Abstract
Aims
Ventricular arrhythmias (VAs) from the left ventricular outflow tract (LVOT) can originate from within or below the aortic sinus of valsalva (ASV). Mapping and ablation below the ASV is challenging and there are limited data predicting VA origins using electrocardiographic and electrophysiological features.
Methods
Thirty-four patients (56.7 ± 15.2 years; 19 males) with symptomatic VAs were analyzed. VA origins were determined by successful ablation. Patients were classified into 2 groups (group 1, VAs within the ASV; group 2, VAs below the ASV). Local activation and QRS morphology were compared between these 2 groups.
Results
Twelve patients were classified as group 1 and 22 as group 2. Presystolic potentials (PPs) during VAs were present in 11 patients (91 %) in group 1 and 3 (13 %) in group 2. S-wave amplitude and duration in lead I were lower and shorter in group 1 vs. group 2, respectively. Q-wave aVL/aVR ratio (Q-aVL/aVR) was smaller in group 1 vs. group 2. No group 1 patients had Q-aVL/aVR >1.45. PPs in the ASV was the strongest independent predictor for VAs originating within the ASV (OR: 30.003, P = 0.006).
Conclusion
Deeper and longer S-waves in lead I and Q-aVL/aVR >1.45 suggest VAs originating below the ASV. Local PPs strongly suggest an origin within the ASV. ECG characteristics combined with local PPs can be a practical guide for ablating LVOT-VAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventricular arrhythmias (VAs) including ventricular tachycardia (VT) and frequent premature ventricular complexes (PVC) can originate from the left ventricular outflow tract (LVOT) [1–4]. These can originate from the aortic sinus of valsalva (ASV), as well as the LVOT below the ASV such as the aortomitral continuity, superior basal septum, left ventricular (LV) summit and epicardial myocardium [5–10]. VAs originating from these locations are amenable to catheter ablation [11–13]; however, mapping and ablation below the ASV is still challenging via both trans-aortic and trans-septal approaches [14]. LVOT-VAs are generally classified anatomically as originating within or below the ASV. In the present study, we analyzed electrophysiological and electrocardiographic features of LVOT-VAs originating within and below the ASV to determine predictors of localization.
Methods
Study subjects
Between June 2011 and September 2013, 121 consecutive patients referred to our center for VAs ablation of the right or left ventricular outflow tract were assessed (Fig. 1). Ablation was not performed due to the earliest ventricular activation site being located near the left-sided His in 1 patient, and due to no spontaneous or provoked clinical VAs during the procedures in 2 patients. Therefore, ablation success rate was in 115 of the remaining 118 patients (97.5 %) (Fig. 1). The successful site of catheter ablation was located in the right ventricular outflow tract (RVOT) in 65, the LVOT in 50 patients. In the 50 patients with LVOT origins, 9 patients were excluded due to the VA origin located at the aortomitral continuity in 4, near the left His bundle in 2 and epicardially in 3 patients. Additionally, pathologic substrate with low amplitude and fractionated potentials in the LVOT was identified in 7 patients with sustained macro-reentrant VT [large anterior myocardial infarction = 4; idiopathic dilated cardiomyopathy (DCM) = 3]. These 7 patients were excluded from this study due to macro-reentrant tachycardia. Therefore, 34 patients (56.7 ± 15.2 years; 19 male) with symptomatic LVOT-VAs (PVC = 28, non-sustained VT = 6) were included in this study. Clinical characteristics are shown in Table 1. Patients were classified into 2 groups based only on the anatomical location of VAs. Group 1 was defined as patients with VAs originating within the ASV and group 2 with VAs below the ASV. Previous Failed ablation was attempted in 3 patients in group 1 and 4 patients in group 2 in other centers. All patients were highly symptomatic (palpitation: 34/34 patients, dyspnea with motion: 5/34, presyncope: 2/34). Additionally, the majority of patients were refractory to oral ß-blocker (Table 1).
Electrophysiological study
The study was approved by our institutional review committee on human research and all patients provided written informed consent prior to the procedure. Antiarrhythmic drugs (AADs) were withheld for at least five half-lives. Procedures were performed while patients were awake or sedated with intravenous propofol and midazolam. Catheters were positioned in the right ventricle via femoral veins and advanced into the great cardiac vein (GCV) from the coronary sinus (CS) via left subclavian or femoral veins. Twelve-lead surface ECGs and intracardiac electrograms were recorded simultaneously by a digital multichannel system (EPMed, SJM), filtered at 30–400 Hz for bipolar and 0.05–400 Hz for unipolar electrograms. If clinical arrhythmias did not occur spontaneously, programmed stimulation was performed. The standard protocol consisted of ventricular stimulation at two basic drive cycle lengths with up to two extrastimuli, to a minimal coupling interval of 230 ms. If VAs were not inducible, intravenous isoproterenol infusion (2–5 µg/min) was administered to provoke clinical arrhythmias.
Mapping and radiofrequency ablation
3-D electroanatomical mapping of the RVOT and GCV via the CS was initially performed, then the aortic root. LV access was achieved via a trans-aortic approach and/or a trans-septal approach [14]. Point-by-point mapping was performed using a steerable 7.5 French (Fr), D-curve catheter with a 3.5-mm irrigated-tip electrode (Navi-Star ThermoCool, Biosense Webster, Diamond Bar, CA, USA). The site of earliest ventricular activation was annotated on the 3-D mapping system. Aortic root angiography was performed either with a 5 Fr pigtail catheter or the irrigated ablation catheter to confirm the catheter’s anatomical location. No intracardiac echocardiography was used in this study. Pacemapping using the lowest pacing output (2–20 mA) and pulse width (0.5–10 ms) to capture the ventricular myocardium was performed at the site of earliest ventricular activation before ablation. During the procedure, unfractionated heparin was administered to maintain an activated clotting time between 250 and 300 s.
If ablation of VAs was performed within the ASV, the irrigated catheter was switched to non-irrigated RF mode without flow rate. RF energy was used starting at 20 Watts (W) and titrated to a maximal of 30 W and temperature limit of 55 °C. If VAs terminated during the first 10 s of application, RF energy was maintained for 120 s. When VAs were performed below the ASV, irrigated RF energy was delivered with irrigation at 20 ml/min, maximum power of 40 W and temperature limit of 43 °C. An extra RF application, applying the same RF settings, was deployed in all VAs below the ASV. After successful ablation, intravenous isoproterenol administration and programmed stimulation were performed to provoke clinical VAs.
Electrocardiographic analysis
Detailed analysis of clinical VAs was performed offline using either the EPMed System (SJM) with a recording speed of 50–100 mm/sec, or 12-lead ECGs at 25–50 mm/sec.
The following parameters of clinical PVCs or the first beat of VT were analyzed:
-
1.
QRS duration, precordial lead transition zone, presence of S-wave in V6
-
2.
Amplitude and duration of R-waves and S-waves in each lead, duration from onset to peak of each QRS
-
3.
Ratio in lead aVL and aVR Q-wave amplitudes (Q-aVL/aVR), ratio in lead III and II R-wave amplitudes (R-III/II)
-
4.
V1 and V2 R/S-wave amplitude ratios, R/S-wave amplitude index
-
5.
R-wave duration index (QRS duration/the longer R-wave duration in lead V1 or V2)
-
6.
Maximum deflection index (MDI) (duration from QRS onset to maximum positive or negative deflection of any precordial lead/QRS duration). A MDI > 0.55 was used as a cut-off value [15]
-
7.
Peak deflection index (PDI) (in the inferior lead with the tallest R-wave: Time from QRS onset to peak deflection/QRS duration) [16]. A PDI > 0.6 was used as a cut-off value.
All measurements were performed by two investigators blinded to the site of VA origin; discrepancies were adjudicated by a third investigator.
Follow-up
Ablation success was defined as; (1) absence of spontaneous or provoked clinical VAs at the end of the procedure, and (2) absence of VAs on 48-h ECG monitoring post-ablation off AADs. VA burden was documented on 24-h holter monitoring before and after ablations. All patients were followed up at 1, 3, 6, 9 and 12 months after the ablation procedure.
Statistical analysis
Continuous data were analyzed using independent-samples t test and presented as mean ± standard deviation (SD). A Chi-square test was used to compare dichotomous data, presented as numbers and percentages. Differences between two groups were detected by Wilcoxon rank-sum test with two-tailed P values <0.05. Multivariate logistic regression analysis was used to investigate the association between covariates and the incidence of VAs originating within the ASV. Variables with statistical significance (P < 0.05) in univariate analyses were entered into the multivariate analysis to obtain the final model. Receiver-operating characteristic (ROC) analysis was performed to calculate sensitivity, specificity, area under the ROC curve and the optimal cut-off with 95 % confidence limits. All analyses were performed with SPSS for Windows, version 17.0 (SPSS Inc., Chicago, Illinois). All statistical tests were 2-sided. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Clinical characteristics are shown in Table 1. There was a trend toward group 2 for structural heart disease [8/22 (36 %) vs. 2/10 (16 %), P > 0.05] and non-sustained VT (NSVT) [5/22 (23 %) vs. 1/12 (8 %), P > 0.05]. NSVT occurred mainly in patients with ischemic heart disease and DCM. LV ejection fraction (LVEF) tended to be lower in group 2 compared to group 1 (49.8 ± 11.8 vs. 55.6 ± 7.4 %, P > 0.05). 4/12 patients (33 %) in group 1 and 8/22 (36 %) in group 2 had undergone previous failed procedures.
Mapping and ablation
Electrophysiological study was performed under sedation in 19 patients and in 13 patients who were awake. In the 19 sedated patients, VAs were completely suppressed in 4 and intravenous isoproterenol was required to induce clinical VAs. Clinical VAs were not inducible by programmed stimulation.
In group 1, mapping and ablation was performed only via the trans-aortic approach in all 12 patients. In group 2, the procedure was performed only via the trans-aortic approach in 4 patients (before March 2012) and via a combined trans-aortic and trans-septal approach in the other 18/22 patients. In group 2, ablation was successfully achieved via the trans-aortic approach in 4 and via the trans-septal approach in the remaining 18 patients.
The anatomical location of VA origins was identified within the ASV in 12 patients and below the ASV in 22 patients. There was no difference in the activation time preceding the QRS (group 1; 36.4 ± 6.9 ms vs. group 2; 39.5 ± 15.7 ms, P > 0.05). VA origins in group 1 were the right coronary cusp (RCC) in 3, left coronary cusp (LCC) in 5, and LCC/RCC junction in 4; anatomical locations in group 2 were below the RCC in 6, below the LCC in 9, and below the LCC/RCC junction in 7 patients. The distance from the VA origin to the closest ASV was 4.4 ± 2.6 mm.
In group 1, a presystolic potential (PP) was seen preceding the QRS on bipolar recordings in 11 patients (Fig. 2a, b). No PP was identified in one patient, who had VAs originating from the RCC. Pacemapping was attempted in 11 patients, 10 of these 11 patients had paced QRS morphologies similar to the clinical VAs. A paced QRS morphology identical to clinical VA QRS morphology was not found in any patient. All VAs were successfully eliminated with 3.3 ± 2.9 RF applications. The time to VA disappearance during the successful RF application was 6.3 ± 3.0 s.
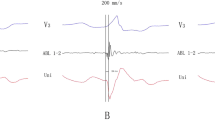
a Left upper panel an aortogram in RAO 30°. Right upper panel the ablation catheter location in RAO 30°. The catheter tip is located in the left coronary cusp (LCC). A brown and pink tag demonstrates the successful ablation site and the left main coronary artery, respectively. b A local potential from the LCC in group 1. The first beat is sinus rhythm (SR) with a small atrial potential followed by a ventricular potential, then a short duration of fractionation. The third beat is a PVC. The fractionated potential recorded in SR is reversed and precedes the ventricular potential (presystolic potential) by 37 ms
In group 2, complete mapping of the aortic root was achieved in all 22 patients. PPs were seen in the ASV in only 3 patients (Fig. 3), in whom the earliest activation in the ASV preceded the QRS by 32.0 ± 16.7 ms (range 22–47 ms). In these 3 patients, RF delivery in the ASV failed to eliminate the clinical VAs. Mapping below the ASV demonstrated an earlier ventricular activation, preceding the QRS by 53.0 ± 15.1 ms (range 35–72 ms). The site with earliest ventricular activation below the ASV was successfully reached via the trans-aortic approach in 4 and trans-septal approach in 18 patients (Fig. 4a–c). Pacemapping was successfully achieved in all patients at the site of earliest local ventricular activation; however, the QRS morphology during pacemapping did not match the clinical VAs in any patient from group 2. All VAs were successfully ablated with 5.9 ± 3.7 irrigated RF applications. The time to VA disappearance during the successful RF application was 8.6 ± 5.8 s.
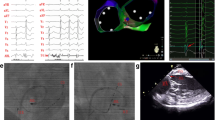
a Catheter location of successful ablation below the LCC/RCC junction in RAO 30° (top panel) and LAO 50° (bottom panel). A trans-septal approach was used to access the anatomical location. b Earliest local activation in patients with VAs originating from below the LCC/RCC junction. An early ventricular activation potential preceding the QRS onset by 35 ms without a presystolic potential is represented. c 3-D electroanatomical map in a patient with VA origin arising from the LCC/RCC junction. Blue point the successful ablation site
Ventricular arrhythmias with different morphologies (non-clinical VAs) were infrequently observed in one patient from group 1 and 4 patients from group 2. Non-clinical VAs were not targeted for ablation.
QRS analysis during clinical VAs on surface ECG
ECG parameters are shown in Table 2. Twenty-nine patients had VAs with left bundle branch block (LBBB) morphology and 5 with right bundle branch block (RBBB) morphology. In clinical VAs with RBBB, 2 originated from within the ASV and 3 below the ASV. S-waves in V5–6 on surface ECGs were seen in only one patient, in whom a small S-wave in V6 was documented only on the EP recording system, and the VA origin was located superolateral to the LCC. There are no significant differences in the QRS duration, R-wave amplitudes in lead I and the inferior leads, duration of QRS onset to peak of R-wave or nadir of S-wave in all leads, R- or S-wave duration in the inferior leads, lead V2 and V3, the R-wave duration in the inferior leads, lead V2 and V3, the R/S amplitude index and V2 R-wave duration index between group 1 and 2. There were statistically significant differences between the two groups in the S-wave depth and duration and R-wave duration in lead I, S-wave amplitude in aVL, R-wave amplitude, duration and R-wave duration index in V1, R-III/II and Q-aVL/aVR (Table 2). Of note, no patient in group 1 had a Q-aVL/aVR >1.45, however 11 patients in group 2 had a Q-aVL/aVR >1.45.
In addition, ROC curve analysis of all parameters was performed to distinguish VAs originating within ASV from that below the ASV. S-wave duration in lead I was excluded, because of no statistically significant difference during ROC curve calculations. The cut-off value of S-wave amplitude in lead I (S in I) was 0.19 mV (68.2 % sensitivity, 83.3 % specificity), of the R-wave duration in lead V1 (Rd in V1) was 66 ms (72.7 % sensitivity, 66.7 % specificity), of the Q-aVL/aVR was 1.35 (50.0 % sensitivity, 99.2 % specificity), of the R-III/II was 0.93 (86.4 % sensitivity, 66.7 % specificity), of the S-wave amplitude in lead aVL (S in aVL) was 0.61 mV (90.9 % sensitivity, 66.7 % specificity), of the R-wave amplitude in lead V1 (R in V1) was 0.18 mV (77.3 % sensitivity, 75.0 % specificity), of the R-wave duration in lead I (Rd in I) was 100 ms (66.7 % sensitivity, 81.8 % specificity) and of the R-wave duration index in lead V1 (Rd index in V1) was 0.37 (77.3 % sensitivity, 67.7 % specificity) (Fig. 5a–h).
ROC curve analysis for S-wave amplitude in I (S in I: a), R-wave duration in V1 (Rd in V1: b), aVL/aVR Q-wave amplitude ratio (Q-aVL/aVR: c), III/II R-wave amplitude ratio (R-III/II: d), S-wave amplitude in aVL (S in aVL: e), R-wave amplitude in V1 (R in V1: f), R-wave duration in I (Rd in I: g) and R-duration index in V1 (Rd ind of V1: h). The Area under the curve (AUC) for S in I was 0.807 (0.670–0.933, P = 0.002); Rd in V1, 0.748 (0.581–0.915, P = 0.011); Q-aVL/aVR, 0.73 (0.556–0.906, P = 0.028); R-III/II, 0.767 (0.619–0.914, P = 0.006); S in aVL 0.746 (0.585–0.906, P = 0.014); R in V1 0.765 (0.607–0.924, P = 0.007); Rd in I 0.711 (0.526–0.896, P = 0.031); Rd index in V1 0.735 (0.555–0.914, P = 0.016)
Predictors for successful ablation site within or below the valve
In univariate analysis, S in I, S in aVL, R in V1, Rd in 1, Sd in I, Rd in V1, Rd index in V1, R-III/II, Q-aVL/aVR and PPs within the ASV were associated with predicting VAs originating within the ASV using the unadjusted odds ratio (OR) (Table 3). Multivariate analysis was performed using covariates that had significance in univariate analysis. After adjustment of covariates, the existence of PPs within the ASV remained an independent predictor (OR: 30.003, 95 % confidence interval (CI): 2.638–341.245, P = 0.006). This indicates that the absence of PPs at the earliest site in the ASV strongly predicts VAs originating below the ASV.
Clinical outcome
Procedure duration was 136.8 ± 55.4 min in group 1 vs. 154.2 ± 73.9 min in group 2 (P > 0.05) with fluoroscopic time of 11.3 ± 2.5 min in group 1 vs. 16.2 ± 2.7 min in group 2 (P > 0.05). Procedure-associated hematomas not requiring surgical intervention occurred in one patient from group 1 and one from group 2. During a mean follow-up of 10.1 ± 5.2 months, no patient had clinical VA recurrence and VA burden was much reduced from 20,327 ± 7,015/24-h before ablation to 547 ± 804/24-h 3 months after ablation.
Discussion
The current study in patients with left ventricular outflow tract-VAs describes: (1) Electrophysiological findings during mapping at the aortic sinus of valsalva, (2) ECG QRS morphology differences in VAs within and below the aortic sinus of valsalva, and (3) Predictors to identify the successful ablation site within or below the aortic sinus of valsalva.
Electrophysiological findings during mapping at the aortic root
Ventricular arrhythmias frequently occur from the aortic root and the left ventricular endocardial and epicardial myocardium adjacent to the GCV and left anterior descending artery (LAD), near the mitral anulus (MA) [1, 4–9]. In VAs within the aortic sinus of valsalva, a low-amplitude, high-frequency presystolic potential can be found in the majority of patients [1, 4, 5, 17]. However, there was limited information about mapping below the aortic sinus of valsalva. We demonstrated a PP in 12/13 patients with VAs within the aortic sinus of valsalva and in only 3/22 with VAs below the aortic sinus of valsalva. This significant difference can provide important information during ablation of left ventricular outflow tract-VAs. The PPs in the aortic sinus of valsalva can be explained by slow conduction areas with preferential conduction between the ventricle and left aortic sinus of valsalva [1, 17], which can produce a different QRS morphology on surface ECG. The existence of preferential conduction near the left ventricular outflow tract with slow conduction may explain why a perfect pacemap match to the clinical VA QRS morphology is very difficult to achieve in clinical practice [8, 17].
QRS morphology during VAs on Surface ECG
In our study, VAs predominantly had LBBB morphology (LBBB = 29, RBBB = 5), in line with previous studies [18, 19]. A V6 S-wave indicates an origin close to the aortomitral continuity, but not in the LCC or left ventricular summit, which was consistent with our finding where only one patient had a tiny S-wave in V6 [18]. Hachiya et al. studied the electrocardiographic characteristics of left ventricular outflow tract-VT originating from the supra-valvular region of the coronary cusps and infra-valvular endocardial region of the aortic sinus of valsalva within the left ventricle of 17 patients. They showed that a S-wave in lead I and V6, and a taller R-wave in V1 or V2 suggest VAs originating below the valve [19]. Abularach et al. [8] recently concluded that a Q-aVL/aVR <1.45 can be used as a predictor for successful ablation either from the aortic sinus of valsalva or the endocardial left ventricle.
Using quantitative ECG analysis, we found that VAs originating from below the aortic sinus of valsalva had deeper S-wave amplitudes in lead I and aVL and taller R-wave amplitudes in lead V1 compared to that within the aortic sinus of valsalva. An arrhythmia origin below the aortic sinus of valsalva produces a slightly different vector due to the anatomical location being more anterosuperior and leftward. In this study, the mean distance from the VA origin to the closest aortic sinus of valsalva valve in group 2 was 4.4 ± 2.6 mm (Fig. 4c). Furthermore, using the ROC curves and cut-off values, we found a significant difference between the 2 groups. However, this difference was very small, with large overlap, and may not be clinically practical (Fig. 5a–h). Previous studies have demonstrated that a Q-aVL/aVR >1.4 or 1.45 indicated that the VA origin was far from left aortic sinus of valsalva leading to ablation failure within the aortic sinus of valsalva [20]. In the present study, A Q-aVL/aVR >1.45 was seen only in group 2 patients, and this confirms the results of the previous studies that the Q-aVL/aVR can be useful to predict VAs originating from below the aortic sinus of valsalva. However, again due to much overlap in patients with Q-aVL/aVR <1.45 between group 1 and 2, this index alone is insufficient to distinguish VAs originating from within or below the aortic sinus of valsalva. Therefore, it is necessary to develop a strong set of predictors using a combination of electrophysiological and ECG features.
Predictor for successful ablation of left ventricular outflow tract-VAs
Catheter ablation is effective in abolishing VAs from the aortic root [1, 2, 4, 12, 14]. The LCC is the most common location, followed by the RCC, then LCC/RCC junction [4, 14, 18], which is consistent with our study. However, VAs originating below the aortic sinus of valsalva are generally difficult to ablate [7, 8, 14, 21, 22]. Our new technique via a trans-septal approach using a reversed S-curve of the ablation catheter allows improved accessibility, and can abolish the majority of VAs from below the aortic sinus of valsalva [14]. Therefore, a simple method to differentiate VAs originating within aortic sinus of valsalva from that below the aortic sinus of valsalva can allow planning and preparation prior to obtaining access to this area. Therefore, in this study, a combination of ECG and intracardiac electrogram features within the aortic sinus of valsalva was investigated to develop a simple method that predicts the origin of left ventricular outflow tract-VAs. In addition, local PP at the aortic sinus of valsalva suggests a VA origin within the aortic sinus of valsalva in the majority of patients. Using multivariate logistic regression analysis, the existence of PPs within the aortic sinus of valsalva is the strongest independent predictor for VAs originating within the aortic sinus of valsalva.
Conclusion
VAs within and below ASV present with similar ECG features. Some of these parameters showed significant differences that can discriminate VAs originating within the ASV from that below the ASV. However, due to much overlap between group 1 and 2, these parameters should be used with caution. The existence of PPs at the earliest site in the ASV strongly predicts a VA origin within the ASV, whereas the absence of PPs within the ASV as well as a Q-aVL/aVR >1.45 strongly indicates VAs originating below the ASV.
References
Ouyang F, Fotuhi P, Ho SY, Hebe J, Volkmer M, Goya M, Burns M, Antz M, Ernst S, Cappato R, Kuck KH (2002) Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: electrocardiographic characterization for guiding catheter ablation. J Am Coll Cardiol 39:500–508
Kumagai K, Fukuda K, Wakayama Y, Sugai Y, Hirose M, Yamaguchi N, Takase K, Yamauchi Y, Takahashi A, Aonuma K, Shimokawa H (2008) Electrocardiographic characteristics of the variants of idiopathic left ventricular outflow tract ventricular tachyarrhythmias. J Cardiovasc Electrophysiol 19:495–501
Ito S, Tada H, Naito S, Kurosaki K, Ueda M, Shinbo G, Oshima S, Nogami A, Taniguchi K (2005) Simultaneous mapping in the left sinus of valsalva and coronary venous system predicts successful catheter ablation from the left sinus of valsalva. Pacing Clin Electrophysiol 28:150–154
Yamada T, McElderry HT, Doppalapudi H, Murakami Y, Yoshida Y, Yoshida N, Okada T, Tsuboi N, Inden Y, Murohara T, Epstein AE, Plumb VJ, Singh SP, Kay GN (2008) Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. J Am Coll Cardiol 52:139–147
Chun KR, Satomi K, Kuck KH, Ouyang F, Antz M (2007) Left ventricular outflow tract tachycardia including ventricular tachycardia from the aortic cusps and epicardial ventricular tachycardia. Herz 32:226–232
Yamada T, McElderry HT, Doppalapudi H, Okada T, Murakami Y, Yoshida Y, Yoshida N, Inden Y, Murohara T, Plumb VJ, Kay GN (2010) Idiopathic ventricular arrhythmias originating from the left ventricular summit: anatomic concepts relevant to ablation. Circ Arrhythm Electrophysiol 3:616–623
Obel OA, d’Avila A, Neuzil P, Saad EB, Ruskin JN, Reddy VY (2006) Ablation of left ventricular epicardial outflow tract tachycardia from the distal great cardiac vein. J Am Coll Cardiol 48:1813–1817
Jauregui Abularach ME, Campos B, Park KM, Tschabrunn CM, Frankel DS, Park RE, Gerstenfeld EP, Mountantonakis S, Garcia FC, Dixit S, Tzou WS, Hutchinson MD, Lin D, Riley MP, Cooper JM, Bala R, Callans DJ, Marchlinski FE (2012) Ablation of ventricular arrhythmias arising near the anterior epicardial veins from the left sinus of Valsalva region: ECG features, anatomic distance, and outcome. Heart Rhythm 9:865–873
Steven D, Roberts-Thomson KC, Seiler J, Inada K, Tedrow UB, Mitchell RN, Sobieszczyk PS, Eisenhauer AC, Couper GS, Stevenson WG (2009) Ventricular tachycardia arising from the aortomitral continuity in structural heart disease: characteristics and therapeutic considerations for an anatomically challenging area of origin. Circ Arrhythm Electrophysiol 2:660–666
Christoph M, Sandfort V, Arya A, Wunderlich C (2014) Epicardial ablation cures electrical storm caused by cardiac involvement in myotonic dystrophia type 2. Clin Res Cardiol 103:315–317
Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DM, Trusso J, Carlson M, Luceri R, Kopelman H, Wilber D, Wharton JM, Stevenson W (2000) Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol 35:1905–1914
Coggins DL, Lee RJ, Sweeney J, Chein WW, Van Hare G, Epstein L, Gonzalez R, Griffin JC, Lesh MD, Scheinman MM (1994) Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol 23:1333–1341
Della Bella P, Baratto F, Tsiachris D, Trevisi N, Vergara P, Bisceglia C, Petracca F, Carbucicchio C, Benussi S, Maisano F, Alfieri O, Pappalardo F, Zangrillo A, Maccabelli G (2013) Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation 127:1359–1368
Ouyang FM, Mathew S, Wu S, Kamioka M, Metzner A, Xue Y, Ju W, Yang B, Zhan X, Rillig A, Lin T, Rausch P, Deiß S, Lemes C, Tönnis T, Wissner E, Tilz R, Kuck K, Chen M (2014) Ventricular arrhythmias arising from the left ventricular outflow tract below the aortic sinus cusps: mapping and catheter ablation via transseptal approach and electrocardiographic characteristics. Circ Arrhythm Electrophysiol 7:445–455
Daniels DV, Lu YY, Morton JB, Santucci PA, Akar JG, Green A, Wilber DJ (2006) Idiopathic epicardial left ventricular tachycardia originating remote from the sinus of Valsalva: electrophysiological characteristics, catheter ablation, and identification from the 12-lead electrocardiogram. Circulation 113:1659–1666
Hachiya H, Hirao K, Sasaki T, Higuchi K, Hayashi T, Tanaka Y, Kawabata M, Isobe M (2010) Novel ECG predictor of difficult cases of outflow tract ventricular tachycardia: peak deflection index on an inferior lead. Circ J 74:256–261
Yamada T, Murakami Y, Yoshida N, Okada T, Shimizu T, Toyama J, Yoshida Y, Tsuboi N, Muto M, Inden Y, Hirai M, Murohara T, McElderry HT, Epstein AE, Plumb VJ, Kay GN (2007) Preferential conduction across the ventricular outflow septum in ventricular arrhythmias originating from the aortic sinus cusp. J Cardiovasc Electrophysiol 21:170–176
Yamada T, McElderry HT, Okada T, Murakami Y, Doppalapudi H, Yoshida N, Yoshida Y, Inden Y, Murohara T, Epstein AE, Plumb VJ, Kay GN (2010) Idiopathic left ventricular arrhythmias originating adjacent to the left aortic sinus of valsalva: electrophysiological rationale for the surface electrocardiogram. J Cardiovasc Electrophysiol 21:170–176
Hachiya H, Aonuma K, Yamauchi Y, Harada T, Igawa M, Nogami A, Iesaka Y, Hiroe M, Marumo F (2000) Electrocardiographic characteristics of left ventricular outflow tract tachycardia. Pacing Clin Electrophysiol 23:1930–1934
Ito S, Tada H, Naito S, Kurosaki K, Ueda M, Hoshizaki H, Miyamori I, Oshima S, Taniguchi K, Nogami A (2003) Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol 14:1280–1286
Yamada T, Litovsky SH, Kay GN (2008) The left ventricular ostium: an anatomic concept relevant to idiopathic ventricular arrhythmias. Circ Arrhythm Electrophysiol 1:396–404
Reents T, Stilz S, Herold U, Deisenhofer I (2014) Transapical access for catheter ablation of left ventricular tachycardia in a patient with mechanical aortic and mitral valve prosthesis. Clin Res Cardiol 103:1025–1027
Conflict of interest
None declared.
Ethical standard
The study was approved by our institutional review committee on human research and all patients provided written informed consent prior to the procedure.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Kamioka and S. Mathew authors contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Kamioka, M., Mathew, S., Lin, T. et al. Electrophysiological and electrocardiographic predictors of ventricular arrhythmias originating from the left ventricular outflow tract within and below the coronary sinus cusps. Clin Res Cardiol 104, 544–554 (2015). https://doi.org/10.1007/s00392-015-0817-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0817-4