Abstract
Background
The use of non-vitamin K antagonists (NOACs), uninterrupted (uVKA) and interrupted vitamin K antagonists (iVKA) are common periprocedural oral anticoagulation (OAC) strategies for atrial fibrillation (AF) ablation. Comparative data on complication rates resulting from OAC strategies for solely persistent AF (persAF) undergoing ablation are sparse. Thus, we sought to determine the impact of these OAC strategies on complication rates among patients with persAF undergoing catheter ablation.
Methods
Consecutive patients undergoing persAF ablation were included. Depending on preprocedural OAC, three groups were defined: (1) NOACs (paused 48 h preablation), (2) uVKA, and (3) iVKA with heparin bridging. A combined complication endpoint (CCE) composed of bleeding and thromboembolic events was analyzed.
Results
Between 2011 and 2014, 1440 persAF ablation procedures were performed in 1092 patients. NOACs were given in 441 procedures (31 %; rivaroxaban 57 %, dabigatran 33 %, and apixaban 10 %), uVKA in 488 (34 %), and iVKA in 511 (35 %). Adjusted CCE rates were 5.5 % [95 % confidence interval (CI) (3.1–7.8)] in group 1 (NOACs), 7.5 % [95 % CI (5.0–10.1)] in group 2 (uVKA), and 9.9 % [95 % CI (6.6–13.2)] in group 3. Compared to group 1, the combined complication risk was almost twice as high in group 3 [odd’s ratio (OR) 1.9, 95 % CI (1.0–3.7), p = 0.049)]. The major complication rate was low (0.9 %). Bleeding complications, driven by minor groin complications, are more frequent than thromboembolic events (n = 112 vs. 1, p < 0.0001).
Conclusions
Patients undergoing persAF ablation with iVKA anticoagulation have an increased risk of complications compared to NOACs. Major complications, such as thromboembolic events, are generally rare and are exceeded by minor bleedings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, oral anticoagulation (OAC) has been revolutionized by the introduction of non-vitamin K antagonists (NOACs) as an alternative option to vitamin K antagonists (VKA). For patients (pts) undergoing atrial fibrillation (AF) catheter ablation, it is, therefore, essential to establish safe periprocedural anticoagulation strategies, weighing out bleeding, and thromboembolic risks.
The first usage of periprocedural OAC was via the interruption of VKAs and bridging with low-molecular-weight heparin (LMWH). Subsequently, continuous periprocedural VKAs entered clinical practice [1, 2], followed by NOACs [3–10]. Despite their now routine usage, strategies regarding the continuation, discontinuation, and bridging of OACs before and after AF ablation are not uniform [3].
Recently published, non-randomized prospective registry data indicate that the use of continuous NOACs as compared to uninterrupted VKAs (uVKA) is feasible and safe [4–6, 8, 9, 11]. In addition, the interruption of OAC during ablation seems feasible [12]. Regarding VKAs, interruption of VKAs and heparin bridging leads to an increased risk of overall and major bleeding rates [2, 13]. In uVKA therapy, an optimal international normalized ratio (INR) of 2.0–2.5 is recommended [14].
All the above-cited studies included pts with paroxysmal and persistent atrial fibrillation (persAF). However, comparative data on the influence of OAC strategies on complication rates of ablation procedures for solely persAF are rare. Pts with persAF are known to undergo longer and more complex ablation procedures compared to pts with paroxysmal AF [15, 16]. Consequently, the probability for periprocedural complications increases. Furthermore, pts with underlying cardiomyopathies appear more often with persAF, and comorbidities are more frequently present [17]. In addition, it is known that pts with paroxysmal AF with a moderate to high stroke risk are less likely prescribed to an appropriate OAC therapy compared to persAF pts [18].
Therefore, we sought to determine periprocedural complication rates among three OAC strategies [NOACs, uVKAs, and interrupted VKAs (iVKAs)] in pts undergoing solely persAF ablation.
Methods
Study population and anticoagulation strategy
We retrospectively analyzed consecutive persAF ablation procedures undertaken in our electrophysiology department. The study was approved by the institutional review board, and informed consent was obtained from all patients. We included all symptomatic pts with persAF, indication for catheter ablation and with a preprocedural anticoagulation in the form of NOACs or VKAs. Pts without OAC prior to ablation were excluded from the analysis. Depending on the preprocedural oral anticoagulation prescribed by prior physicians, three groups were defined: group 1) NOACs, group 2) uVKA (INR range 2.0–3.0), and group 3) iVKA [discontinuation of VKA and bridging with heparin (LMWH/unfractionated heparin)]. In group 1, we recommended the last intake of the NOAC to be 48 h before the ablation procedure according to the clinical practice and the guideline recommendation at that time [19]. This group is heterogeneous, including rivaroxaban, dabigatran, or apixaban. Discontinuation of the NOAC 48 h prior to ablation was recommended in all the types of NOACs. There was no heparin bridging in the NOAC group, and NOACs were restarted in the evening after the ablation procedure in all NOACs. In addition, patients in group 2 received their maintenance dose of VKA in the evening of the procedure day. In group 3, patients received weight-adapted LMWH doses in the evening of the procedure. Before hospital discharge, VKA was restarted in overlap with heparin.
Catheter ablation procedure
Before ablation, all pts underwent transesophageal echocardiography to rule out thrombus formation in the left atrial appendage. Ablation procedures were performed under deep sedation using continuous infusion of propofol (propofol, 1 mg/ml, B. Braun, Melsungen, Germany) as well as boluses of fentanyl (Fentanyl, 0.1 mg/ml, Rotexmedica, Trittau, Germany), [20].
Four diagnostic and ablation catheters were inserted through both Vv. femoralis. A double transseptal puncture using a modified Brockenbrough technique was performed (BRK Needle, St. Jude Medical Inc., St. Paul, MN, USA). To maintain an activated clotting time (ACT) of more than 300 s, intravenous unfractionated heparin (Heparin sodium, 25000 IU/5 ml, Rotexmedica) was administered immediately after the transseptal puncture.
After conduction of pulmonary vein (PV) angiograms, a three-dimensional electroanatomic mapping system [CARTO® (Biosense Webster, South Diamond Bar, CA, USA) or EnSite™ NavX™ (St. Jude Medical Inc.)] was used to define the left atrial anatomy. All ablation procedures were performed using radiofrequency current (RFC) energy with an irrigated-tip catheter [Thermocool®, Thermocool® Smarttouch® (Biosense Webster), TactiCath™ Quartz (St. Jude Medical)].
Ablation procedures followed the so-called “stepwise approach” aiming for AF termination [16, 21].
Definition of complications
We analyzed all complications occurring during periprocedural hospitalization. Periprocedural complications were categorized in major and minor bleedings, thromboembolic events (stroke and TIA), and further complications, such as pericardial effusion without hemodynamical relevance due to inflammatory response (no intervention required, <1 cm on echocardiography), myocardial infarction (MI), cardiac surgery, pacemaker implantation, cardiac or respiratory insufficiency, aortic mal-puncture, PV stenosis, and phrenic nerve palsy and death. Endpoints, such as MACE (major adverse cardiac event: death and MI) and MACCE (major adverse cardiac and cerebrovascular event: death, MI and stroke), were additionally recorded [17].
Major bleedings were defined according to current guidelines as: (1) hemodynamically relevant pericardial tamponades requiring pericardiocentesis, (2) groin complications [e.g., arteriovenous fistula (AVF), pseudoaneurysm, and hematoma] requiring blood transfusion or vascular surgery, and (3) resulting in a 20 % or greater fall in hematocrit [22]. Minor bleedings were defined as all bleeding complications not requiring invasive treatment, such as minor groin complications (AVF, pseudoaneurysm, and hematoma), hematuria, epistaxis, or hemoptysis. Furthermore, we defined a combined complication endpoint (CCE) as composed of any bleeding complications and thromboembolic events (stroke/TIA). The CCE was the primary basis of the statistical analysis in this study. This endpoint is based on several studies that focus on OAC-related complications during atrial fibrillation ablation. In these studies, solely bleeding and thromboembolic complications were analyzed [1, 2, 13, 14, 23].
Statistical analysis
All continuous values were expressed as mean ± standard deviation. Binary-coded and categorical data were described by the use of the median, interquartile range, and relative frequencies. For group comparisons, unpaired and paired T tests (for continuous data) as well as Fisher’s exact test (for binary and categorical data) were used.
Models for the linear regression analysis with pairwise comparisons of adjusted predictions with consideration of repetitive measurements were constructed. Eight confounders (age, hypertension, coronary artery disease, BMI, gender, procedure duration, number of procedure, and year of procedure) were implemented in the regression model to make up for unequal patient characteristics due to non-randomization.
A p value <0.05 defined statistical significance. Statistics were calculated using a scientific graphic (GraphPad, Version 6, GraphPad Software Inc., La Jolla, CA, USA) and statistical software (Stata, Release 14, StataCorp LP, College Station, TX, USA).
Results
Baseline characteristics
A total of 1092 symptomatic patients with persAF treated between January 2011 and December 2014 were included in this analysis. The baseline characteristics are summarized in Table 1. Furthermore, mean duration of hospitalization (including the day of admission) was 4.0 ± 2.3 days.
Procedural data
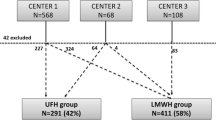
We analyzed data from 1440 persAF ablation procedures, which were consecutively performed at our center. This resulted in 1.3 procedures per pt. Prior to the analysis, we ruled out 197 procedures due to the absence of OAC (Fig. 1). These 1440 procedures had the following distribution: n = 441 in group 1 (31 %; distribution of NOACs 57 % rivaroxaban, 33 % dabigatran, and 10 % apixaban), n = 488 (34 %) in group 2, and n = 511 (35 %) in group 3.
Procedural data are detailed in Table 2: initially, all pts included in this study suffered from persAF. This study also included re-procedures following prior persAF ablation, so that the specific indication of the current procedure varies and was primarily persAF (n = 882, 61.3 %) followed by consecutive atrial tachycardias (n = 445, 30.9 %). Complete PVI or Re-PVI were the major electrophysiological approaches performed during ablation (78 %) followed by ablation of CFAEs (n = 742, 52 %), ablation lines (n = 381, 26 %), focal ablation (n = 303, 21 %), and CTI (n = 317, 22 %). Preprocedural INR values were significantly different between all groups (p < 0.0001, Table 2).
Figure 2 demonstrates procedural complication rates in relation with preprocedural INR values in patients prescribed to VKA (including solely group 2 and 3). Lowest complication rates (6.59 and 6.79 %) were found within an INR range of 2.0–2.99. INR ranges lower than 2.0 demonstrate higher complications rates (11.1 and 11.1 %).
Conducting linear regression, adjusted intraprocedural ACT levels were significantly higher in group 2 [324.3 s, 95 % Confidence Interval (CI) (320.7–327.9)] as compared to group 1 [293.0 s; 95 % CI (289.5–296.5); p < 0.0001] and group 3 [291.5 s, 95 % CI (288.3–294.7); p < 0.0001]. There was no difference in ACT values between group 1 and group 3 (p = 0.52).
Adjusted intraprocedural heparin doses were significantly different among all groups (group 1 vs. 2 p < 0.0001, 2 vs. 3 p < 0.0001, 1 vs. 3 p < 0.0001) with highest doses in group 1 [11,364 IU; 95 % CI (10,959–11,783)] and lowest in group 2 [7749 IU; 95 % CI (7519–7988); group 3: 9212 IU; 95 % CI (8902–9533)].
Complication rates
The overall complication rate was 210 out of 1440 procedures (14.6 %) and is detailed in Table 3.
There were 13 major complications (0.90 %), including one stroke (group 2), four hemodynamically relevant pericardial tamponades requiring pericardiocentesis, one cardiac intervention (mitral clipping in a decompensated pt, group 3), and seven major groin complications requiring vascular surgery and/or blood transfusion. Furthermore, there was one major bleeding event caused by hemoptysis requiring interventional bronchoscopy and blood transfusion (group 3). There was no death. There were 113 (7.84 %) combined complication endpoints (CCE), mainly attributable to bleeding complications (n = 112, 7.78 %). A single stroke occurred (0.07 %).
Bleeding complications were the most frequent complication overall, led by minor groin complications (96/112; 85.7 %) not requiring any treatment. All bleeding complications combined, as well as major bleedings only, occurred significantly more often than thromboembolic events [all bleedings n = 112 (7.78 %) vs. 1 stroke (0.07 %), p < 0.0001; 13 major bleedings vs. one stroke, p = 0.0018). The highest overall bleeding rate was observed in group 3 at 10.8 %. The occurrence of complications led to a significantly longer in-hospital stay [4 days (q1-3: 3–7) with vs. 3 days (q1-3: 3–4) without complication, p = 0.0002].
Group comparison
Adjusted CCE rates were 5.5 % [95 % CI (3.1–7.8)] in group 1, 7.5 % [95 % CI (5.0–10.1)] in group 2, and 9.9 % [95 % CI (6.6–13.2)] in group 3. There was a significant difference in CCE rates among the groups: compared to group 1, the combined complication risk was almost twice as high in group 3 [odd’s ratio (OR) 1.9, 95 % CI (1.0–3.7), p = 0.049]. There was no difference between group 1 and group 2 [OR 1.4, 95 % CI (0.8–2.5), p = 0.23] or group 2 and group 3 [OR 1.4, 95 % CI (0.8–2.4), p = 0.30]. Of all confounders, only age showed an influence on CCE rates (Fig. 3).
Forest plot of the linear regression model: difference of combined complication rates among the three periprocedural oral anticoagulation groups and influence of the confounders. Age, BMI body mass index, gender, hypertension, CAD coronary artery disease, CCE combined complication endpoint, NOAC non VKA, VKA Vitamin K antagonist
The HAS-BLED score was predictive for bleedings (major and minor) in the whole study cohort [n = 1440; p = 0.02, OR = 1.16; 95 % CI (1.01–1.49)]. The same accounts for the CHA2DS2-VASC score [p = 0.04, OR = 1.23; 95 % CI (1.03–1.31)], respectively. In a subgroup analysis of group 3, the HAS-BLED score was not significantly predictive for bleeding [p = 0.2, OR = 1.19; 95 % CI (0.92–1.53)].
Absolute intravenous heparin doses and ACT values did not differ among procedures with and without complications (heparin 10,198 ± 3874 IU vs 9856 ± 3773 IU, p = 0.2254; ACT 303.9 ± 32.7 vs. 303.4 ± 39.7 s, p = 0.54).
NOACs
Rivaroxaban was the most frequent NOAC prescribed in this study population [253 pts, 57 %; of these 232 pts (92 %) were prescribed to the 20 mg dose, 19 pts (7.5 %) to 15 mg, and two pts (0.5 %) to 10 mg], followed by dabigatran [147 pts, 33 %; of these 110 pts (75 %) were prescribed to the 150 mg dose and 37 pts (25 %) to 110 mg twice daily] and apixaban [41 pts, 10 %; of these 39 pts (95 %) were prescribed to the 5 mg dose and two pts to 2.5 mg (5 %) twice daily]. Nine (15 %) patients subscribed to a low-dose NOAC have suffered from a bleeding event in the past. A reduced glomerular filtration rate was present in 13 (21 %) of the 61 pts that were subscribed to the lower dose regime of NOACs. There was no thromboembolic complication in the NOAC group with the lowest bleeding rates (n = 23, 5.22 %, Table 3).
Adjusted CCE rates were 12.0 % [95 % CI (6.9–17.2)] for dabigatran, 13.3 % [95 % CI (8.8–17.8)] for rivaroxaban, and 2.4 % [95 % CI (0.7–3.2)] for apixaban. There was no significant difference in adjusted complication rates among the different NOACs.
The last intake of the NOAC was recommended to be 48 h before the procedure. However, the real timeframe was spread between 2 and 168 h with a median of 48 h (q1–q3 48–72). The timeframe was not different in procedures with and without complications (median 48 h (q1–3: 48–72) in both the groups, p = 0.445; Fig. 4).
Discussion
Major findings
In our study, we found that the iVKA strategy was associated with an increased risk of periprocedural complications (CCE) when compared to NOACs in pts with persAF undergoing catheter ablation. Another major finding was that thromboembolic events are rare and were exceeded by minor bleeding complications. Furthermore, these complications led to a prolonged hospital stay.
There were no thromboembolic events when NOACs were paused 48 h preablation in this persAF cohort. Despite significantly different intraprocedural administered heparin doses among the groups, no effect of heparin dose was found between procedures with and without periprocedural complications.
Combined complication endpoint
Our data show an increased CCE rate in the iVKA as compared to the NOAC group. This rate is primarily determined by minor bleeding complications, as there was only one stroke reported in group 2. Higher bleeding rates in iVKAs are a well described issue, as previously reported by other authors [2, 13, 23]. An explanation for this could be the difficulty in reliably quantifying a patients’ coagulation status, especially when restarting the VKA and simultaneously administering LMWH. It is, therefore, understandable that the periprocedural strategy of iVKAs has nowadays been abandoned by many electrophysiology centers.
However, the above-mentioned studies have only compared uVKAs to iVKAs. Another study has compared iVKAs to rivaroxaban and dabigatran [12]. They found fewer groin complications in pts treated with dabigatran compared to iVKAs and no differences between iVKAs and rivaroxaban. Our data extend on these findings by revealing an increased complication rate in iVKAs compared to NOACs (including apixaban). Regarding INR values, in pts taking VKAs, our data show lower complication rates when INR values range between 2.0 and 2.99. Kim et al. reported similar results with an optimal INR range of 2.1–2.5 [14].
In this study, the major complication rate was low (0.9 %) and comparable to similar studies [12, 23]. Bleeding complications accounted for a major part of the total complication rate and were caused mainly by minor groin complications that did not require treatment. Yet, periprocedural complications did lead to prolonged hospital stays.
An increase in age was associated with a higher CCE rate in our study. Data regarding age are discrepant across current literature, ranging from a non-increased to an increased risk of complication in aged pts undergoing catheter ablation [24–26]. Yet, the cause of this discrepancy remains unknown. It might be explained by a multifactorial genesis, e.g., different patient populations, previous anticoagulation, comorbidities, and experience of ablation center.
The CHA2DS2-VASc and HAS-BLED scores are predictive for bleeding complications in our study cohort. In our study, there was only one thromboembolic event. Therefore, the influence of the CHA2DS2-VASc score on stroke rates cannot be determined in our study. Other studies reported more frequent postablation thromboembolic event rates in high-risk pts with increased CHA2DS2-VASc scores [27, 28]. The follow-up time in these studies was much longer (up to 489 days after ablation) as compared to our study. We only analyzed in-hospital, postablation thromboembolic events.
The HAS-BLED Score was not predictive for increased bleeding risks in group 3. A reason why the HAS-BLED score is predictive in the whole study cohort but not in the subgroup analysis (only group 3) could be the fact that the sample size is smaller in the subgroup analysis and statistical significance cannot be reached.
NOACs
This study included the NOACs dabigatran, rivaroxaban, and apixaban. Despite pausing NOACs 48 h preablation in pts with persAF, not a single thromboembolic event occurred in 441 pts. Rillig et al. reported similar findings with no occurrence of any cerebrovascular event in 444 pts on NOACs with any type of AF (paroxysmal, persistent, and longstanding persistent), [29]. The median CHA2DS2-VASc score in this group was 2. This result is consistent with our findings.
In addition, we verified the recommended 48-h NOAC withholding period by directly questioning all pts about their last medication intake. When comparing procedures with and without periprocedural complications, there were no differences in timeframes of the last NOAC intake. Based on the results of our study, as well as those of Rillig et al. pausing NOACs 48 h before ablation, according to 2013’s practical guidelines, seem feasible and safe [19].
The latest anticoagulation studies for ablation urge the continuous use of periprocedural OAC [8, 9, 11]. Furthermore, the current updated guidelines recommend withholding NOACs 18–24 h before ablation; NOACs should then be restarted 6 h post ablation [30]. It remains unknown whether continuous NOAC administration leads to higher bleeding complications, as bleedings are already the most common complication found in our study. Bleedings under continuous NOACs could be more difficult to manage as antidotes either do not exist or are not routinely implemented. Furthermore, it is unknown if uninterrupted NOAC therapy during ablation will lead to a decrease in periprocedural stroke rates. Due to the low incidence of periprocedural strokes, larger patient populations are needed to resolve this question.
We found non-significantly lower complication rates in pts taking the pre-OAC apixaban as compared to rivaroxaban and dabigatran. Only 10 % of pts in group 1 were subscribed to apixaban in our study. Hence, case numbers are low and a definitive conclusion cannot be drawn. Di Biase et al. investigated pts on continuous apixaban versus on uVKA [11]. There were no statistical differences with regard to complications between apixaban and VKA. However, there was no comparison with other NOACs. Rillig et al. observed no difference in major complications between pts prescribed to dabigatran, rivaroxaban, or apixaban [29]. Further data are required to follow up on our findings.
Intraprocedural heparin administration
In our study, the lowest ACT levels were observed in the NOAC group, despite having the highest intraprocedural heparin doses. In the uVKA group, however, low heparin doses led to higher ACT levels. These findings have been reported previously by other authors [4, 31, 32]. The reason for higher heparin requirements in NOACs remains uncertain. A multifactorial genesis seems to be the underlying cause, and the above-mentioned authors have generated various hypotheses, such as: when pausing the NOAC, the intensity of therapeutic OAC at the time of ablation could be lower than under uVKA therapy. Furthermore, NOACs bind to antithrombin or factor-Xa, both of which are heparin targets. This could lead to competition at the binding site and to increased heparin administration.
Unfractionated heparin doses were higher in OAC with NOACs compared to uVKAs and iVKAs, without an increase in complication rate. We therefore hypothesize that periprocedural complications, especially bleeding complications, are not dependent on the amount of heparin administered. Possible explanations could be the short acting time of unfractionated heparin or the fact that intraprocedural coagulation status is controlled by ACT levels. Complications seem therefore influenced by the periprocedural OAC strategy and not by the dose of intraprocedural heparin. In clinical practice, this could reduce the fear of administering higher heparin doses when low ACT levels are measured.
At latest, our data represent a real-world cohort from a high-volume center and give valuable insight into daily clinical practice.
Study limitations
The two primary limitations of this study were that the data come from only one center and that the analysis of the persAF cohort was done in a retrospective fashion over the years 2011–2014. As a result, there was a bias caused by historical changes in OAC strategies. However, we accounted for this bias by including the survey year in the linear regression model. Furthermore, we included numerous confounders in the model to account for differences in patient characteristics due to non-randomization. In addition, no pts in this study cohort were prescribed edoxaban, as this medication had not received market approval at the time the study was conducted. Moreover, our trial focused on persAF; pts suffering from PAF were not included in this study.
Conclusion
Patients undergoing persistent atrial fibrillation ablation with interrupted vitamin K antagonists are exposed to an increased risk of complications compared to NOACs. Thromboembolic events are in general rare and are exceeded by minor bleeding complications. Complications lead to a prolonged hospital stay. Despite pausing NOACs 48 h before ablation in patients with persistent atrial fibrillation, not a single thromboembolic event occurred in this cohort.
References
Santangeli P, Di Biase L, Horton R, Burkhardt JD, Sanchez J, Al-Ahmad A et al (2012) Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications evidence from a meta-analysis. Circ Arrhythmia Electrophysiol 5(2):302–311
Nairooz R, Sardar P, Payne J, Aronow WS, Paydak H (2015) Meta-analysis of major bleeding with uninterrupted warfarin compared to interrupted warfarin and heparin bridging in ablation of atrial fibrillation. Int J Cardiol 187:426–429
Weitz JI, Healey JS, Skanes AC, Verma A (2014) Periprocedural management of new oral anticoagulants in patients undergoing atrial fibrillation ablation. Circulation 129(16):1688–1694
Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M et al (2013) Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythmia Electrophysiol 6(3):460–464
Kim J-S, She F, Jongnarangsin K, Chugh A, Latchamsetty R, Ghanbari H et al (2013) Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm 10(4):483–489
Kaess B, Ammar S, Reents T, Dillier R, Lennerz C, Semmler V et al (2015) Comparison of safety of left atrial catheter ablation procedures for atrial arrhythmias under continuous anticoagulation with apixaban versus phenprocoumon. Am J Cardiol 115(1):47–51
Garg J, Chaudhary R, Krishnamoorthy P, Shah N, Natale A, Bozorgnia B (2016) Safety and efficacy of uninterrupted periprocedural rivaroxaban in patients undergoing atrial fibrillation catheter ablation: a metaanalysis of 1362 patients. Int J Cardiol 203:906–908
Nagao T, Inden Y, Shimano M, Fujita M, Yanagisawa S, Kato H et al (2015) Feasibility and safety of uninterrupted dabigatran therapy in patients undergoing ablation for atrial fibrillation. Intern Med 54(10):1167–1173
Lakkireddy D, Reddy YM, Di Biase L, Vallakati A, Mansour MC, Santangeli P et al (2014) Feasibility and safety of uninterrupted rivaroxaban for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol 63(10):982–988
Ewen S, Rettig-Ewen V, Mahfoud F, Böhm M, Laufs U (2014) Drug adherence in patients taking oral anticoagulation therapy. Clin Res Cardiol 103(3):173–182
Di Biase L, Lakkireddy D, Trivedi C, Denke T, Martinek M, Mohanty S (2015) Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Hear Rhythm 12:1162–1168
Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA (2014) Peri-procedural interrupted oral anticoagulation for atrial fibrillation ablation: comparison of aspirin, warfarin, dabigatran, and rivaroxaban. Europace 16(10):1443–1449
Siegal D, Yudin J, Kaatz S, Douketis JD, Lim W, Spyropoulos AC (2012) Periprocedural heparin bridging in patients receiving Vitamin K antagonists: systematic review and meta-analysis of bleeding and thromboembolic rates. Circulation 126(13):1630–1639
Kim J-S, Jongnarangsin K, Latchamsetty R, Chugh A, Ghanbari H, Crawford T et al (2013) The optimal range of international normalized ratio for radiofrequency catheter ablation of atrial fibrillation during therapeutic anticoagulation with warfarin. Circ Arrhythmia Electrophysiol 6(2):302–309
Vogler J, Willems S, Sultan A, Schreiber D, Lüker J, Servatius H et al (2015) Pulmonary vein isolation versus defragmentation. J Am Coll Cardiol 66(24):2743–2752
Schreiber D, Rostock T, Frohlich M, Sultan A, Servatius H, Hoffmann BA et al (2015) Five-year follow-up after catheter ablation of persistent atrial fibrillation using the stepwise approach and prognostic factors for success. Circ Arrhythm Electrophysiol 8(2):308–317
Hoffmann BA, Kuck K-H, Andresen D, Spitzer SG, Hoffmann E, Schumacher B et al (2014) Impact of structural heart disease on the acute complication rate in atrial fibrillation ablation: results from the German Ablation Registry. J Cardiovasc Electrophysiol 25(3):242–249
Hsu JC, Chan PS, Tang F, Maddox TM, Marcus GM (2015) Differences in anticoagulant therapy prescription in patients with paroxysmal versus persistent atrial fibrillation. Am J Med 128(6):654.e1–654.e10
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J et al (2013) European heart rhythm association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace 15(5):625–651
Salukhe TV, Willems S, Drewitz I, Steven D, Hoffmann BA, Heitmann K et al (2012) Propofol sedation administered by cardiologists without assisted ventilation for long cardiac interventions: an assessment of 1000 consecutive patients undergoing atrial fibrillation ablation. Europace 14(3):325–330
Lankveld T, Zeemering S, Scherr D, Kuklik P, Hoffmann BA, Willems S et al (2016) Atrial fibrillation complexity parameters derived from surface ECGs predict procedural outcome and long-term follow-up of stepwise catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 9(2):e003354
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen S-A et al (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 9(4):632–696
Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R et al (2014) Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of Coumadin in preventing thromboembolism in atrial fibrillation (AF) patient. Circulation 129(25):2638–2644
Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS et al (2010) Long-term clinical efficacy and risk of catheter ablation for atrial fibrillation in octogenarians. Pacing Clin Electrophysiol 33:146–152
Nademanee K, Amnueypol M, Lee F, Drew CM, Suwannasri W, Schwab MC et al (2015) Benefits and risks of catheter ablation in elderly patients with atrial fibrillation. Heart Rhythm 12(1):44–51
Spragg DD, Dalal D, Cheema A, Scherr D, Chilukuri K, Cheng A et al (2008) Complications of catheter ablation for atrial fibrillation: incidence and predictors. J Cardiovasc Electrophysiol 19(6):627–631
Nührich JM, Kuck KH, Andresen D, Steven D, Spitzer SG, Hoffmann E et al (2015) Oral anticoagulation is frequently discontinued after ablation of paroxysmal atrial fibrillation despite previous stroke: data from the German Ablation Registry. Clin Res Cardiol 104(6):463–470
Kornej J, Kosiuk J, Hindricks G, Arya A, Sommer P, Rolf S et al (2015) Sex-related predictors for thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Clin Res Cardiol 104(7):603–610
Rillig A, Lin T, Plesman J, Heeger C-H, Lemes C, Metzner A et al (2016) Apixaban, rivaroxaban and dabigatran in patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol 27(2):147–153
Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W et al (2015) Updated European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 17(10):1467–1507
Nagao T, Inden Y, Yanagisawa S, Kato H, Ishikawa S, Okumura S et al (2015) Differences in the activated clotting time among uninterrupted anticoagulants during the periprocedural period of atrial fibrillation ablation. Heart Rhythm 12(9):1972–1978
Bin Abdulhak A, Kennedy K, Gupta S, Giocondo M, Ramza B, Wimmer A (2015) Effect of pre-procedural interrupted apixaban on heparin anticoagulation during catheter ablation for atrial fibrillation: a prospective observational study. J Cardiovasc Electrophysiol 44(2):91–96
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Gunawardene, M., Willems, S., Schäffer, B. et al. Influence of periprocedural anticoagulation strategies on complication rate and hospital stay in patients undergoing catheter ablation for persistent atrial fibrillation. Clin Res Cardiol 106, 38–48 (2017). https://doi.org/10.1007/s00392-016-1021-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-016-1021-x