Abstract
Purpose
Catheter ablation of premature ventricular contractions (PVCs) is highly successful and has become the hallmark treatment for symptomatic or highly prevalent cases. However, few studies exist that evaluate the outcomes of ablation and likely mechanisms of PVC recurrence beyond 1 year of follow-up.
Methods
This study is a retrospective analysis of patients who underwent catheter ablation for symptomatic PVCs with acute procedural success and had clinical follow-up ≥ 12 months.
Results
Forty-four patients (24 women; age 53.5 ± 4.8 years) following acutely successful PVC ablation with long-term follow-up were studied. At a mean of 36 ± 6 months, overall long-term ablation success was 75% (33/44 patients). Notably, recurrence of the targeted PVC focus was low (6.8%, 3/44 patients); the majority of recurrences were from a new source location (18.2%, 8/44 patients). The time course for targeted versus de novo PVC recurrences was significantly different: recurrence of a PVC similar to the targeted PVC morphology occurred at a mean of 5.0 ± 2.0 months, while recurrence of a PVC different from the index case occurred at a mean of 35.8 ± 17.1 months (p = 0.01). Non-ischemic cardiomyopathy was associated with increased risk of PVC recurrence (odds ratio [OR] 14.50 (95% confidence interval [CI] 1.92–109.33, p = 0.01)) and was a significant negative prognostic factor in multivariate analysis for PVC recurrence survival (hazard ratio [HR] 4.63, 95% CI 1.03–20.74, p = 0.04).
Conclusions
The majority of long-term PVC recurrences occur late in follow-up, at locations remote from the targeted PVC source or sources. Such sites may represent ongoing substrate evolution; additional work is required to determine the precise substrate alterations which promote such arrhythmogenic changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Frequent premature ventricular contractions (PVCs) are common and may occur in 1–4% of the general population [1]. An elevated PVC burden may cause significant palpitations and lead to left ventricular dysfunction or enlargement [1,2,3,4,5]. Prior seminal work has demonstrated that catheter ablation of PVCs is feasible and safe and may reverse LV dysfunction due to tachycardia-mediated cardiomyopathy. Current guidelines recommend PVC ablation for drug-refractory cases with significant symptoms or LV dysfunction [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20].
The success of PVC ablation at 6 months has been reported as 80–95%. However, limited data exist regarding the outcomes of PVC ablation beyond 1 year. Additionally, risk factors for late PVC recurrence are uncertain. We hypothesized that ongoing substrate changes may result in late PVC recurrences at sites remote from the index procedure. The purpose of this study was to investigate the mode and timing of long-term PVC recurrence following successful ablation.
2 Methods
2.1 Study design
This study was conducted under an institution review board-approved protocol for the retrospective analysis of patients following catheter ablation of PVCs between May 2010 and May 2015 at the University of California San Diego Medical Center. Data were obtained from the UCSD procedural database (Perminova, Inc., San Diego, CA, USA) and electronic medical record (Epic Systems Corporation, Verona, WI, USA).
2.2 Patient characteristics
Patients with symptomatic or frequent PVCs who underwent acutely successful ablation with long-term follow-up greater than or equal to 12 months were included. Acutely successful ablation was defined as elimination of PVCs without intraoperative recurrence on or off an isoproterenol challenge. Recurrence was defined as any of the following: recurrent symptoms due to PVCs at clinical follow-up, ECGs demonstrating PVC recurrence during follow-up visits, or ambulatory monitors (24/48 Holter, 2-week event monitoring) showing > 1% PVC burden. Patients were excluded if clinical follow-up was less than 12 months.
2.3 Mapping and ablation
A standard electrophysiology study was performed in all patients. Monitored anesthesia care was used to avoid PVC suppression by general anesthesia. Three-dimensional electroanatomic mapping was used in all subjects. PVC mapping and ablation technique and equipment were left to the discretion of the attending electrophysiologist.
In general, the clinical PVC templates were captured for pace-map matching prior to sedation. If no PVCs were seen at baseline, then isoproterenol and/or phenylephrine was given to induce PVCs. A combination of pace-map matching and activation mapping was performed when possible to localize PVC sources. After successful ablation of the PVC site, a 30-min waiting period was observed, during which high-dose isoproterenol (up to 30 μg/min) was administered. Ablation success was defined as the absence of intraprocedural PVC recurrence.
2.4 Follow-up
Post-ablation PVC burdens were monitored by 24/48 Holter within the first 3 months after the initial procedure. Subsequent follow-up and quantitative measurements of PVC burden depended on the clinician’s judgment of symptoms, presence of coexisting arrhythmias or other cardiac conditions, and implantable device interrogation.
2.5 Comparison of index versus recurrent PVC source locations
Index source locations were identified as the site of PVC termination from intraprocedural mapping and ablation. If the patients had clinical recurrence of PVCs during long-term follow-up, recurrent source location was similarly defined as the site of successful PVC termination during repeat ablation. If repeat ablation was not pursued, source location was determined using standard 12-lead ECG PVC localization technique [2, 4, 11, 12, 17, 19,20,21,22,23,24,25,26,27,28,29,30]. For all patients with PVC recurrence, we searched for available cardiac magnetic resonance imaging (CMRI) studies and reviewed these studies for the presence of delayed gadolinium enhancement at either the index or recurrent PVC source location.
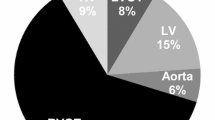
For statistical analysis, PVC sources were grouped into the following categories: (1) right ventricular outflow tract (RVOT), (2) right ventricle (non-outflow tract), (3) left ventricular outflow tract and coronary cusps (LVOT), (4) left ventricle (non-outflow tract), and (5) LV papillary muscle.
2.6 Statistical analysis
Continuous variables were expressed as mean ± standard deviation and were compared with Student’s t test. Categorical variables were compared with the χ2 test; Fisher’s exact test was used when expected values in contingency tables were less than 5. One-way ANOVA was used to determine the effect of source location on probability of PVC recurrence, using Bonferroni’s correction for multiple comparisons. Multivariate logistic regression was used to determine predictors which were associated with PVC recurrence within the entire study population. Predictors exhibiting differences between groups with a p value of < 0.10 in the univariate analysis were incorporated into the multivariate model. Univariate analysis of PVC source locations was performed via logistic regression with the RVOT location as the reference group. A two-tailed p value of < 0.05 in the multivariate regression was considered statistically significant. In the subset of patients with PVC recurrence, univariate survival analysis was performed using the Kaplan-Meier log-rank test for categorical variables and Cox regression for continuous variables to determine which factors were associated with recurrence of PVCs at a location different than the targeted site. Characteristics exhibiting differences between survival distributions with a p value of < 0.10 in the univariate analysis were incorporated into the multivariate model. Cox regression was used for multivariate survival analysis of PVC recurrence. A two-tailed p value of < 0.05 in Cox regression was considered statistically significant. The RVOT was chosen as the reference group for logistic regression and Cox regression of PVC source location, as it is the most common PVC source location with the highest ablation success in the general population. Statistical analysis was performed using Stata (Statacorp, College Station, TX, USA).
3 Results
The study included 44 patients (age 53.5 ± 4.8 years, 24 female, mean ejection fraction 56.4 ± 3.8%) with acutely successful PVC ablation and greater than or equal to 12 months of clinical follow-up. PVC burden by Holter monitoring was reduced from an average of 19.7 ± 3.3% PVCs at 3.3 ± 1.1 months pre-ablation to 2.3 ± 1.7% PVCs at 4.6 ± 2.8 months following successful ablation. Subsequent PVC burden was recorded at a mean of 10.7 ± 18.9 months and at a mean of 22.6 ± 26.6 months post-ablation. Patient characteristics were similar between the long-term ablation success and failure groups for age, co-morbidities, echocardiographic parameters, and antiarrhythmic drugs (Table 1).
Long-term ablation success (mean follow-up time after index ablation 36.1 ± 5.9 months) of the index PVC was achieved in 93.2% (41/44) of cases. Conversely, 8/44 (18.2%) had symptomatic recurrence from different PVC versus the index case. Thus, overall 3-year freedom from PVCs was 75% (33/44) following ablation. Of these patients, 4/33 (12%) required antiarrhythmic medications for other coexisting arrhythmias: atrial fibrillation (1/4), atrial flutter (2/4), and ventricular tachycardia (1/4). All recurrences were detected with repeat Holter monitoring. To date, 8 of 11 patients with PVC recurrence (72.7%) have presented for repeat ablation.
3.1 Index PVC source location and procedural success
The most common PVC source locations identified and ablated were the RVOT (50%) and LVOT/coronary cusps (30%, Table 2). The RVOT source location had a 95% (21/22) long-term ablation success rate.
3.2 Mode, location, and time course of PVC recurrence
Three patients (3/44, 6.8%) had recurrence of the index procedure PVC location at a mean 5.0 ± 2.0 months. Of the three recurrences of the same PVC, 2/3 were from the LVOT/coronary cusp and 1 in the RVOT. In contrast, 8 patients (8/44, 18.2%) presented with recurrence of a PVC source location distinct from the index procedure at a mean on 35.8 ± 17.1 months (p = 0.01), Table 3. The average annual risk of new PVC source development was 6.1%.
Of the 8 patients with recurrence of a new PVC, the LVOT/coronary cusp location during the index case (4/8, 50%) was most common. None of the recurrent PVC sources occurred at a LV papillary muscle location. None of the recurrent PVC sources were identified or mapped during the index procedure.
Notably, the follow-up interval between the 33 patients with successful long-term outcome and the 11 patients with unsuccessful long-term outcome was not statistically different (p = 0.643). The patients with successful outcome had a follow-up interval of mean 35.5 ± 36.9 months, and the patients with unsuccessful outcome had a follow-up interval of mean 38.7 ± 45.1 months.
3.3 Findings from cardiac imaging
In the group of 11 patients with PVC recurrence, CMRI scans were obtained in 3 (same-source-recurrence patient 1, and distant-source-recurrence patients 1 and 3 (Table 3)). Notably, the CMRI scans did not show evidence of delayed myocardial enhancement at either the index PVC or recurrent PVC site.
3.4 Logistic regression: Univariate analyses
Univariate logistic regression analyses of baseline characteristics identified three predictors of PVC recurrence or non-recurrence (Table 4). Non-ischemic cardiomyopathy was associated with a greater than 5-fold risk of PVC recurrence (RR of 5.29, 95% CI 1.71–16.3,p = 0.01). The LVOT and coronary cusp location was associated with a greater than 6-fold risk of PVC recurrence compared to RVOT (RR of 6.77, 95% CI (0.92–18.04), p = 0.06). Non-outflow tract right ventricular location was associated with an 11-fold risk of PVC recurrence compared to RVOT (RR of 11.00, 95% CI (0.70–21.31), p = 0.08).
3.5 Logistic regression: Multivariate analyses
Non-ischemic cardiomyopathy remained statistically significant in multivariate logistic regression analysis (Table 5). Non-ischemic cardiomyopathy significantly increased the odds of PVC recurrence (OR 14.50, 95% CI 1.92–109.33, p = 0.01).
3.6 Survival analysis
Univariate survival analysis identified differences in survival distributions of PVC recurrences in three variables: ejection fraction (p = 0.02), non-ischemic cardiomyopathy (p < 0.01), and PVC source location (p < 0.01) (Table 6). In the multivariate survival analysis (Table 7), non-ischemic cardiomyopathy was the only significant factor affecting recurrence with a hazard ratio (HR) of 4.63 (95% CI 1.03–20.74, p = 0.04).
3.7 Comparison of patients with and without long-term follow-up
We evaluated whether there were differences between patients with and without long-term follow-up following PVC ablation; results are detailed in the online supplement, Table S1. In summary, there were no observed differences between groups.
4 Discussion
There are three major findings from this long-term study of PVC ablation outcomes. First, we found that while the long-term freedom from the index PVC focus remains excellent at 3 years (approximately 93%), the majority of PVC recurrences at 3 years were due to new foci developing remote from the targeted site (18%). Second, we found that the recurrence of the index PVC focus primarily occurs within the first 6 months, while new PVC source development occurs after 6 months, at a rate of approximately 6.1% per year. Third, we found that non-ischemic cardiomyopathy is both a significant risk factor for PVC recurrence after ablation and has a greater risk of PVC recurrence at a site distant from the targeted PVC source. These findings represent novel insight into the natural history of PVCs following ablation and may help to inform decisions for patients undergoing this procedure.
4.1 Long-term PVC ablation outcomes
Since the seminal studies of PVC ablation, the excellent acute success of the procedure has been well documented [2, 4]. Baser and colleagues reported an 80% success at 3 months in a general population undergoing PVC ablation [31]. In a study limited to RVOT PVC ablation, Zhang and colleagues found an 86.8% long-term success [32]. However, the long-term results, modes, and timings of recurrence in a general population undergoing PVC ablation remained uncertain.
In this study, we focused on patients presenting with symptomatic or frequent PVCs who had acute procedural success and long-term follow-up (≥ 12 months). During extended follow-up, we found that recurrence of the original PVC source remains low, < 7%. These results suggest durable long-term success for most patients undergoing PVC ablation which continues long beyond the previously reported studies of 3–12 months.
Importantly, with extended long-term follow-up, we found a significant amount (18.2%) of patients had symptomatic PVC recurrence from a different PVC location. None of these recurrent PVCs were identified or mapped during the initial ablation. Because the identified anatomic locations were distant from the index PVC source, our work strongly supports that they are not the result of “changing the exit” from the index procedure but, rather, likely represent ongoing changes in ventricular substrate. Notably, in the patients with CMRI, such substrate changes were either not extensive or dense enough to be visualized with current CMRI technology in this study. Our mechanistic hypothesis is that ongoing ventricular stress and fibrosis create new regions capable of automaticity. Further work is required to define precise pathophysiologic mechanisms which result in proarrhythmic substrate and to develop imaging technology capable of detecting this substrate; such work is ongoing in our laboratory.
4.2 Time course of PVC recurrence
The limited follow-up in prior work had not detailed the time course or mode of PVC recurrence following successful catheter ablation. As a result, it had been unclear whether such cases represented ablation failure or development of new sources.
In this work, we methodically localized index PVC sources and compared these sites with those of recurrent PVCs. We were thus able to identify a bimodal pattern for the mechanism of PVC recurrence following ablation. Early in follow-up, recurrent PVCs are most likely to be due to resumption of activity of the index PVC site. This may represent an incomplete, or “missed,” ablation in which ablation stunned or suppressed the PVC source, but did not completely eliminate the triggering tissue, similar to studies of atrial fibrillation source ablation [33].
In contrast, de novo sources predominate in late PVC recurrence, consistent with work in other arrhythmias supporting a link between ongoing substrate remodeling and arrhythmia risk [34]. Such data are useful in counseling patients regarding PVC ablation. Furthermore, this finding may be hypothesis generating in that future work may study lifestyle interventions and risk factor modifications to potentially slow or prevent subsequent PVC source development, as has been shown in AF [35].
4.3 Risk factors for PVC recurrence
Finally, we analyzed clinical factors associated with greater risk of PVC recurrence. Notably, patients with non-ischemic cardiomyopathy, defined as reduced ejection fraction (EF < 50%) without patient history of myocardial infarction or echocardiographic evidence of regional scar, are significantly more likely to have PVC recurrence (Table 5). These findings holds true even with differential follow-up between individual patients, as evidenced by the time-to-event analysis (Table 7). This finding is consistent with prior work in PVC ablation demonstrating that PVC sources were often associated with remodeled substrate [36]. Notably, prior work has demonstrated that the ventricles of patients with non-ischemic cardiomyopathy are significantly remodeled [37] and have elevated levels of biomarkers associated with myocyte stretch and necrosis [38] compared with patients without non-ischemic cardiomyopathy. Thus, substrate progression in such patients may occur at an accelerated rate, and is thus detectable in our cohort of patients during the timescale of this study.
It is also possible that the non-ischemic cardiomyopathy cases in this study may have been PVC-induced cardiomyopathy situations. From the 7/44 patients with non-ischemic cardiomyopathy, 6 of them (86%) had a significant improvement in their LV ejection fraction following successful PVC ablation. Notably, 3 out of these 6 patients had recurrent PVCs. In addition, recurrence of non-clinical PVCs seemed to result in a drop in ejection fraction in 2 out of the 11 patients.
These factors may play an important role in pre-ablation counseling and education as they represent clinical characteristics that predict PVC recurrence. Such data can help inform a more precise discussion of the expected success rate of an invasive PVC ablation.
4.4 Limitations
The main limitation of this study was a small study population due to our strict inclusion criteria of a minimum of 12 months of follow-up after successful PVC ablation. Despite this limitation, we were able to identify sufficient patients to detect the bimodal pattern and timing of PVC recurrence (e.g., index versus de novo). Furthermore, there was no statistical difference in our database between patients who did and did not have long-term follow-up. This provides reassurance regarding the generalizability of our findings. Ongoing prospective series are required to confirm these results. In addition, the four patients with antiarrhythmic medications after ablation for other arrhythmias may have contributed to their long-term recurrence-free success.
5 Conclusions
Long-term success of PVC ablation is quite good; the majority of patients remain PVC free at a mean follow-up of 3 years. Most recurrences after the first year occur at a site anatomically distinct from the targeted focus. Non-ischemic cardiomyopathy is associated with increased PVC recurrence at locations distant from the targeted site.
Abbreviations
- ECG:
-
Electrocardiogram
- PVC:
-
Premature ventricular contraction
- RVOT:
-
Right ventricular outflow tract
- LVOT:
-
Left ventricular outflow tract
References
Latchamsetty R, Bogun F. Premature ventricular complexes and premature ventricular complex induced cardiomyopathy. Curr Probl Cardiol. 2015;40:379–422.
Badhwar N, Scheinman MM. Idiopathic ventricular tachycardia: diagnosis and management. Curr Probl Cardiol. 2007;32:7–43.
Chugh SS, Shen WK, Luria DM, Smith HC. First evidence of premature ventricular complex-induced cardiomyopathy: a potentially reversible cause of heart failure. J Cardiovasc Electrophysiol. 2000;11:328–9.
Hoffmayer KS, Gerstenfeld EP. Diagnosis and management of idiopathic ventricular tachycardia. Curr Probl Cardiol. 2013;38:131–58.
Yarlagadda RK, Iwai S, Stein KM, Markowitz SM, Shah BK, Cheung JW, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–7.
Bala R, Garcia FC, Hutchinson MD, Gerstenfeld EP, Dhruvakumar S, Dixit S, et al. Electrocardiographic and electrophysiologic features of ventricular arrhythmias originating from the right/left coronary cusp commissure. Heart Rhythm : Off J Heart Rhythm Soc. 2010;7:312–22.
Bogun F, Crawford T, Reich S, Koelling TM, Armstrong W, Good E, et al. Radiofrequency ablation of frequent, idiopathic premature ventricular complexes: comparison with a control group without intervention. Heart Rhythm : Off J Heart Rhythm Soc. 2007;4:863–7.
Chinushi M, Aizawa Y, Ohhira K, Fujita S, Shiba M, Niwano S, et al. Repetitive ventricular responses induced by radiofrequency ablation for idiopathic ventricular tachycardia originating from the outflow tract of the right ventricle. Pacing Clin Electrophysiol : PACE. 1998;21:669–78.
Good E, Desjardins B, Jongnarangsin K, Oral H, Chugh A, Ebinger M, et al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm : Off J Heart Rhythm Soc. 2008;5:1530–7.
Jauregui Abularach ME, Campos B, Park KM, Tschabrunn CM, Frankel DS, Park RE, et al. Ablation of ventricular arrhythmias arising near the anterior epicardial veins from the left sinus of Valsalva region: ECG features, anatomic distance, and outcome. Heart Rhythm : Off J Heart Rhythm Soc. 2012;9:865–73.
John RM, Stevenson WG. Catheter-based ablation for ventricular arrhythmias. Curr Cardiol Rep. 2011;13:399–406.
Joshi S, Wilber DJ. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol. 2005;16(Suppl 1):S52–8.
Kottkamp H, Chen X, Hindricks G, Willems S, Haverkamp W, Wichter T, et al. Idiopathic left ventricular tachycardia: new insights into electrophysiological characteristics and radiofrequency catheter ablation. Pacing Clin Electrophysiol : PACE. 1995;18:1285–97.
Kumagai K, Yamauchi Y, Takahashi A, Yokoyama Y, Sekiguchi Y, Watanabe J, et al. Idiopathic left ventricular tachycardia originating from the mitral annulus. J Cardiovasc Electrophysiol. 2005;16:1029–36.
Latif S, Dixit S, Callans DJ. Ventricular arrhythmias in normal hearts. Cardiol Clin. 2008;26:367–80 vi.
Movsowitz C, Schwartzman D, Callans DJ, Preminger M, Zado E, Gottlieb CD, et al. Idiopathic right ventricular outflow tract tachycardia: narrowing the anatomic location for successful ablation. Am Heart J. 1996;131:930–6.
Tada H, Ito S, Naito S, Kurosaki K, Kubota S, Sugiyasu A, et al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol. 2005;45:877–86.
Tada H, Tadokoro K, Ito S, Naito S, Hashimoto T, Kaseno K, et al. Idiopathic ventricular arrhythmias originating from the tricuspid annulus: prevalence, electrocardiographic characteristics, and results of radiofrequency catheter ablation. Heart Rhythm :Off J Heart Rhythm Soc. 2007;4:7–16.
Yamada T, McElderry HT, Doppalapudi H, Murakami Y, Yoshida Y, Yoshida N, et al. Idiopathic ventricular arrhythmias originating from the aortic root prevalence, electrocardiographic and electrophysiologic characteristics, and results of radiofrequency catheter ablation. J Am Coll Cardiol. 2008;52:139–47.
Yamada T, McElderry HT, Okada T, Murakami Y, Doppalapudi H, Yoshida N, et al. Idiopathic focal ventricular arrhythmias originating from the anterior papillary muscle in the left ventricle. J Cardiovasc Electrophysiol. 2009;20:866–72.
Betensky BP, Park RE, Marchlinski FE, Hutchinson MD, Garcia FC, Dixit S, et al. The V(2) transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57:2255–62.
Buxton AE, Waxman HL, Marchlinski FE, Simson MB, Cassidy D, Josephson ME. Right ventricular tachycardia: clinical and electrophysiologic characteristics. Circulation. 1983;68:917–27.
Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Electrocardiographic patterns of superior right ventricular outflow tract tachycardias: distinguishing septal and free-wall sites of origin. J Cardiovasc Electrophysiol. 2003;14:1–7.
Hachiya H, Aonuma K, Yamauchi Y, Igawa M, Nogami A, Iesaka Y. How to diagnose, locate, and ablate coronary cusp ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:551–6.
Ito S, Tada H, Naito S, Kurosaki K, Ueda M, Hoshizaki H, et al. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2003;14:1280–6.
Lin D, Ilkhanoff L, Gerstenfeld E, Dixit S, Beldner S, Bala R, et al. Twelve-lead electrocardiographic characteristics of the aortic cusp region guided by intracardiac echocardiography and electroanatomic mapping. Heart Rhythm :Off J Heart Rhythm Soc. 2008;5:663–9.
Sekiguchi Y, Aonuma K, Takahashi A, Yamauchi Y, Hachiya H, Yokoyama Y, et al. Electrocardiographic and electrophysiologic characteristics of ventricular tachycardia originating within the pulmonary artery. J Am Coll Cardiol. 2005;45:887–95.
Yamada T, Doppalapudi H, McElderry HT, Okada T, Murakami Y, Inden Y, et al. Idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: prevalence, electrocardiographic and electrophysiological characteristics, and results of the radiofrequency catheter ablation. J Cardiovasc Electrophysiol. 2010;21:62–9.
Yamada T, Yoshida N, Murakami Y, Okada T, Muto M, Murohara T, et al. Electrocardiographic characteristics of ventricular arrhythmias originating from the junction of the left and right coronary sinuses of Valsalva in the aorta: the activation pattern as a rationale for the electrocardiographic characteristics. Heart Rhythm :Off J Heart Rhythm Soc. 2008;5:184–92.
Yoshida N, Yamada T, McElderry HT, Inden Y, Shimano M, Murohara T, et al. A novel electrocardiographic criterion for differentiating a left from right ventricular outflow tract tachycardia origin: the V2S/V3R index. J Cardiovasc Electrophysiol. 2014;25:747–53.
Baser K, Bas HD, Belardi D, Yokokawa M, Good E, Latchamsetty R, et al. Predictors of outcome after catheter ablation of premature ventricular complexes. J Cardiovasc Electrophysiol. 2014;25:597–601.
Zhang F, Yang B, Chen H, Ju W, Kojodjojo P, Cao K, et al. Noncontact mapping to guide ablation of right ventricular outflow tract arrhythmias. Heart Rhythm : Off J Heart Rhythm Soc. 2013;10:1895–902.
Lalani GG, Coysh T, Baykaner T, Zaman J, Hopper K, Schricker AA, et al. Organized sources are spatially conserved in recurrent compared to pre-ablation atrial fibrillation: further evidence for non-random electrical substrates. J Cardiovasc Electrophysiol. 2016;27:661–9.
Dimitri H, Ng M, Brooks AG, Kuklik P, Stiles MK, Lau DH, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm : Off J Heart Rhythm Soc. 2012;9:321–7.
Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. 2014;64:2222–31.
El Kadri M, Yokokawa M, Labounty T, Mueller G, Crawford T, Good E, et al. Effect of ablation of frequent premature ventricular complexes on left ventricular function in patients with nonischemic cardiomyopathy. Heart Rhythm : Off J Heart Rhythm Soc. 2015;12:706–13.
Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5.
Wijeysundera HC, Hansen MS, Stanton E, Cropp AS, Hall C, Dhalla NS, et al. Neurohormones and oxidative stress in nonischemic cardiomyopathy: relationship to survival and the effect of treatment with amlodipine. Am Heart J. 2003;146:291–7.
Financial support
This study was financially supported by the UCSD Clinical Translational Research Institute (GEM Grant), the University of California Center for Accelerated Innovation, the UCSD Rady MEET Grant to DEK, and National Institutes of Health (Grant 5T35HL007491) to DL.
Author information
Authors and Affiliations
Contributions
Derek Lee: Data analysis/interpretation, drafting article, statistics.
Kurt S. Hoffmayer: Critical revision of article, statistics, approval of article.
Jonathan C. Hsu: IRB approval secured by, data collection, approval of article.
Amir Schricker: Data generation, approval of article.
Ulrika Birgersdotter-Green: Critical revision of article, approval of article.
Farshad Raissi: Data generation, approval of article.
Gregory K. Feld: Data generation, approval of article.
David E. Krummen: Funding secured by, concept/design, data generation, drafting article, critical revision of article, approval of article.
Corresponding author
Ethics declarations
This study was conducted under an institution review board-approved protocol for the retrospective analysis of patients following catheter ablation at the University of California San Diego Medical Center.
Conflict of interest
Lee: This author declares that he has no conflict of interest.
Hsu: Honoraria from Medtronic, St. Jude Medical, Boston Scientific, and Biotronik. Consulting for 3DT Holdings. Research grants from Biotronik, Biosense Webster.
Feld: Equity and Board of Directors for Perminova, Inc.
Krummen: Consulting for Abbott Laboratories. Equity in Vektor Medical.
Hoffmayer, Schricker, Hsu, Birgersdotter-Green, Raissi, Feld, and Krummen: EP Fellowship support from Abbott Laboratories, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 21 kb)
Rights and permissions
About this article
Cite this article
Lee, D., Hoffmayer, K.S., Hsu, J.C. et al. Long-term mode and timing of premature ventricular complex recurrence following successful catheter ablation. J Interv Card Electrophysiol 55, 153–160 (2019). https://doi.org/10.1007/s10840-019-00520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-019-00520-3