Abstract
Purpose
The aim of the study was to compare short- and long-term outcomes of laparoscopic surgery and conventional open surgery for colorectal cancer.
Methods
Published randomized controlled trial (RCT) reports of laparoscopic surgery and open surgery for colorectal cancer were searched, and short- and long-term factors were extracted to perform meta-analysis.
Results
A total of 15 RCT reports (6,557 colorectal cancer patients) were included in this study. Blood loss of laparoscopic surgery was less by 91.06 ml than open surgery (p = 0.044). Operation time was longer by 49.34 min (p = 0.000). The length of hospital stay was shorter by 2.64 days (p = 0.003). Incisional length was shorter by 9.23 cm (p = 0.000). Fluid intake was shorter by 0.70 day (p = 0.001). Bowel movement was earlier by 0.95 day (p = 0.000). Incidence of complications, blood transfusion, and 30 days death were significantly lower in laparoscopic surgery than in open surgery (p = 0.011, 0.000, 0.01). But there was no significant difference in lymph nodes (p = 0.535) and anastomotic leak (p = 0.924). There was also no significant difference in 3 and 5 years overall survival (p = 0.298, 0.966), disease-free survival (p = 0.487, 0.356), local recurrence (p = 0.270, 0.649), and no difference in 5 years distant recurrence (p = 0.838).
Conclusions
Laparoscopic surgery is a mini-injured approach which can cure colorectal cancer safely and radically, and it is not different from conventional open surgery in long-term effectiveness, so laparoscopic surgery can be tried to widely use in colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is a common disease which is the fourth reason resulted to patients’ death [1]. Since the first laparoscopic colorectal surgery was operated successfully in the year 1991 [2], laparoscopic surgery is widely performed in the colorectal cancer, and the skill is becoming more and more mature. Its’ security, feasibility, and short-term curative effect have already been verified [3, 4]. Some randomized controlled trials (RCTs) have gotten the result that laparoscopic colorectal surgery (LCS) had the better short-term outcomes than open colorectal surgery (OCS), for example, less blood loss, better quality life, less pain, the shorter time of return to normal life and shorter length of hospital stay, and so on [5, 6]. But the post-operation recurrence is the most important problem which we should consider. And there are few reports about meta-analysis results of post-operation recurrence between laparoscopic and open surgery, while it is essential first-class evidence of evidence-based medicine, so several RCTs comparing LCS and OCS’s short- and long-term outcomes were selected to have been done meta-analysis. And the factors of 3 and 5 years following up period below were concluded to evaluate the long-term results of LCS.
Materials and methods
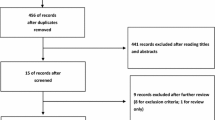
We looked up many materials about RCTs of colorectal cancer comparing LCS and OCS which were published from January 1991 to June 2013 and searched the major medical databases such as Pubmed, Embase, Ovid, ScienceDirect, Springer, Interscience, and so on. The search terms were used: “laparoscopy surgery,” “colorectal cancer,” “open surgery,” “randomized controlled trial,” and so on. Furthermore, we limited our search to those studies that involved a following up period of 3 or 5 years to evaluate the long-term outcomes of LCS. We conducted a meta-analysis for the short and long term. For the short-term analysis, we collected data of the operation time, blood loss, number of patients requiring blood transfusion, number of harvested lymph nodes, time of fluid intake, bowel movement, anastomotic leak, length of hospital stay, length of operation incision, complications, and 30 days death. For the long-term analysis, we used data of the rate of 3 years local recurrence, 3 years overall survival rate, 3 years disease-free survival rate, 5 years overall survival rate, 5 years disease-free survival rate, 5 years local recurrence rate, and 5 years distant recurrence.
Statistical analysis
Weighted mean difference (WMD) and odds ratio (OR) were used for the variables analysis of continuous and dichotomous, respectively. χ 2 test was used to evaluate heterogeneity among the studies, and I 2 was used to quantify the inconsistency (there were two models: fixed effect model and random effect model. The fixed effect model was used when the effects were deemed to be homogeneous (p > 0.1, I 2 < 50 %); otherwise, the random effects model was used). And Z test was used to compare the overall difference. The confidence interval (CI) was established at 95 %, and p values of less than 0.05 were considered to indicate statistical significance. Begg’s test and Egger’s test were performed in order to evaluate the publication bias (in Begg’s test p > 0.05 and in Egger’s test p > 0.05 and 95 % CI includes 1; it is thought that there was no publication bias). Statistical analyses were performed using the stata12.0 (meta module) software.
Results
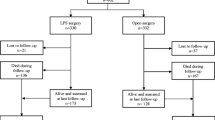
At last 15 papers of RCTs that compared LCS and OCS for colorectal cancer [5–20] were selected. The characteristics of each RCT are presented in Table 1. This meta-analysis included 6,557 patients with colorectal cancer in all, of which 3,509 had performed LCS and 3,048 had OCS. The results of the short and long term are shown in Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, and 18, respectively, and the data are presented in Tables 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and 19.
Short-term outcomes
The blood loss for LCS was significantly less than for OCS, by an average volume of 91.06 ml (WMD = −91.06; 95 % CI = −179.66 to −2.46; p = 0.044); six of the 15 RCTs included data of blood loss. Operation time for LCS was significantly longer than for OCS, by 49.34 min (WMD = 49.34; 95 % CI = 29.57 to −69.12; p = 0.000); five of the 15 RCTs included data of operation time. The length of hospital stay for LCS was significantly shorter than for OCS, by 2.64 days (WMD = −2.64; 95 % CI = −4.41 to −0.87; p = 0.003); six of the 15 RCTs included data of the length of hospital stay. The incisional length for LCS was significantly shorter than for OCS, by an average of 9.23 cm (WMD = −9.23; 95 % CI = −13.77 to −4.68; p = 0.000); four of the 15 RCTs included data of incisional length. The bowel movement time for LCS was significantly shorter than for OCS, by an average of 0.95 day (WMD = −0.95; 95 % CI = −1.18 to −0.73; p = 0.000); three of the 15 RCTs included data of bowel movement. The fluid intake for LCS was significantly shorter than for OCS, by 0.70 day (WMD = −0.70; 95 % CI = −1.11 to −0.29; p = 0.001); four of the 15 RCTs included data of the fluid intake. There were no significant differences in lymph nodes between the LCS group and the OCS group for treatment of the colorectal cancer. The rate of perioperative complications for patients in the LCS group was significantly lower than for those in the OCS group in this analysis of the pooled data for colorectal cancer treatment (OR = 0.86; 95 % CI = 0.77–0.97; p = 0.011). Twelve of the 15 RCTs included data of perioperative complications. The number of blood transfusion in the LCS group was significantly lower than that in the OCS group in this analysis of the pooled data for colorectal cancer treatment (OR = 0.46; 95 % CI = 0.32–0.65; p = 0.000). Three of the 15 RCTs included data of blood transfusion. There were no significant differences in anastomotic leak between the LCS group and the OCS group for the treatment of the colorectal cancer.
The rate of 30 days death in the LCS group was significantly lower than in the OCS group in this analysis of the pooled data for colorectal cancer treatment (OR = 0.58; 95 % CI = 0.38–0.88; p = 0.01). Seven of the 15 RCTs included data of 30 days death.
Long-term outcomes
We found no significant differences in the rate of 3 years local recurrence between the surgery groups when we pooled data for the treatment of the colorectal cancer. Our analysis of the 5 years of local and distant recurrence between the LCS group and the OCS group for the treatment of the colorectal cancer indicated no significant difference. There were also no significant differences between the surgery groups for the overall survival in the 3 and 5 years. We also found no significant differences in the 3- and 5-year disease-free survival rates between patients who underwent LCS and OCS.
Heterogeneity
In the short-term period, significant heterogeneity was detected among studies with respect to the following six factors: blood loss, the length of hospital stay, operation time, time of fluid intake, the rate of perioperative complications, and the number of blood transfusion. In the long-term period, significant heterogeneity was detected among studies with respect to the following factors: 3 years disease-free survival, 3 years local recurrence, 5 years overall survival, 5 years disease-free survival, and 5 years distant recurrence. Random effect model was used in the above given factors. Fixed effect model was used in the rest factors. Begg’s test and Egger’s test were performed, respectively. And all factors below passed the tests (p > 0.05 and 95 % CI includes 1).
Discussion
The biggest advantage of LCS than OCS lies to its minor injury. And many studies concluded that LCS had lower complications, less pain, shorter hospital stay, and less time to return to normal life than ORS in short-term period [9, 21, 22]. But the recurrence is the focus of debate laparoscopic approach and conventional open approach for the treatment of colorectal cancer. Therefore, we examined the results of LCS and compared to those of OCS in short- and long-term periods by a meta-analysis of 15 RCTs.
From the data meta-analysis, it is indicated that in short-term period, LCS has less blood loss, lower length of hospital stay, lower incisional length, less time bowel movement, lower rate of perioperative complication, lower number of blood transfusion, and lower number of 30 days death than OCS. It fits to the LCS’s consistent advantage. LCS is prior obviously to OCS in post-operation recovery. The length of hospital stay and time of bowel movement can be shown. But the operation time of LCS is longer than OCS because laparoscopic approach is more difficult than conventional open approach. And with surgeons’ richer and richer experience, the operation time will decrease. LCS is similar to OCS with no significant differences in lymph nodes and anastomotic leak, while the number of lymph nodes is one of the most important factors of prognosis of colorectal cancer patients.
Long-term effectiveness is the basic criterion to evaluate the tumor radical operation. Long-term survival and recurrence are acknowledged standard criterion to detect if it is radical surgery-based disease free. There are no significant differences between LCS and OCS in 3 and 5 years overall survival and disease-free survival. There are also no significant differences in 3 and 5 years local and distant recurrence between two groups. So it can be concluded that there are similar long-term effectiveness between LCS and OCS.
It is considered that the hospital charges of LCS are higher than those of OCS [23, 24]. The use of disposable surgical instruments, the high cost of intraoperative anesthesia, and the higher technical operation requirements made the charges of LCS higher than those of OCS. But YS Choi et al. ever separated charge from cost. Cost encompassed anesthesia, laboratory, radiology, pharmacy, nursing, medical therapy, and consumables charges, so total hospital charges should be evaluated by cost-effectiveness analysis. JS Park et al. also provided that total hospital charges for laparoscopic surgery were higher than those of open surgery only during the early learning period and became similar during the experienced period. So it is hoped the emergence of reusable materials which can reduce the costs and the shortening of the learning period to achieve cost-effective. It is also expected to increase the intensity of insurance of consumables.
In conclusion, this meta-analysis shows that LCS has the advantage of less blood loss, lower length of hospital stay, earlier bowel movement, and lower rate of complications than OCS in the short-term period. And LCS is similar to OCS with no significant differences in the long-term results. LCS can safely cure colorectal cancer; anyway, this article also has shortcomings, due to the lack of relevant data reported on the application condition of LCS. We at least put forward a bold attempt; at the same time, it is also hoped that more scholars and researchers can come together to explore and apply laparoscopic surgery routinely to the treatment of colorectal cancer.
References
Ohtani H, Tamamori Y, Azuma T et al (2011) A meta-analysis of the short-and long-term results of randomized controlled trials that compared laparoscopy-assisted and conventional open surgery for rectal cancer. J Gastrointest Surg 15(8):1375–1385
Jacobs M, Verdeja J, Goldstein H (1991) Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc Per Tech 1(3):144–150
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726
Lord SA, Larach SW, Ferrara A et al (1996) Laparoscopic resections for colorectal carcinoma. Dis Colon Rectum 39(2):148–154
Lacy AM, García-Valdecasas JC, Delgado S et al (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359(9325):2224–2229
Milsom JW, Böhm B, Hammerhofer KA et al (1998) A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: a preliminary report. J Am Coll Surg 187(1):46–54
Braga M, Frasson M, Zuliani W et al (2010) Randomized clinical trial of laparoscopic versus open left colonic resection. Br J Surg 97(8):1180–1186
Fujii S, Ota M, Ichikawa Y et al (2010) Comparison of short, long-term surgical outcomes and mid-term health-related quality of life after laparoscopic and open resection for colorectal cancer: a case-matched control study. Int J Colorectal Dis 25(11):1311–1323
Gong J, Shi D-B, Li X-X et al (2012) Short-term outcomes of laparoscopic total mesorectal excision compared to open surgery. World J Gastroenterol WJG 18(48):7308
Green B, Marshall H, Collinson F et al (2013) Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 100(1):75–82
Jayne D, Thorpe H, Copeland J et al (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97(11):1638–1645
Jayne DG, Guillou PJ, Thorpe H et al (2007) Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 25(21):3061–3068
Jeng Y-M (2007) Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol 14(1):109–117
Lacy AM, Delgado S, Castells A et al (2008) The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg 248(1):1–7
Nelson HSD, Wieand HS et al (2004) Laparoscopically assisted colectomy is as safe and effective as open colectomy in people with colon cancer Abstracted from: Nelson H, Sargent D, Wieand HS, et al.; for the Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 350:2050–2059, Cancer Treat Rev 30(8):707–709
Neudecker J, Klein F, Bittner R et al (2009) Short-term outcomes from a prospective randomized trial comparing laparoscopic and open surgery for colorectal cancer. Br J Surg 96(12):1458–1467
Pappas-Gogos G, Tellis C, Lasithiotakis K et al (2013) Oxidative stress markers in laparoscopic versus open colectomy for cancer: a double-blind randomized study. Surg Endosc 27(7):2357–2365
Park IJ, Choi G-S, Lim K-H et al (2009) Laparoscopic resection of extraperitoneal rectal cancer: a comparative analysis with open resection. Surg Endosc 23(8):1818–1824
Schwandner O, Schiedeck T, Killaitis C et al (1999) A case–control-study comparing laparoscopic versus open surgery for rectosigmoidal and rectal cancer. Int J Colorectal Dis 14(3):158–163
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 14:210–218
Chan AC, Poon JT, Fan JK et al (2008) Impact of conversion on the long-term outcome in laparoscopic resection of colorectal cancer. Surg Endosc 22(12):2625–2630
Park JS, Choi G-S, Jun SH et al (2013) Long-term outcomes after laparoscopic surgery versus open surgery for rectal cancer: a propensity score analysis. Ann Surg Oncol 20:2633–2640
Choi YS, Lee S-I, Lee T-G et al (2007) Economic outcomes of laparoscopic versus open surgery for colorectal cancer in Korea. Surg Today 37(2):127–132
Park J-S, Kang S-B, Kim S-W et al (2007) Economics and the laparoscopic surgery learning curve: comparison with open surgery for rectosigmoid cancer. World J Surg 31(9):1827–1834
Conflict of interest
This article was supported by grants from the Medical Science Foundation of health ministry in Dalian city (no.2013-80).
Author information
Authors and Affiliations
Corresponding author
Additional information
Chun-Li Wang and Gang Qu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, CL., Qu, G. & Xu, HW. The short- and long-term outcomes of laparoscopic versus open surgery for colorectal cancer: a meta-analysis. Int J Colorectal Dis 29, 309–320 (2014). https://doi.org/10.1007/s00384-013-1827-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-013-1827-1