excess of patients with structural heart disease or patients without. In 35 % of patients, a provocation with orciprenaline was necessary (Table 3; Figs. 1, 2).
Ablation procedure
In 64 % of patients, no 3D mapping system was used, and there was no difference between patients with structural heart disease and without (p = 0.63). Of the 3D systems used, 90 % were Carto (Biosense Webster, Diamant Bar, USA), and 10 % Ensite Navx (St. Jude Medical, Saint Paul, USA). 94.6 % of procedures were performed manually, 4.8 % using the magnetic Stereotaxis system (Stereotaxis, St. Louis, USA), and 0.6 % using the Hansen robotic system (Hansen Medical, Mountain View, USA). The procedure was performed without sedation in 31 % of patients; in 67 %, analgo-sedation was used. General anesthesia was not used. In 99.5 % of patients, radiofrequency was used as the ablation method. In 86 %, a 4 mm tip catheter was used, and in 5 %, an 8 mm tip catheter was used. An irrigated tip was used in 37 % of patients compared with a non-irrigated tip in 63 % of patients. The procedural results did not differ between patients with and without structural heart disease.
Ablation procedure: acute outcome
Acute success of the procedure was 82 % in all patients and localizations. In patients without structural heart disease, acute success was significantly higher (86 % compared to 74 %, p = 0.002). When comparing the different localizations, acute success showed no significant difference (p = 0.35). (For procedural data, see Table 4).
The majority of patients had a RVOT origin of the PVCs. These patients were younger [50 (41–64) years vs. 59 (48–70) years, p < 0.001], more often female (61 vs. 47 %, p = 0.005), and suffered less often from structural heart disease (24 vs. 45 %, p < 0.001). Acute success was higher in patients with RVOT origin than in patients with other origin (86 vs. 79 %, p = 0.052).
Ablation procedure: adverse events
All patients could be discharged alive after a median of 3 days. Patients with structural heart disease had longer hospital stay compared with patients without structural heart disease [median 3 (2–6) vs. 2 (2–4) days, p = 0.039]. No patient suffered an acute myocardial infarction, stroke, or major bleeding. Moderate adverse events occurred in 2.1 % of patients (1.2 % aneurysm spuria of the groin, 0.5 % of significant pericardial effusion, 0.3 % of pneumothorax). Minor adverse events were reported in 1.0 % of patients (1.0 % minor bleeding) (Table 5). No other complications have been recorded. Patients who experienced an adverse event had significantly longer hospital stay compared with patients without adverse event [median stay 4 (3–11) vs. 2 (2–4) days, p < 0.001).
Follow-up
Follow-up data were obtained from 403 out of 408 patients (99 %). Four patients died during follow-up of 596 days, three with structural heart disease and one without; of these deaths, two were sudden, one was non-cardiac, and one was unknown. After a follow-up of 12 months, a significant difference in mortality between patients with structural heart disease compared with without was detected (2.3 vs. 0 %, p = 0.012, Fig. 3). MACCE (death, myocardial infarction, stroke) occurred in 1.0 % of patients, 2.3 % with structural heart disease and 0.4 % without (p = 0.065). (For more information, see Table 6).
Severe non-fatal adverse events (myocardial infarction, stroke, and major bleeding) occurred in 0.8 % with no significant differences between both groups. Moderate adverse events (syncope, TIA, systemic embolism, pulmonary embolism, deep vein thrombosis, CPR, moderate bleeding, groin complications, and revascularization) occurred in 7.3 % with no significant difference between both groups (p = 0.13). In patients with RVOT origin compared with patients without RVOT origin, no significant difference could be noted.
After a follow-up of 12 months, 27 % of patients reported a symptomatic relapse of arrhythmia, in 24 % the arrhythmia could be documented by their treating physician; there was no significant difference between patients with structural heart disease and without (p = 0.26). Patients with RVOT origin had a significant better outcome with 21 % documented relapses compared to 31 % of patients without RVOT origin (p = 0.027). During follow-up 7.7 % of patients underwent repeat ablation procedure; 8.1 % of patients with structural heart disease and 7.5 % of patients without (p = 0.83).
Patients who initially had successful ablation procedure reported relapse of documented arrhythmia in 23 % showing no difference between patient with and without structural heart disease. In addition, the rate of repeat ablation procedure was with 6.8 % comparable in the entire cohort.
After 12 months, 15.5 % of patients took antiarrhythmic medication: 6.8 % took class I antiarrhythmic medication, and 5.7 % class III. There was no significant difference between patients with structural heart disease and without regarding overall antiarrhythmic medication intake; however, use of class III antiarrhythmic medication was significantly more often in patients with structural heart disease (p < 0.001). Patients with RVOT origin used significantly less antiarrhythmic medication compared with patients without RVOT origin (p = 0.007). 31.1 % of patients were hospitalized during follow-up; patients with structural heart disease were hospitalized significantly more often compared with patients without (41.7 vs. 26.0 %, p = 0.002) (Table 6).
Discussion
Main results
408 patients who underwent ablation for premature ventricular contractions (PVC) were included in the German Ablation Registry. Patients were divided into two subgroups: one group with structural heart disease (131 patients, 32 %) and another group without structural heart disease (277 patients, 68 %). In the group without structural heart disease, the main localization of PVCs was in the right ventricular outflow tract (RVOT, 70 %); other localizations like the LVOT/LV/RV/Aorta occurred in less than 10 %. In the group with structural heart disease, RVOT was again the main localization (50 %); however, compared with patients without structural heart disease, a significantly higher proportion of PVCs was located in the LV and LVOT (29 and 17 %, p < 0.0001, p < 0.05). The acute success of the procedure was significantly higher in patients without structural heart disease (86 vs. 74 %, p = 0.002). After a follow-up of 12 months, 99 % of patients were still alive and 76 % had significantly improved symptoms. Myocardial infarction, stroke, and major bleeding during follow-up occurred in a minority of patients.
Localization of PVCs in patients with and without structural heart disease
Patients with and without structural heart disease showed different localizations of PVCs. Patients with RVOT origin only had structural heart disease in a minority of cases (25 %), whereas this percentage was far higher (49 %) in patients with LVOT origin. As expected, patients with PVC origin in the left ventricle showed a high prevalence of structural heart disease (59 %).
There is only limited data on this subject so far. In accordance with our data, Penela et al. demonstrated that patients with left-sided PVCs had a higher prevalence of structural heart disease compared with patients with right-sided PVCs [2]. In another retrospective study by Latchamsetty et al., patients with RVOT origin showed significant higher rates of success compared with patients without RVOT origin (p < 0.01) [9]. In our cohort, similar results towards a higher success in patients with RVOT origin were seen (p = 0.027). In addition, patients without structural heart disease in our cohort showed significantly higher success rates compared with patients with structural heart disease (p = 0.002). In conclusion, patients with RVOT PVC compared with LVOT PVC location seem to represent different patient subgroups.
Safety of the procedure
In this registry, a low rate of complications was observed. In summary, a total of 100 % of patients could be discharged alive after a median of 3 days. No patient suffered an acute myocardial infarction, stroke, or major bleeding. Moderate adverse events occurred in 2.1 % of patients and minor adverse events occurred in 1.0 % of patients. Up until now, one prospective randomized trial comparing RVOT ablation with antiarrhythmic medication is available; in accordance with our data, adverse events were low (1.8 %, [5]). In a retrospective multicenter study by Latchamsetty et al., including 1185 patients, adverse events were low with 5.2 %, including 2.4 % with serious adverse events [9]. In a metaanalysis of 712 patients by Zang et al. [10], most of the included studies had very low complication rates ranging between 0 and 3 %. In another study, including only patients with PVCs from LVOT or aortic cusp, complication rate was also very low with only two occurring groin hematomas [11].
Success after ablation
Acute success of the procedure was 82 % in all patients and localizations; in patients without structural heart disease, acute success was significantly higher (86 vs. 74 %, p = 0.002) compared with patients with structural heart disease. After a follow-up of 12 months, 99 % of patients were still alive and MACE rates were very low, highlighting the benign nature of PVCs in this cohort. In addition, 76 % of patients reported improved symptoms. Up until now, only one prospective randomized trial comparing PVC ablation to antiarrhythmic medication is available [5]. In this study, 80 % of patients in the ablation arm and 11 % in the antiarrhythmic medication arm showed no relevant PVC burden on Holter-ECG after 12 months of follow-up. In a multicenter retrospective study by Latchamsetty et al., including 1185 patients, acute success was similar with 84 %; after 12 months, 71 % of patients reported a significant improvement in symptoms or had no relevant PVC burden on Holter-ECG off antiarrhythmic medication [9]. In an additional retrospective study [12], a similar acute success rate of 76 % could be achieved; after a follow-up of 12 months 13 % of patients needed a repeat ablation procedure. This is in line with our data, where 8 % of patients underwent repeat ablation procedure within 12 months after the index procedure. In another study by Penela et al., acute success was 85 % and success rate after 12 months of follow-up was 66 % [2]. In a metaanalysis, success rates ranged between 66 and 80 % [10].
Ablation of PVCs also in patients with preserved ejection fraction?
In the EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias from 2009, which was the effective consensus at the time of data acquisition, ablation of PVCs was mainly recommended in patients with reduced ejection fraction [7]. However, in highly symptomatic patients with preserved ejection fraction, a small trial could show a significant improvement in quality-of-life questionnaire SF 36 after ablation of PVCs [13]. The other treatment option is antiarrhythmic medication. Therefore, Ling et al. [5] conducted a prospective randomized trial comparing catheter ablation with antiarrhythmic medication. After 12 months, 80 % in the ablation arm and 11 % in the antiarrhythmic medication arm showed no significant burden of PVC on Holter-ECG. Zhong et al. [6] did a retrospective analysis of 510 patients with frequent PVCs; of which, 215 patients underwent ablation and 295 received antiarrhythmic medication. In the follow-up, a significant greater reduction of PVCs on Holter-ECG was achievable with catheter ablation compared with antiarrhythmic medication (−21 000 vs. −8000 PVCs/24 h p < 0.0001). In addition, patients with reduced ejection fraction showed a significant improvement in LVEF only after ablation. The data on this subject are limited. However, antiarrhythmic medication has many side effects and antiarrhythmic class Ic medication can lead to increased mortality, especially in patients with structural heart disease [14]. In addition, in young patients without structural heart disease, a long-term antiarrhythmic therapy is badly accepted. Therefore, in the new 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death [15], ablation is recommended in patients with structural heart disease and frequent symptomatic PVCs with or without LV dysfunction. In addition, in patients with outflow tract PVCs, ablation is now recommended with symptomatic PVCs also without LV dysfunction.
Limitations
No data on the number of PVCs on Holter-ECG before and after the ablation procedure were available; however, the main goal of the ablation procedure is symptom improvement. Acute success was not previously defined but individually defined by the operator. Participation in the registry was voluntary and dependent on informed consent of the patients, so peri-procedural complications might be underrepresented. Patients were supposed to be consecutively included in the registry; however, 50 % of centers only included less than 10 patients. Heart failure symptoms were only recorded in patients with structural heart disease. After 12 months, only data through telephonic interview were available; therefore, no data on PVC burden and no data on echocardiographic assessment of left ventricular function are available. In addition, patients were asked if arrhythmia relapse was documented by ECG, this ECG documentation were not reviewed by the study team. Success after 12 months was gathered through patient’s interview. Patients were asked if an ECG documentation of arrhythmia relapse was available, but these ECG documentation were not evaluated through IHF, and therefore, success rate after 12 months relies solely on patients understanding.
Conclusion
Our data have shown that the ablation of PVCs is a safe and efficient procedure with an excellent outcome and improved symptoms after 12 months. In the future, it seems appropriate to perform an ablation procedure also in patients who are highly symptomatic with a preserved ejection fraction.
References
Dukes JW, Dewland TA, Vittinghoff E et al (2015) Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol 66:101–109. doi:10.1016/j.jacc.2015.04.062
Penela D, Taxis CV, Aguinaga L et al (2013) Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: a prospective multicenter study. J Am Coll Cardiol 62:1195–1202. doi:10.1016/j.jacc.2013.06.012
Yokokawa M, Good E, Crawford T et al (2013) Recovery from left ventricular dysfunction after ablation of frequent premature ventricular complexes. Heart Rhythm 10:172–175. doi:10.1016/j.hrthm.2012.10.011
Bogun F, Crawford T, Reich S et al (2007) Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm 4:863–867. doi:10.1016/j.hrthm.2007.03.003
Ling Z, Liu Z, Su L et al (2014) Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythmia Electrophysiol 7:237–243. doi:10.1161/CIRCEP.113.000805
Zhong L, Lee YH, Huang XM et al (2014) Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm 11:187–193. doi:10.1016/j.hrthm.2013.10.033
Aliot EM, Stevenson WG, Almendral-Garrote JM et al (2009) EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); i. Heart Rhythm 6:886–933. doi:10.1016/j.hrthm.2009.04.030
Chun KR, Schmidt B, Kuck KH et al (2013) Catheter ablation of atrial fibrillation in the young: insights from the German Ablation Registry. Clin Res Cardiol 102:459–468. doi:10.1007/s00392-013-0553-6
Latchamsetty R, Yokokawa M, Morady F et al (2015) Multicenter outcomes for catheter ablation of idiopathic premature ventricular complexes. JACCCEP 1:116–123. doi:10.1016/j.jacep.2015.04.005
Zang M, Zhang T, Mao J et al (2014) Beneficial effects of catheter ablation of frequent premature ventricular complexes on left ventricular function. Heart 100:787–793. doi:10.1136/heartjnl-2013-305175
Kamioka M, Mathew S, Lin T et al (2015) Electrophysiological and electrocardiographic predictors of ventricular arrhythmias originating from the left ventricular outflow tract within and below the coronary sinus cusps. Clin Res Cardiol 104:544–554. doi:10.1007/s00392-015-0817-4
Baser K, Bas HD, Yokokawa M et al (2014) Infrequent intraprocedural premature ventricular complexes: implications for ablation outcome. J Cardiovasc Electrophysiol 25:1088–1092. doi:10.1111/jce.12454
Pytkowski M, Maciag A, Jankowska A et al (2012) Quality of life improvement after radiofrequency catheter ablation of outflow tract ventricular arrhythmias in patients with structurally normal heart. Acta Cardiol 67:153–159
Echt DS, Liebson PR, Mitchell LB et al (1991) Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. doi:10.1056/NEJM199103213241201
Priori SG, BlomstroLundqvist C, Mazzanti A, Bloma N, Borggrefe M, Camm J et al (2015) 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. doi:10.1093/europace/eul108
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest for all authors.
Rights and permissions
About this article
Cite this article
Fichtner, S., Senges, J., Hochadel, M. et al. Safety and efficacy in ablation of premature ventricular contraction: data from the German ablation registry. Clin Res Cardiol 106, 49–57 (2017). https://doi.org/10.1007/s00392-016-1022-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1022-9