Abstract
Purpose
Effective intraprocedural anticoagulation is considered essential to minimize the risk of thromboembolism in catheter ablation (CA) of atrial fibrillation (AF). The effect of interrupted apixaban on intraprocedural heparin dosing requirements and levels of achieved anticoagulation with heparin has not been well studied. The purpose of the present study was to compare heparin administration and activated clotted times (ACTs) for patients undergoing CA for AF treated with interrupted apixaban before the procedure with patients on uninterrupted warfarin.
Methods
Consecutive patients undergoing CA for AF treated with interrupted apixaban or uninterrupted warfarin were prospectively enrolled. Heparin administration determined by a standard protocol and normalized to patient weight and procedure duration, as well as rapidity, and degree of anticoagulation with heparin (as measured by mean ACT, peak ACT, time to ACT ≥300 s, and time to ACT ≥350 s) were compared between the groups.
Results
Forty-eight patients were enrolled (25 apixaban and 23 warfarin). Heparin administered by bolus (51.3 ± 21.5 vs 27.8 ± 9.6 units/kg/h; p < 0.001) and mean heparin drip rate (25.3 ± 3.6 vs 20.7 ± 2.4 units/kg/h; p < 0.001) were significantly higher in the apixaban group compared to the warfarin group. Despite greater heparin administration, apixaban patients achieved a significantly lower mean ACT (332.3 ± 17.0 vs 384.5 ± 53.9; p < 0.001) and peak ACT (369.5 ± 22.6 vs 432.3 ± 75.8, p < 0.001) compared to the warfarin group. The time to ACT ≥350 s (66.7 ± 35.8 vs 26.9 ± 34.0; p < 0.001) was significantly longer for apixaban-treated patients. Outcome differences persisted after analysis using linear models and Cox proportional hazard regression with adjustment for propensity scores.
Conclusions
A standard intraprocedural heparin protocol results in delayed and lower levels of anticoagulation as measured by the ACT for interrupted apixaban-treated patients in comparison to those on uninterrupted warfarin during CA of AF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Catheter ablation has become a common treatment for atrial fibrillation in patients unresponsive to or intolerant of antiarrhythmic therapy. Minimizing the risk of peri-procedural thromboembolism is a central safety focus for the procedure. Stroke and transient ischemic attack (TIA) rates have declined with the use of uninterrupted and more aggressive anticoagulation [1–7]. A common strategy now is to perform the procedure with the patient on therapeutic warfarin and to begin intravenous heparin prior to trans-septal catheterization (target activated clotting time (ACT) often 350 s or longer) and continue for the duration of the procedure [2–7]. Several recent alternatives to warfarin have been shown to be effective for stroke prevention in non-valvular atrial fibrillation [8]. However, a study performed at our institution found that reaching target ACTs was significantly delayed despite greater heparin administration when patients had been treated with the direct thrombin inhibitor dabigatran [9, 10]. Because of the findings of this study, our heparin dosing protocol for patients treated with dabigatran was modified, resulting in higher initial bolus administration and drip rates than for patients on warfarin.
More recently, apixaban, a factor Xa inhibitor, has been approved by the FDA for thromboembolism prevention in non-valvular atrial fibrillation. In a large clinical trial, apixaban was superior to warfarin in prevention of stroke and systemic embolism, bleeding risk, and survival [11]. However, the effect if any of interrupted apixaban on intraprocedural anticoagulation with intravenous heparin is unknown.
2 Objective
The objective of this study was to compare heparin administration and ACTs achieved for patients undergoing catheter ablation for atrial fibrillation treated with interrupted apixaban before the procedure with patients on uninterrupted warfarin.
3 Methods
3.1 Study design
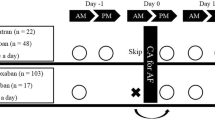
Consecutive patients undergoing catheter ablation for atrial fibrillation treated with warfarin or apixaban at our institution were prospectively enrolled from March 2013 through August 2014. Warfarin was continued without interruption peri-procedurally. Apixaban was held beginning the night before the procedure and resumed 3 h post-hemostasis at the vascular access site(s). Heparin dosing (normalized to patient weight and procedure duration), as well as rapidity, and level of anticoagulation with heparin (as measured by mean ACT, peak ACT, and time to ACT ≥350 s) were compared between the two groups. ACTs were measured using a commercially available analyzer (Hemochron® Response, ITC, Edison, NJ).
3.2 Intraprocedural anticoagulation protocol
After venous sheaths were placed and prior to trans-septal catheterization, all patients were given an initial intravenous heparin bolus (70 units/kg) and a maintenance heparin drip was started at 20 units/kg/h with a target ACT of ≥350 s. The ACT was tested every 20 min with administration of additional heparin boluses and titration of the heparin drip based on the results and according to the judgment of the operating physician. Typically, additional heparin boluses are 1000–4000 units and adjustments to the drip rate are either up or down by 100–400 units/h.
3.3 Statistical analysis
General characteristics and outcomes of the treatment groups were compared using the chi-square test for categorical variables and the t test for continuous variables. To account for differences between the treatment groups and possible selection bias, propensity score adjustment was used to evaluate the effect of treatment on the outcomes. Probabilities (propensity scores) for treatment assignment were derived by creating a logistic regression of treatment on baseline patient characteristics [12].
The independent association of the warfarin and apixaban groups was examined using multivariable linear regression for heparin administration measures, mean and peak ACTs, and time to ACT ≥350 s, with the propensity score used as a covariate adjustment. Additionally, time to ACT ≥350 s was plotted using Kaplan–Meier curves.
Further analyses were performed using longitudinal random-effect multivariable models to compare the velocity curves of ACT over procedure time for warfarin as compared with apixaban. The variables included in the model were treatment assignment, propensity scores of the treatment assignment, procedure time, and interactions between treatment and time. In order to incorporate the nonlinear time effects, restricted cubic spline terms and their interactions with treatment were included in the model. The intercept and time effects were estimated with the use of random effects, and the mean velocity curves for each treatment group were plotted using the estimated coefficients from the model.
All statistical analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC) and R Version 2.11.1 (Free Software Foundation, Boston, MA). Two-tailed tests were used for statistical significance at the level of 0.05.
4 Results
4.1 General characteristics
A total of 48 patients were enrolled in the study. Twenty-three patients were on uninterrupted warfarin and 25 patients were treated with interrupted apixaban. Table 1 lists the baseline clinical characteristics of both groups. The warfarin group was older (mean age 64.0 ± 7.0 vs 58.1 ± 12.3 years in the apixaban group; p = 0.047) and had more prior ablations, a larger left atrial size, and more coronary artery disease. Otherwise, the groups were similar with no significant difference in gender distribution, race, clinical history, and medications. The international normalized ratio (INR) was 2.3 ± 0.6 for warfarin and 1.0 ± 0.1 for apixaban patients (p < 0.001). Approximately 60 % of the ablations were done by radiofrequency ablation (the remainder by cryoballoon ablation), and there was no significant difference between treatment groups (Table 1). The procedure duration was shorter for apixaban-treated patients compared to those on warfarin (270.5 ± 60.0 vs 222.4 ± 41.21; p = 0.002). There was no significant difference in complication rates between the groups. One patient from the warfarin group developed a small pericardial effusion which did not require drainage, and two patients from the apixaban group had diaphragmatic paralysis. The diaphragmatic paralyses were diagnosed by loss of phrenic nerve capture during cryoballoon ablation and confirmed with a sniff test the following day. The pericardial effusion was diagnosed by echocardiogram. All patients were monitored with overnight in-hospital observation after their procedures. Diagnostic testing was performed when there was a clinical suspicion for a complication.
4.2 Heparin administration
Heparin administered by bolus (51.3 ± 21.5 vs 27.8 ± 9.6 units/kg/h; p < 0.001) and mean heparin drip rates (25.3 ± 3.6 vs 20.7 ± 2.4 units/kg/h; p < 0.001) were significantly higher in the apixaban group compared to the warfarin group. Despite this, apixaban patients achieved a significantly lower mean ACT (332.3 ± 17.0 vs 384.5 ± 53.9; p < 0.001) and peak ACT (369.5 ± 22.6 vs 432.3 ± 75.8, p < 0.001). The time to ACT ≥350 s (66.7 ± 35.8 vs 26.9 ± 34.0; p < 0.001) was significantly longer for apixaban-treated patients. Five patients in the apixaban group failed to achieve the target ACT ≥350 s during the entire procedure, in contrast to one patient in the warfarin group.
4.3 Multivariate adjustment
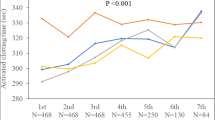
The observed differences persisted after adjustment using multivariable linear models and Cox proportional hazard regression adjusted for propensity scores of treatment groups. The mean differences between warfarin and apixaban were heparin bolus −38.5 units/kg/h, 95 % CI (−57.7, −19.2); p = 0.0002; mean heparin drip rate −6.2 units/kg/h, 95 % CI (−9.8, −2.6), p = 0.001; mean ACT 64.4 s, 95 % CI (15.6, 113.2), p = 0.01; peak ACT 82.6 s, 95 % CI (14.2, 151.0), p = 0.02; and time to ACT ≥350 −56.5 min, 95 % CI (−97.1, −15.9), p = 0.008, as shown in Fig. 1. The adjusted hazard ratio of warfarin vs apixaban for an ACT ≥350 s was 5.8 (95 % CI 1.4, 25.3), p = 0.02, indicating more than a fivefold increased likelihood of reaching an ACT ≥350 s during the procedure for patients on warfarin (associated Kaplan-Meier curves are shown in Fig. 2).
Mean ACT velocity curves for warfarin and apixaban plotted using the estimated coefficients from the longitudinal random-effect multivariable model are shown in Fig. 3. The curves show a relatively slow rise and prolonged time to the target ACT of 350 s in the apixaban group (p = 0.0002).
5 Discussion
In this study of heparin anticoagulation during CA of AF, patients treated with interrupted apixaban received significantly more heparin by bolus and drip, and yet achieved lower mean and peak ACTs, and had prolonged times to ACTs of 350 s. These differences persisted after multivariate adjustment.
Our findings are similar to what was previously observed with dabigatran-treated patients during catheter ablation of AF. Pre-procedural dabigatran was associated with higher heparin dosing and difficulties achieving target ACTs in comparison to uninterrupted warfarin [9].
Uninterrupted warfarin likely facilitates achieving target ACTs, with potential mechanisms including direct prolongation of the ACT, suppression of vitamin K-dependent prothrombin, and a neutral effect on antithrombin, preserving its availability to inhibit thrombin [9]. The exact mechanisms by which apixaban and dabigatran are associated with increased intraprocedural heparin requirements and lower levels of heparin anticoagulation as measured by the ACT are unknown. Interruption of the oral anticoagulant prior to catheter ablation in the case of apixaban and dabigatran may play a role. However, several recent studies, though reporting unadjusted data with more limited analyses of heparin dosing and ACTs, showed higher heparin administration and lower ACTs with uninterrupted apixaban in CA of AF in comparison to uninterrupted warfarin [13–15]. A direct negative effect of dabigatran has been suggested as a possible explanation for the observed difficulties of achieving target ACTs through downregulation of antithrombin, a substrate for heparin to exert its anticoagulant effect [8, 13]. There may be unidentified direct effect(s) of apixaban as well. Additionally, apixaban-treated patients have shown a diminished response to heparin compared to dabigatran possibly due to the fact that apixaban inhibits factor Xa activity, which heparin also uses to exert its action [13].
Uninterrupted warfarin with a therapeutic INR is a widely implemented strategy to decrease the risk of thromboembolic complications associated with CA of AF and has been suggested to be the most favorable approach [7]. Furthermore, some patients display delayed kinetics in response to intravenous heparin [16] and ACTs less than 300 s during the procedure have been associated with silent cerebral ischemia [17]. Although data are thus far lacking to indicate a higher peri-procedural thromboembolic risk with apixaban in comparison to warfarin, interruption of the anticoagulant (due to non-availability of a reversal agent) in addition to delayed and sub-therapeutic heparinization, both raise concern regarding potential increased thromboembolic risk, including silent cerebral ischemia [17, 18].
5.1 Clinical significance
Delayed and often sub-target anticoagulation in interrupted apixaban-treated patients raises concern regarding intraprocedural thromboembolic risk with use of apixaban in CA of AF. Whether sub-therapeutic anticoagulation can and should be overcome with more aggressive heparin administration remains to be determined.
5.2 Limitations
This study is small, not controlled, and observational and subject to inherent biases of such study design. However, rigorous statistical methods were used to adjust for possible confounders and biases. Though the sample size was small, the results were robust, consistent, and statistically significant even after adjustments. In addition, the findings are consistent with another recently reported study [18]. Nagao et al. reported that patients treated with uninterrupted apixaban in comparison to uninterrupted warfarin during catheter ablation of atrial fibrillation required more intraprocedural heparin, achieved lower ACTs, and required a longer time to reach target ACTs [18]. The current study was not powered to assess outcomes such as stroke, mortality, and bleeding.
6 Conclusion
A standard intravenous heparin protocol failed to achieve a similar rapidity and degree of anticoagulation as assessed by the ACT in interrupted apixaban-treated patients in comparison to patients on uninterrupted warfarin during CA of AF. Further studies are needed to confirm these findings, demonstrate potential mechanisms, and determine if there is an effect on outcomes.
References
Oral, H., Chugh, A., Ozaydin, M., Good, E., Fortino, J., et al. (2006). Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation, 114, 759–765.
Kwak, J., Pak, H., Jang, J., Kim, S., Park, J., Choi, J., et al. (2010). Safety and convenience of continuous warfarin strategy during the periprocedural period in patients who underwent catheter ablation of atrial fibrillation. Journal of Cardiovascular Electrophysiology, 21, 620–625.
Bruce, C., Friedman, P., Narayan, O., Munger, T., Hammill, S., Packer, D., et al. (2008). Early heparinization decreases the incidence of left atrial thrombi detected by intracardiac echocardiography during radiofrequency ablation for atrial fibrillation. Journal of Interventional Cardiac Electrophysiology, 2(3), 211–219.
Di Biase, L., Burkhardt, J., Mohanty, P., Sanchez, J., Horton, R., Gallinghouse, G., et al. (2010). Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation, 121(23), 2550–2556.
Gopinath, D., Lewis, W. R., Biase, L. D., & Natale, A. (2011). Pulmonary vein antrum isolation for atrial fibrillation on therapeutic coumadin: special considerations. Journal of Cardiovascular Electrophysiology, 22(2), 236–239.
Hussein, A., Martin, D., Saliba, W., Patel, D., Karim, S., Batal, O., et al. (2009). Radiofrequency ablation of atrial fibrillation under therapeutic international normalized ratio: a safe and efficacious periprocedural anticoagulation strategy. Heart Rhythm, 10, 1425–1429.
Di Biase, L., Burkhardt, J. D., Santangeli, P., Mohanty, P., Sanchez, J. E., & Horton, R. (2014). Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of Coumadin In Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing CatheterAblation (COMPARE) randomized trial. Circulation, 129, 2638–2644.
Sattari, M., & Lowenthal, D. (2011). Novel oral anticoagulants in development: dabigatran, rivaroxaban, and apixaban. American Journal of Therapeutics, 18, 332–338.
Konduru, S., Cheema, A., Jones, P., Li, Y., Ramza, B., & Wimmer, A. (2012). Heparinization during catheter ablation for atrial fibrillation: a comparison of patients treated with dabigatran vs patients maintained on warfarin. Journal of Interventional Cardiac Electrophysiology, 35(3), 277–284.
Snipelisky, D., Ray, J. C., Ung, R., Duart, M., Kauffman, C., & Kusumoto, F. (2014). A comparison of bleeding complications between warfarin, dabigatran, and rivaroxaban in patients undergoing cryoballoon ablation. Journal of Interventional Cardiac Electrophysiology, 41, 231–236.
Granger, C., Alexander, J., & McMurray, J. (2011). Apixaban versus warfarin patients with atrial fibrillation. New England Journal of Medicine, 365, 981–992.
D’Agostino, R., Jr. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine, 17(19), 2265–2281.
Nagao, T., Inden, Y., Shimano, M., Fujita, M., Yanagisawa, S., Kato, H., et al. (2015). Efficacy and safety of apixaban in the patients undergoing the ablation of atrial fibrillation. Pacing and Clinical Electrophysiology, 38(2), 155–163.
Di Biase, L., Lakkireddy, D., Trivedi, C., Deneke, T., Martinek, M., Mohanty, S., et al. (2015). Feasibility and safety of uninterrupted periprocedural apixaban administration in patients undergoing radiofrequency catheter ablation for atrial fibrillation: results from a multicenter study. Heart Rhythm, 12(6), 1162–1168.
Kaess, B. M., Ammar, S., Reents, T., Dillier, R., Lennerz, C., Semmler, V., et al. (2015). Comparison of safety of left atrial catheter ablation procedures for atrial arrhythmias under continuous anticoagulation with apixaban versus phenprocoumon. American Journal of Cardiology, 115, 47–51.
Gabus, V., Rollin, A., Maury, P., Forclaz, A., Pascale, P., Dhutia, H. et al. (2015) Short-term heparin kinetics during catheter ablation of atrial fibrillation. PACE 0: 1–9.
Di Biase, L., Gaita, F., Toso, E., Mohanty, P., Rutledge, N., & Yan, X. (2014). Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm, 11(5), 791–798.
Nagao, T., Inden, Y., Yanagisawa, S., Kato, H., Ishikawa, S., Okumura,S.,et al. (2015). Differences in activated clotting time among uninterrupted anticoagulants during the periprocedural period of atrial fibrillation ablation. Heart Rhythm.
Acknowledgments
We would like to thank Laura Oaks and Lijia Lyles for their invaluable support in data acquisition and maintaining our AF ablation database.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bin Abdulhak, A.A., Kennedy, K.F., Gupta, S. et al. Effect of pre-procedural interrupted apixaban on heparin anticoagulation during catheter ablation for atrial fibrillation: a prospective observational study. J Interv Card Electrophysiol 44, 91–96 (2015). https://doi.org/10.1007/s10840-015-0048-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-015-0048-7