Abstract
Aims
Atrial fibrillation (AF) is the most common cause of ischemic stroke. Recent data suggest that AF patients after successful ablation have the same risk for thromboembolic events (TE) as patients without AF. Despite current guideline recommendations it is still under debate if oral anticoagulation (OAC) can be safely discontinued after ablation. We analyzed follow-up (FU) after ablation of paroxysmal AF (PAF) in a high- (previous stroke; group 1) and a low-risk group (no previous stroke; group 2) based on data from the German Ablation Registry to reveal real-life prescription behavior.
Methods
Overall 29 centers in Germany participated by performing AF-ablation. Between April 2008 and April 2011, 83 patients in group 1 and 377 patients in group 2 with a first ablation of PAF were included in the registry.
Results
Mean CHA2DS2-VASc-Score was 4.2 ± 1.4 (group 1) vs. 1.6 ± 1.2 (group 2) (p < 0.0001). No peri-interventional TE was observed. Arrhythmia recurrence was seen in 47.4 vs. 48.4 % (p = 0.79) during a median FU of 489 (453–782) days, resulting in a repeat procedure in 20.0 vs. 20.7 % (p = 0.88), respectively. OAC was discontinued in 38.6 % in group 1 vs. 66.3 % in group 2 (p < 0.0001) during FU. TE during FU occurred more often in group 1 than in group 2 (4.3 vs. 0.3 %, p < 0.05).
Conclusion
Even in patients with previous stroke, OAC was frequently discontinued during FU after PAF ablation in this observational study. However, TE occurred significantly more frequent in these high-risk patients. These data argue against OAC discontinuation after ablation in patients with previous stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is the most common cause of ischemic strokes [1]. Data from the Framingham study report a twofold increased relative risk of stroke in patients with AF compared to the general population [1]. In clinical routine the CHADS2/CHA2DS2-VASc-Score is used for individual risk stratification [2]. Oral anticoagulation (OAC) for the prevention of thromboembolic events (TE) is recommended in patients with a CHADS2-Score ≥1 or a CHA2DS2-VASc-Score ≥2.
Pulmonary vein isolation (PVI) has become an established curative therapeutic option in patients with paroxysmal atrial fibrillation (PAF) and achieves 5-year success rates up to 80 % [3, 4]. Despite this fact, the guidelines recommend to continue OAC after AF-ablation depending on the individual CHADS2/CHA2DS2-VASc-Score, irrespective of the procedural success [2].
However, recent data suggest that patients after successful AF-ablation have the same risk for TE as patients without AF [5–7]. Hunter et al. [6] observed an annual stroke rate of 0.5 % per year after ablation. Still, most of these studies only included patients with a low CHADS2-Score and therefore a low risk for TE. Only Guiot et al. [8] included patients aged ≥65 years, thus having an increased risk for TE. The annual stroke rate after AF-ablation in this cohort was 1.1 %. Of note, mean CHADS2-Score was 1.1 ± 0.9 in these patients, which is not markedly increased.
It is yet unclear, whether patients at high risk, with previous stroke, develop TE after ablation more often than patients at low risk. Furthermore—despite current guidelines—it is still under debate whether OAC can be safely discontinued after ablation of PAF. Thus, we performed a centralized follow-up (FU) regarding the frequency of discontinuation of OAC after AF-ablation in clinical routine. A high-risk group, defined by a history of previous stroke before ablation, was compared to a low-risk group without previous stroke. Furthermore, we were interested in TE after ablation of PAF.
Methods
Recruiting sites
A total of 55 German electrophysiological centers agreed to participate in this prospective multicenter registry. 29 centres performed ablation of atrial fibrillation and included patients for this study.
Patient population
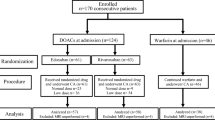
All patients aged ≥18 years who underwent a de novo catheter ablation of PAF in their respective centers were enrolled between April 2008 and April 2011 after written informed consent was obtained. Patients with a relevant valvular heart disease (Grade ≥ 2) or undergoing atrioventricular node ablation were excluded. The minimum FU was 12 months. A total cohort of 460 patients was divided into two groups: a high-risk group with previous stroke before ablation (n = 83, 18.0 %), and a low-risk group without previous stroke (n = 377, 82.0 %).
Catheter ablation procedure
The mode of the catheter ablation, such as the ablation catheter, ablation energy, non-fluoroscopic navigation system, magnetic or remote navigation, as well as the pre- and peri-procedural management were chosen at the physician’s discretion according to the institutional standards. All ablation strategies aimed at electrical isolation of the pulmonary veins which probably impacts both trigger and substrate of AF [9, 10].
Transesophageal echocardiography was performed routinely before the ablation procedure to exclude intracardiac thrombus formation.
OAC was administered at the discretion of the treating physician for an individual period, but at least for 3 months. Antiarrhythmic drugs were also continued at the discretion of the treating physician.
Follow-up
The postinterventional monitoring regarding arrhythmia recurrences and adverse events was chosen at the physician’s discretion according to the institutional standards.
A centralized FU was conducted via telephone interviews. A thromboembolic event was defined as either stroke or transient ischemic attack (TIA).
Registry management
This registry was carried out by the Institute for Research in Myocardial Infarction (Ludwigshafen, Germany) which was responsible for project development, project management, data management, clinical monitoring and the telephone interviews. The institute was the central contract research organization for the study. Documentation and data management were paperless and carried out as an internet-based case report form system. All site information was confidential and transmitted data were encrypted with a secure socket layer. The development of the biometric model as well as planning and performing all statistical analyses was also done by the institute.
Statistical analysis
Data are shown as absolute values, percentages, means with standard deviation or medians with 25 and 75 % quartiles (interquartile range, IQR). For statistical comparisons, the Chi-square test was used with categorical or dichotomous variables and the non-parametric Kruskal–Wallis test with metric variables. All statistical comparisons were two sided, with p < 0.05 being accepted as statistically significant. All analyses were performed using the Statistical Analysis System (SAS, Version 9.2, SAS Institute Inc., Cary, NC, USA).
Results
Baseline data
Between April 2008 and April 2011, 83 patients in the high-risk group and 377 patients in the low-risk group with a de novo catheter ablation of non-valvular PAF were included in the registry. The baseline data of these patients are shown in Table 1. Mean CHADS2-Score was 0.7 ± 0.7 vs. 2.9 ± 0.7 (p < 0.0001), mean CHA2DS2-VASc-Score was 1.6 ± 1.2 vs. 4.2 ± 1.4 (p < 0.0001) in the low- vs. the high-risk group, respectively. 59.5 % (100/168) of those patients with a CHA2DS2-VASc-Score >1 in the low-risk group were female. Mean age [60 years (52–67) vs. 66 years (62–72), p < 0.0001] was higher and a greater portion of patients aged ≥75 years (3.4 vs 8.4 %, p < 0.05) was found in the high-risk group. The respective proportions of the different score groups are shown in Fig. 1. The FU was significantly longer in the high-risk group (625.5 vs. 493.0 days, p < 0.0001).
Procedural data
The procedural data are shown in Table 2. There were no significant differences between the two groups, neither regarding the ablation technique, the energy source, nor the procedure related complications. The procedural success was also comparable. No peri-interventional TE was observed. Severe procedural-related complications (defined as myocardial infarction, stroke, major bleeding) occurred in 1.6 vs. 2.4 % (p = 0.61), in the high-risk group vs. the low-risk group, respectively. Of note, only major bleeding was observed while no procedural-related myocardial infarction or stroke occurred. Moderate procedural-related complications (defined as aneurysm, pneumothorax, cardiac tamponade, 3rd degree atrioventricular (AV)-block) occurred in 3.0 vs. 3.7 % (p = 0.75) and minor procedural-related complications (defined as minor bleeding, 1st and 2nd degree AV-block) in 5.2 vs. 4.9 % (p = 0.92), in the high-risk group vs. the low-risk group, respectively. No 1st or 2nd degree AV-block was observed in this registry cohort. No atrial esophageal fistula was observed.
Arrhythmia recurrence
Recurrence of any atrial arrhythmia was seen in 47.4 vs. 48.4 % (p = 0.79, Fig. 2), resulting in a repeat procedure in 20.0 vs 20.7 % (p = 0.88), in the high- vs. the low-risk group, respectively. At the 1-year follow-up, 69.8 vs 74.3 % (p = 0.46) patients were on betablocker-, 14.8 vs 11.4 % (p = 0.46) on class-I-AAD- and 12.7 vs. 11.4 % (p = 0.77) on class-III-AAD-treatment in the high- vs the low-risk-group, respectively.
Anticoagulation during FU
At the time of hospital discharge all patients were on OAC or bridging low-weight heparin therapy. During FU, OAC was—despite current guideline recommendation—discontinued in 38.6 % in the high-risk group vs. 66.3 % in the low-risk group (p < 0.0001) as shown in Fig. 3. At the same time, the proportion of patients on acetylsalicylic acid (ASA) medication increased, this increase was more pronounced in the low-risk group (18.6 vs 33.4 %, p < 0.05).
A combined therapy with ASA and OAC was present in 3 vs. 9 patients with ASA therapy. Thus, 24 % of patients in the high-risk group neither received anticoagulation nor platelet inhibition during FU.
Table 3 shows frequency in OAC discontinuation with respect to arrhythmia recurrence.
Thromboembolic and other adverse events during FU
TE during FU occurred more often in the high-risk group than in the low-risk group (Table 4; Fig. 4). The calculated annual thromboembolic stroke rate was 1.4 vs. 0 % in the high- vs. the low-risk group, respectively. Data of the four individuals with TE during FU are shown in Table 5.
Other adverse events, such as myocardial infarction (1.4 vs. 0 %, p < 0.05) or combined endpoints such as MACCE (major adverse cardiac and cerebrovascular events: death, myocardial infarction, thromboembolic stroke; 4.3 vs. 0.6 %, p < 0.05) or severe non-fatal adverse events (myocardial infarction, thromboembolic stroke, major bleeding; 5.8 vs. 0.03 %, p < 0.001) also occurred more often in the high-risk group, while there was no significant difference in major bleeding (1.4 vs. 0.3 %, p = 0.21). Two sudden cardiac deaths occurred in the low-risk group, while no death was observed in the high-risk group (p = 0.5).
Discussion
Main findings
We report on the continuation of OAC as well as the occurrence of TE after ablation of PAF in a large observational registry. First of all, OAC was discontinued after AF-ablation in over 60 % in the low-risk group and in nearly 40 % of high-risk patients despite having a history of previous stroke. In addition, recurrent TE occurred significantly more often in the high-risk group, that is with previous stroke before ablation, of this registry cohort.
The proportion of patients with stroke prior to ablation in this cohort is higher than in other population-base cohorts. In comparison, the Leipzig Heart Center AF-ablation registry found 9 % of their AF-ablation patients to have had a previous stroke [11]. Of note, an additional FU of patients with previous stroke which otherwise would not have been able to be included because of a too short FU was conducted. Therefore, patients in the low- (29.04.2008–26.10.2010) and in the high-risk group were included during two different periods of time (10.02.2009–18.04.2011).
Anticoagulation during FU
Surprisingly, this real life registry showed that OAC was discontinued frequently after AF-ablation in both groups, despite the current guideline recommendations [2]. A current survey in Canada also found that physicians are likely to discontinue OAC when there is no evidence for arrhythmia recurrence [12]. As to be expected and in line with previous studies [8], withdrawal of OAC occurred more often in low-risk patients. Still, these patients had a mean CHADS2-Score of 0.7 ± 0.7 and were thus predominantly recommended to receive ongoing OAC after AF-ablation regardless of the procedural outcome.
Furthermore, even in the high-risk group—that is in patients with previous stroke and thus a minimum CHADS2-Score of 2—OAC was discontinued in over a third of patients, although a previous stroke is one of the strongest predictors for recurrent TE [13]. Whether to receive OAC or not should be decided regardless of procedural outcome, in part because the risk factors for AF and stroke overlap and those risk factors predominantly remain even after successful AF-ablation. AF might even only be a risk marker for TE rather than the cause [14].
Moreover, as procedural success rates of AF-ablation still need to improve and even late recurrences after several years are observed [15], the definition of long-term ablation success remains difficult. Therefore, patients who were considered to have had a successful ablation procedure may experience late arrhythmia recurrence and are then again at high risk for TE due to underlying risk factors. In one study, late recurrences were even more frequent in patients with high CHADS2-Score [15].
Increase of ASA therapy during FU might be related either to changing OAC to ASA or to discontinuation of OAC and prescription of ASA due to comorbidities, such as coronary artery disease. The first explanation however, would not meet the current guidelines as ASA is no longer recommended as an alternative to OAC in patients with low CHA2DS2-VASc-Score [2].
Thromboembolic events
The rate of recurrent TE in this study was higher than previously reported [5, 6]. Of note, the mean CHADS2-/CHA2DS2-VASc-Score and therefore the risk for TE in this cohort, was markedly increased compared to those studies. In addition, in previous studies those patients with higher risk factors and especially patients with previous stroke mostly continued OAC which might underestimate the actual stroke rate [6].
Recurrent TE in this study occurred significantly more often in the high-risk group. This finding is in line with previous studies [16]. However, it has to be kept in mind, that the overall event rate was very low. Despite this low event rate and the differences in FU time, the calculated annual thromboembolic stroke rate was still increased in the high-risk group compared to the low-risk group as well as compared to previous studies and the estimated risk in the general population [6]. Although all patients with recurrent TE also had recurrence of an atrial arrhythmia, the overall recurrence rate of atrial arrhythmias was comparable in both groups. Thus, recurrence of TE might be determined rather by the risk profile than by the arrhythmia recurrence. While we did not observe group differences regarding arrhythmia recurrence, a previous study found a poorer outcome after AF-ablation in patients with a high CHADS2-Score [6, 17, 18].
Interestingly, in those individuals with a TE during FU, OAC was not discontinued. This does not exclude insufficient time in therapeutic range as a cause for these TE. Unfortunately, the INR at the time of the TE cannot be investigated, as it was not included in the case report form. However, this finding also emphasizes that OAC can only reduce the risk for a TE, but not completely prevent it. Keeping those patients at high risk for a TE in sinus rhythm should therefore be pursued.
Study limitations
First of all, the current investigation relies on single-country registry data and was not a prospective randomized trial. Therefore, important data such as the mean time to OAC discontinuation as well as the time in therapeutic range are missing. If OAC can safely be discontinued after AF-ablation or if it really needs to be maintained irrespective of the procedural success has to be answered in randomized clinical trials. Still, the data reflect a real-life scenario in patients with a high risk for thromboembolic events and give insight in clinical routines apart from a study setting. Especially in terms of OAC prescription, this seems to be of particular interest.
Second, as a major limitation the event rate in this cohort was very low. However, the calculated annual thromboembolic stroke rate in the high-risk group was still markedly increased compared to the low-risk group and to the general population without AF. Due to this low event rate in our study no analysis of predicting factors for recurrent TE could be conducted as the study was not powered to detect those.
Third, the FU was significantly longer in the high-risk group. This fact is due to later addition of the variable “previous stroke” to the case report form and therefore low inclusion number of patients in the high-risk group. To increase the number of included patients an additional FU of patients with previous stroke which otherwise would not be able to be included due to FU <12 months was performed.
Finally, included patients only received warfarin as an OAC. However, novel anticoagulants impact on the event rates and the continuation after AF-ablation remains to be investigated.
Conclusion
Despite current guideline recommendations, we demonstrated that OAC is frequently discontinued during FU after AF-ablation. This happens even in patients with previous stroke and therefore a high thromboembolic risk. In addition, TE after ablation of PAF occurred significantly more frequent in these high-risk patients despite similar AF recurrence rates. These data argue against discontinuation of OAC after AF-ablation in patients with previous stroke. The predictive value of the CHA2DS2-VASc-Score seems to persist after ablation irrespective to the arrhythmic outcome. Randomized clinical trials are needed to answer the question whether OAC can safely be discontinued after AF-ablation in high-risk patients or if it needs to be maintained irrespective of the procedural success.
Abbreviations
- AF:
-
Atrial fibrillation
- ASA:
-
Acetylsalicylic acid
- CFAE:
-
Complex fractionated atrial electrogram
- FU:
-
Follow-up
- LVEF:
-
Left ventricular ejection fraction
- MACCE:
-
Major adverse cardiac and cerebrovascular events: death, myocardial infarction, stroke
- Min:
-
Minutes
- OAC:
-
Oral anticoagulation
- PAF:
-
Paroxysmal atrial fibrillation
- PVI:
-
Pulmonary vein isolation
- RF:
-
Radiofrequency
- SAE:
-
Severe non-fatal adverse events: myocardial infarction, stroke, major bleeding
- TE:
-
Thromboembolic events
- TIA:
-
Transient ischemic attack
References
Wolf PA, Abbott RD, Kannel WB (1991) Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 22:983–988
Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De CR, De SJ, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12:1360–1420
Ouyang F, Tilz R, Chun J, Schmidt B, Wissner E, Zerm T, Neven K, Kokturk B, Konstantinidou M, Metzner A, Fuernkranz A, Kuck KH (2010) Long-term results of catheter ablation in paroxysmal atrial fibrillation: lessons from a 5-year follow-up. Circulation 122:2368–2377
Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A, Williams CJ, Sledge I (2009) Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2:349–361
Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, Reich S, Igic P, Elmouchi D, Tschopp D, Wimmer A, Dey S, Crawford T, Pelosi F Jr, Jongnarangsin K, Bogun F, Morady F (2006) Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114:759–765
Hunter RJ, McCready J, Diab I, Page SP, Finlay M, Richmond L, French A, Earley MJ, Sporton S, Jones M, Joseph JP, Bashir Y, Betts TR, Thomas G, Staniforth A, Lee G, Kistler P, Rajappan K, Chow A, Schilling RJ (2012) Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 98:48–53
Themistoclakis S, Corrado A, Marchlinski FE, Jais P, Zado E, Rossillo A, Di BL, Schweikert RA, Saliba WI, Horton R, Mohanty P, Patel D, Burkhardt DJ, Wazni OM, Bonso A, Callans DJ, Haissaguerre M, Raviele A, Natale A (2010) The risk of thromboembolism and need for oral anticoagulation after successful atrial fibrillation ablation. J Am Coll Cardiol 55:735–743
Guiot A, Jongnarangsin K, Chugh A, Suwanagool A, Latchamsetty R, Myles JD, Jiang Q, Crawford T, Good E, Pelosi F Jr, Bogun F, Morady F, Oral H (2012) Anticoagulant therapy and risk of cerebrovascular events after catheter ablation of atrial fibrillation in the elderly. J Cardiovasc Electrophysiol 23:36–43
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm 9:632–696
Ouyang F, Bansch D, Ernst S, Schaumann A, Hachiya H, Chen M, Chun J, Falk P, Khanedani A, Antz M, Kuck KH (2004) Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-Lasso technique in paroxysmal atrial fibrillation. Circulation 110:2090–2096
Kornej J, Hindricks G, Kosiuk J, Arya A, Sommer P, Husser D, Rolf S, Richter S, Piorkowski C, Gaspar T, Lip GY, Bollmann A (2013) Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 6:868–874
Mardigyan V, Verma A, Birnie D, Guerra P, Redfearn D, Becker G, Champagne J, Sapp J, Gula L, Parkash R, Macle L, Crystal E, O’Hara G, Khaykin Y, Sturmer M, Veenhuyzen GD, Greiss I, Sarrazin JF, Mangat I, Novak P, Skanes A, Roux JF, Chauhan V, Hadjis T, Morillo CA, Essebag V (2013) Anticoagulation management pre- and post atrial fibrillation ablation: a survey of canadian centres. Can J Cardiol 29:219–223
Pisters R, Lane DA, Marin F, Camm AJ, Lip GY (2012) Stroke and thromboembolism in atrial fibrillation. Circ J 76:2289–2304
Lip GY (1995) Does atrial fibrillation confer a hypercoagulable state? Lancet 346:1313–1314
Chao TF, Ambrose K, Tsao HM, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Wu TJ, Chen SA (2012) Relationship between the CHADS(2) score and risk of very late recurrences after catheter ablation of paroxysmal atrial fibrillation. Heart Rhythm 9:1185–1191
Chao TF, Lin YJ, Tsao HM, Tsai CF, Lin WS, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Ambrose K, Wu TJ, Chen SA (2011) CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol 58:2380–2385
Bunch TJ, Crandall BG, Weiss JP, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, Day JD (2011) Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 22:839–845
Chao TF, Cheng CC, Lin WS, Tsao HM, Lin YJ, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Liu SH, Hartono B, Wu TJ, Chen SA (2011) Associations among the CHADS(2) score, atrial substrate properties, and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm 8:1155–1159
Acknowledgments
This project was supported by an unrestricted grant from the Institute for Research in Myocardial Infarction (Ludwigshafen, Germany).
Conflict of interest
All authors declare no conflicts of interest regarding this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nührich, J.M., Kuck, KH., Andresen, D. et al. Oral anticoagulation is frequently discontinued after ablation of paroxysmal atrial fibrillation despite previous stroke: data from the German Ablation Registry. Clin Res Cardiol 104, 463–470 (2015). https://doi.org/10.1007/s00392-014-0804-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-014-0804-1