Abstract
Background
Colorectal cancer as well as colorectal surgery is associated with increased oxidative stress through different mechanisms. In this study the levels of different oxidative stress markers were comparatively assessed in patients who underwent laparoscopic or conventional resection for colorectal cancer.
Methods
Sixty patients with colorectal cancer were randomly assigned to undergo laparoscopic (LS) or open surgery (OS). Lipid, protein, RNA, and nitrogen damage was investigated by measuring serum 8-isoprostanes (8-epiPGF2α), protein carbonyls (PC), 8-hydroxyguanosine (8-OHG), and 3-nitrotyrosine (3-NT), respectively. The primary end point of the study was to analyze and compare serum levels of the oxidative stress markers between the groups.
Results
Postoperative serum levels of 8-epiPGF2α, 3-NT, and 8-OHG were significantly lower in the LS group at 24 h after surgery (p < 0.05). At 6 h postoperatively, the levels of 8-epiPGF2α and 3-NT were significantly lower in the LS group (p < 0.05). No difference in the levels of PC was found between the two groups at any time point. In the OS group, postoperative levels of 8-epiPGF2α were significantly lower than the preoperative values (p < 0.01). In the LS group, the postoperative values of 8-epiPGF2α, 3-NT, and 8-OHG were significantly lower than the preoperative values (p < 0.05).
Conclusion
Laparoscopic surgery for colorectal cancer is associated with lower oxidative stress compared to open surgery. 8-epiPGF2α was the most suitable marker for readily defining the oxidative status in patients who underwent surgery for colorectal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Oxidative stress is the condition defined as an imbalance in the cells between oxidants formed by various processes and the antioxidative mechanisms. Abdominal surgery is associated with the generation of oxidative stress. In laparoscopic procedures, damage by reactive species (RS) has been attributed to abdominal inflation/deflation, which is a model of ischemia/reperfusion, and the use of carbon dioxide for the creation of pneumoperitoneum [1–4]. In open procedures, the response to surgical trauma, manipulation of the bowel, and its exposure to room air are responsible for RS generation [5–8]. In general, oxidative stress can damage cellular macromolecules such as DNA, proteins, and lipids, and it is a component of surgical stress. Moreover, RS are implicated in the initiation, promotion, and progression of colon carcinogenesis [9]. It has been shown that colorectal cancer patients exhibit greater oxidative stress than controls [10–13]. Although surgery itself is associated with oxidative stress, the resection of the tumor leads to an overall reduction of oxidative stress markers in patients with colorectal cancer [14, 15].
A recent systematic review of studies comparing oxidative stress in laparoscopic and open procedures revealed inconsistent results. In all but one study, only benign diseases such as cholelithiasis, hiatal hernia, morbid obesity, inguinal hernia, and uterine myomas were included [16]. Colorectal surgery was considered in three studies. Lower levels of blood hydroperoxides [17] and better preservation of gut oxygen tension [18] in laparoscopic colectomies compared with open surgery could be demonstrated, along with lower subcutaneous tissue oxygen tension [19]. Although oxygen tension is related to oxidative damage, it is not a marker of damage by RS. Blood hydroperoxide levels, which were used in those studies, are intermediate products of lipids, peptides, and amino acids and their levels reflect the oxidative injury of the cellular components, but they are not specific markers of oxidative damage. Moreover, those reports included nonhomogeneous populations and did not focus on colorectal cancer.
To date, there has been no report in the literature that compared oxidative stress markers between laparoscopic and open surgery for colorectal cancer. The aim of this double-blinded randomized trial was to compare oxidative stress in patients who underwent laparoscopic and open procedures for colorectal cancer by analyzing the levels of serum markers of lipid peroxidation, protein oxidation, damage by nitrogens, and RNA oxidation.
Materials and methods
Patients
The institutional ethics committee approved this double-blinded randomized trial, and informed patient consent was obtained. Inclusion criteria were age at least 18 years and suitability for elective surgery. Exclusion criteria were ASA (American Society of Anesthesiologists) score of III or higher, body mass index (BMI) ≥ 30, smoking, alcoholism, and the presence of systemic inflammatory disease, diabetes mellitus, autoimmune diseases, stage D (Duke’s classification, Astler-Coller modification) colorectal cancer, acute bowel obstruction, or perforation from cancer. Patients who underwent conversion to the open procedure were excluded from the study. Preoperatively, all patients underwent colonoscopy and computed tomography of the chest and abdomen (CT). Selected patients with rectal cancer underwent magnetic resonance imaging (MRI) and endorectal ultrasound for cancer staging. All patients with stage B2, C1, or C2 rectal cancer (Duke’s classification, Astler-Coller modification) received neoadjuvant chemoradiotherapy.
Randomization was done by an investigator who was not involved in the enrollment. The day before surgery patients were assigned to the laparoscopic (LS) or the open (OS) group by means of sealed envelopes containing random numbers. The surgeon was informed about the procedure type (LS or OS) by opening the envelope assigned to the patient in the operating room. All patients prepared their bowel the day before the operation by intestinal washout with an iso-osmotic solution. Patients were given an enema the morning of the operation. Prophylaxis for deep vein thrombosis was carried out with low-molecular-weight heparin (50 IU/kg/day tinzaparin sodium). For an antibiotic prophylaxis, all patients received 1.5 g of metronidazole per os (in three doses of 500 mg each) the day before surgery and 2 mg of ceftriaxone plus 500 mg of metronidazole the morning of the operation.
Surgery
All patients were premedicated with intravenous paracetamol, ondansetron hydrochloride, and ranitidine. Anesthetic induction was done with propofol (3–4 mg/kg), fentanyl (1.5 μg/kg), and cis-atracurium (0.16 mg/kg). After endotracheal intubation, all patients were provided with mechanical ventilation using sevoflurane (1–2 %) and an air/O2 mixture (30 % O2). The same surgeon performed all surgeries, laparoscopic or conventional. For the laparoscopic group, pneumoperitoneum of 10–12 mmHg was maintained throughout the operation. All precautions to prevent port-site metastasis and the nontouch technique were used for both procedures. All open procedures were performed through an 18–25-cm-long (mean = 21 ± 4 cm) middle-abdomen incision, whereas all laparoscopic procedures were performed with four ports. The specimens were exteriorized through a protected, 3–7-cm-long (mean = 5 cm) minilaparotomy in the right subcostal area for a right colectomy or in the left iliac fossa for a left colectomy. In both groups, the dissections were performed in the medial to lateral direction, with high ligation of the vessels. All anastomoses in both groups were performed using staplers (linear in right colectomies and circular in left colectomies). In right colectomies, a side-to-side stapler anastomosis was performed extracorporeally after the division of the colon. In left colectomies, after the proximal division of the colon, the anvil of the circular stapler was inserted in the proximal colonic stump and secured with a purse-string suture extracorporeally, and a double-stapled anastomosis was performed intracorporeally after the bowel was returned to the abdominal cavity; the pneumoperitoneum was then reestablished. In the open group, the anastomoses in right and left colectomies were performed in the same manner as in the laparoscopic group. In the cases of rectal cancer, total mesorectal excision was performed either laparoscopically or conventionally.
Oxidative stress markers
Plasma levels of 8-isoprostanes (8-epiPGF2α), protein carbonyls (PC), 8-hydroxyguanosine (8-OHG) and 3-nitrotyrosine (3-NT), were determined as specific markers of lipid peroxidation, protein oxidation, RNA and nitrogen damage.
Determination of the levels of 8-isoprostanes (8-epiPGF2α) in plasma samples (expressed as pg/ml) was carried out by means of a competitive enzyme immunoassay (commercial 8-isoprostane EIA kit; Cayman Chemicals, Ann Arbor, MI, USA), following the manufacturer’s instructions. 8-epiPGF2α is considered the best available biomarker of lipid peroxidation in vivo. In humans, the half-life of 8-epiPGF2α was found to be ~16 min. 2,3-Dinor-5,6-dihydro-15-F2t-isoprostane is the major urinary metabolite of 8-epiPGF2α. Although it is not a major product of lipid peroxidation, current methodology is able to readily detect its steady-state level in vivo. It is detectable in esterified form in all normal biological tissues and in free form in normal biological fluids, indicating physiological levels of oxidative stress. Measurement of 8-epiPGF2α as a biomarker of lipid peroxidation has various advantages because it is chemically stable, a specific product of lipid peroxidation, formed in vivo, and detectable in normal or pathological tissues and biological fluids. It thus allows the definition of the normal range and levels increase substantially in animal models of oxidative injury. Levels are unaffected by dietary lipids and might be used as a sensitive basis in order to study the antioxidants [20].
The levels of protein carbonyls (PC) in plasma were determined using the Oxiselect™ Protein Carbonyl ELISA Kit (Cell Biolabs, Inc., San Diego, CA, USA) following the protocol provided by the manufacturer. Absorbance was read at 450 nm using a SpectraMax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). Interassay and intra-assay coefficients of variation were 5.5 and 7.8 %, respectively. PC content is one of the most commonly used markers of protein oxidation. The formation of PC seems to be a common phenomenon of protein oxidation because of the relatively early formation and relative stability of oxidized proteins. Cells degrade oxidized proteins within hours and days. The PC content increases drastically under various pathological conditions of oxidative stress. PCs form early and are circulating for long periods in the blood of patients. Their elevation in serum is stable for at least 4 h. Hence, the chemical stability of carbonyls makes them suitable targets for laboratory measurement and storage [21].
Determination of plasma 3-nitrotyrosine (3-NT) levels was verified using the Oxiselect™ Nitrotyrosine ELISA Kit (Cell Biolabs) following the protocol provided by the manufacturer. Nitric oxide (•NO), produced during inflammation by neutrophils and phagocytes, reacts with superoxide (•O2 −) to generate reactive nitrogen species (NO3 −, NO2 −, and N2O3), including peroxynitrite (ONOO−) and •NO itself, which in turn reacts with tyrosyl radicals to add −NO2 or −NO to tyrosine residues, forming 3-nitrotyrosine (3-NT) residues. Peroxynitrite represents an extremely short-lived reactive nitrogen species (in vivo, <10 ms) that can oxidize proteins, lipids, and low-density lipoproteins, and can also react directly with DNA, leading to mutations and DNA single-strand breaks. 3-NT is a stable product of the action of peroxynitrite on tyrosine-containing proteins. Since this product is stable, the occurrence of 3-NT in blood and tissues can be measured as an indicative marker of formation and activity of the •NO-derived peroxynitrite [22].
Determination of 8-hydroxyguanosine (8-OHG) levels in serum samples (expressed as ng/ml) was carried out by means of a competitive enzyme immunoassay [commercial Oxiselect™ oxidative RNA damage ELISA OHG Kit (Cell Biolabs)], following the manufacturer’s instructions. Oxidative damage to both nucleic acids can lead to malfunctioning and erroneous coding, causing inheritable disease, aging, and cancer. Among the RS, the hydroxyl radical (•HO) represents the most important oxygen-free radical involved in lipids, proteins, RNA, and DNA damage. When it is produced next to cellular and mitochondrial RNA, it can promptly modify RNA because of its high reactivity and easy diffusion. This process leads to the generation of a range of oxidation products. Guanine is particularly vulnerable to oxidation because of its low oxidation potential compared to that of the other nucleosides. In DNA, the interaction of a hydroxyl radical with guanine leads to the formation of 8-hydroxy-2′-deoxyguanosine (8-OHdG). In RNA, oxidation of guanine can generate 8-hydroxyguanine (8-OHG) [23].
Blood sampling
Blood samples were collected the day before surgery, 5 min after pneumoperitoneum deflation (for LS) or 5 min after the distal division of the colon in left colectomies or the division of the small bowel in right colectomies (for OS), and 6 and 24 h after surgery. In the laparoscopic group, deflation was performed immediately after the distal division of the colon in left colectomies and immediately after the intraperitoneal division of the small bowel in right colectomies, resulting in the same time intervals as in the open group. Blood samples were obtained from the superficial veins of the upper extremities. Immediately after collection, the serum was separated by centrifugation at 4,000 rpm for 5 min and stored in cryovials at −80 °C.
Statistical analysis
All patients were analyzed on an intention-to-treat basis. It was estimated that a sample size of 54 would give a 90 % power to detect a 10 % difference in the reduction of 8-epiPGF2α, PC, 3-NT, and 8-OHG between the two groups at an α = 0.05 (GPower 3.1). We included 60 patients, allowing for a dropout rate of ~10 %. Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS) ver. 19.0 (IBM). The normality of quantitative variables was tested using the Kolmogorov–Smirnov test. Since the levels of all parameters studied herein were normally distributed, they were expressed as mean ± standard deviation (SD). Between-groups differences of the parameters were assessed using Student’s t test, while within-groups differences were examined by one-way repeated measures ANOVA (rmANOVA); post hoc analysis was performed using Bonferroni’s correction. The interaction between the type of surgery × time was established by two-way mixed ANOVA. All parameters were tested using Mauchly’s procedure for sphericity. Whenever the data violated the assumption of sphericity, a p value based on a Greenhouse-Geisser correction was reported instead. One-way analysis of covariance (ANCOVA) was also performed to assess between-groups differences, controlling for the preoperative levels. The effect size (ES) of the two surgical procedures on the parameters was described in terms of partial η2. ES ≥ 0.06 represents a moderate effect and ES ≥ 0.14 represents a large effect. The differences in patients’ characteristics between the two groups of patients were assessed using χ2 (gender), Student’s t test (age and BMI), and the Mann–Whitney U-test (operative time, blood loss, and hospital stay). All tests were two-tailed, and statistical significance was considered for p < 0.05.
Results
There were no statistically significant differences in the distribution of gender (p = 0.354), age (p = 0.141), BMI (p = 0.200), and operative time (p = 0.372) between the two groups of patients (Table 1). Blood loss (p = 0.012) and hospital stay (p < 0.001) were significantly lower in patients who underwent LS than those who had OS.
8-epiPGF2α levels
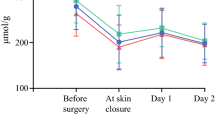
The pre- and postoperative mean 8-epiPGF2α levels of the LS and OS groups are given in Table 2 (Fig. 1A). One-way rmANOVA revealed statistically significant changes over time in the 8-epiPGF2α levels in both groups of patients (LS: p < 0.001, η2 = 0.692; OS: p < 0.001, η2 = 0.614). Post hoc analysis using Bonferroni’s adjustment for the number of comparisons was performed next. In the LS group, a statistically significant stepwise reduction in the 8-epiPGF2α levels from one measurement to the other was observed (Preoperative to T5min: −14.9 %, p = 0.003; T5min to T6h: −8.1 %, p = 0.004; T6h to T24h: −20.0 %, p < 0.001). In the OS group, after the initial significant reduction (p = 0.012) of 8-epiPGF2α levels by −9.0 % (from Preoperative to T5min), the levels remained unchanged until the 6th postoperative hour (p = 1.000); a further significant reduction of 8-epiPGF2α levels by −21.0 % was observed on the T24h measurement (p < 0.001).
Mean levels of oxidative stress markers in patients who underwent laparoscopic resection (LS) and conventional resection (OS) for colorectal cancer. A Levels of 8-epiPGF2α. B Levels of PC. C Levels of 3-NT. D Levels of 8-OHG. aStatistically significant difference compared to the preoperative levels. bStatistically significant difference compared to the previous levels. cStatistically significant difference compared to OS
Throughout the study period, the LS procedure produced an overall statistically significant reduction in 8-epiPGF2α levels by −37.4 % (p < 0.001, η2 = 0.789), while the OS procedure produced a statistically significant reduction by −26.4 % (p < 0.001, η2 = 0.685). The overall reduction in 8-epiPGF2α levels was statistically significantly greater in the LS group than in the OS group [−39.15 ± 20.70 (−37.4 %) vs. −26.59 ± 14.62 (−26.4 %), p = 0.014].
The different course over time of the 8-epiPGF2α levels between the two groups was also disclosed by the two-way mixed ANOVA, which showed a statistically significant type of surgery × time interaction (p = 0.001). In this regard, the changes in the 8-epiPGF2α levels from T5min to T6h were significantly different between the two groups (−7.21 ± 8.99 in LS vs. 2.15 ± 11.03 in OS, p = 0.002).
In the sequence, the levels of 8-epiPGF2α were compared between the two groups of patients on each measurement (Table 2); no statistically significant difference between the LS and OS groups was observed in the Preoperative (p = 0.293) and T5min (p = 0.471) measurements. In the T6h (p < 0.001) and T24h (p = 0.018) measurements, the LS group demonstrated significantly lower levels of 8-epiPGF2α than the OS group. The above differences remained significant (T6h (adjusted mean ± SE): 81.58 ± 1.97 vs. 94.23 ± 1.82, p < 0.001; T24h: 65.37 ± 2.70 vs. 74.39 ± 2.50, p = 0.018) in one-way ANCOVA, controlling for the preoperative levels.
PC levels
The pre- and postoperative mean PC levels of the LS and OS groups are given in Table 2 (Fig. 1B). Neither of the two surgical procedures had a statistically significant effect on the PC levels over time (LS: p = 0.079, η2 = 0.141; OS: p = 0.490, η2 = 0.039). The overall reduction of PC levels was similar between the two groups [(−0.06 ± 0.22 (−9.4 %) in LS vs. -0.04 ± 0.17 (−6.8 %) in OS, p = 0.752]. Neither type of surgery × time interaction reached statistical significance (p = 0.197), although the reduction of PC levels from T5min to T6h was significantly greater in the LS group than in the OS group [−0.11 ± 0.15 (−17.5 %) vs. −0.01 ± 0.11 (−1.7 %), p = 0.037]. Furthermore, there was no statistically significant difference in PC levels on any of the four measurements between the LS and OS groups (Preoperative: p = 0.374; T5min: p = 0.522; T6h: p = 0.259; T24h: p = 0.565).
3-NT levels
The pre- and postoperative mean 3-NT levels of the LS and OS groups are given in Table 2 (Fig. 1C). One-way rmANOVA revealed statistically significant changes over time in the 3-NT levels in both groups (LS: p = 0.011, η2 = 0.184; OS: p = 0.018, η2 = 0.147). In the LS group, post hoc analysis showed that 3-NT levels fluctuated minimally around the preoperative levels during the first six postoperative hours, but they were significantly lower at the T24h measurement (−12.9 %, p = 0.002, compared to T6h). The LS procedure produced an overall statistically significant reduction in 3-NT levels of −18.7 % (p = 0.016, η2 = 0.232). In the OS group, the only significant change in the 3-NT levels was observed at the sixth postoperative hour (26.9 %, p = 0.002, compared to T5min). Overall, the OS procedure was followed by a nonsignificant reduction in 3-NT levels of −0.4 % (p = 1.000, η2 = 0.000). Although the overall reduction of 3-NT levels was greater in the LS group than in the OS group [−0.47 ± 0.87 (−18.7 %) vs. −0.01 ± 1.03 (−0.4 %)], this difference did not reach statistical significance (p = 0.117). The changes of the 3-NT levels over time were not similar between the two groups, as found by the two-way mixed ANOVA, which showed a statistically significant type of surgery × time interaction (p = 0.032); in this regard, although the Preoperative to T5min changes of 3-NT levels were similar between the two groups (−0.15 ± 0.57 vs. −0.30 ± 0.94, p = 0.494), the T5min to T6h elevation of 3-NT levels was significantly greater in the OS group than in the LS group (0.12 ± 0.49 vs. 0.67 ± 0.733, p = 0.004).
When the levels of 3-NT were compared between the two groups on each measurement (Table 2), no statistically significant difference of the Preoperative (p = 0.273) and T5min (p = 0.526) measurements was observed. For the T6h (p = 0.004) and T24h (p = 0.003) measurements, the LS group demonstrated significantly lower levels of 3-NT than the OS group. The above differences remained significant [T6h (adjusted mean ± SE): 2.52 ± 0.14 vs. 3.12 ± 0.15, p = 0.007; T24h: 2.08 ± 0.15 vs. 2.74 ± 0.16, p = 0.005] in one-way ANCOVA, controlling for the preoperative levels.
8-OHG levels
The pre- and postoperative mean 8-OHG levels of the LS and OS groups are given in Table 2 (Fig. 1D). One-way rmANOVA revealed statistically significant changes in the 8-OHG levels over time in the LS group (p = 0.018, η2 = 0.177) but not in the OS group (p = 0.126, η2 = 0.091). The nonsignificant changes of 8-OHG levels during the first six postoperative hours in both groups were followed by a statistically significant (p = 0.008) reduction in 8-OHG levels in the LS group by −12.9 % and an elevation in 8-OHG levels in the OS group by 13.9 %, although the latter did not reach statistical significance (p = 0.181). The LS procedure produced an overall statistically significant reduction in 8-OHG levels of −20.7 % (p = 0.003, η2 = 0.342), while the OS procedure had a nonsignificant elevation in 8-OHG levels of 5.4 % (p = 0.452, η2 = 0.027). Therefore, the overall changes were statistically significantly different between the two groups [−8.56 ± 12.15 (−20.7 %) in LS vs. 2.15 ± 13.16 (5.4 %) in OS, p = 0.007] and the type of surgery × time interaction was statistically significant (p = 0.004).
When the levels of 8-OHG were compared between the two groups on each measurement (Table 2), no statistically significant difference was observed on the Preoperative (p = 0.609), T5min (p = 0.739), and T6h (p = 0.732) measurements. On the T24h (p = 0.001) measurements, the LS group demonstrated significantly lower levels of 8-OHG than the OS group, which remained significant [(adjusted mean ± SE) 32.43 ± 1.81 vs. 41.72 ± 1.85, p = 0.004] in one-way ANCOVA, controlling for the preoperative levels.
Discussion
To our knowledge, this is the first study to show, with the use of specific oxidative stress markers, that laparoscopic surgery for colorectal cancer is associated with significantly lower oxidative stress compared to open surgery. Among the markers used in this study, 8-epiPGF2α was considered the most suitable oxidative stress marker that was able to define early and readily the oxidative status in patients who underwent surgery for colorectal cancer. In fact, 8-epiPGF2α presented a prompt response to both laparoscopic and open surgery. This observation may offer an additional explanation for the well-known benefits of laparoscopic versus open colorectal surgery that has not described so far and may generate hypotheses for further evaluation of the role of OS markers as prognostic markers of the operation, i.e., markers of the quality of the laparoscopic procedure and possibly prognostic markers of the overall outcome of the surgical treatment.
Although the most widely used test for lipid peroxidation is measurement of malondialdehyde (MDA) by a thiobarbituric acid reactive substances (TBARS) assay, the use of the TBARS assay is problematic because MDA is not a specific product of lipid peroxidation and the TBARS assay is not specific for MDA. The accuracy of exhaled isoprostanes as a marker of endogenous lipid peroxidation has been questioned because these hydrocarbon gases are minor end products of peroxidation and their concentrations are influenced by the breakdown rate of peroxides, while lipid hydroperoxides cannot be detected in the circulation, even under severe oxidative stress using a highly accurate and sensitive GC/MS assay [20].
For the first time, RNA oxidation was investigated in patients who underwent surgery for colorectal cancer. Although oxidative damage to DNA has been studied for a number of diseases, including cancer, RNA oxidation has not received much attention. Cellular RNA is more susceptible to oxidative stress than DNA because it is mainly single-stranded and easily accessible to ROS, has relatively little association with proteins, is extensively distributed proximal to mitochondria where the majority of RS are generated, and in contrast to the repair mechanisms for DNA, repair mechanisms for RNA have not been found [23, 24]. Therefore, oxidation of rRNA, mRNA, and tRNA may have serious effects on cellular homeostasis because oxidation of these RNAs could impair the overall integrity of translational processes [25].
There are scarce data regarding the metabolism and clearance of the markers used in this study. In general, lipid peroxidation products are formed and detoxified within minutes, whereas carbonyl and 3-nitrotyrosine protein as well as 8-OHG circulate for a longer period in the blood of patients. Oxidative proteins are degraded within hours and days and their elevation persists in plasma for at least 4 h [20–23]. During surgery for benign disease patients develop oxidative stress, and the oxidative stress markers return to preoperative levels days to weeks after surgery, depending on the type of surgery [26].
Patients with colorectal cancer show a higher oxidative status than healthy subjects. Increased levels of MDA [10, 11], lipid peroxides, and TBARS were observed in tissues of colonic tumor. PCs are also increased in patients with colon cancer [13, 15, 27], while upregulation of the expression of the inducible nitric oxide synthase (iNOS) gene [28], the major gene responsible for the production of NO, was observed in human colorectal carcinoma tissues [29].
Even though surgery is implicated in the generation of oxidative stress, as was demonstrated in surgery for benign diseases [16], patients with malignant diseases who underwent tumor resection exhibited an overall reduction of oxidative stress [14, 15].
Although reduction of oxidative stress was observed in both groups, some of the markers were decreased significantly in the LS group compared to the OS group. This significance reflects the lower oxidative stress generated during the laparoscopic procedure. Assuming that resection of the tumor reduces oxidative stress in both procedures, the lower oxidative stress observed in LS compared to OS may be attributed to the following factors: surgical trauma, manipulation of the intestine, and mesentery traction; Trendelenburg position of the patient during laparoscopic surgery; and exposure of the peritoneal cavity to room air during open surgery.
Surgical trauma is associated with the generation of RS and also with impairment of the biological defense systems against RS attack [7, 14]. Surgical manipulation during laparoscopic procedures causes only minor trauma to the peritoneal environment which could minimize the inflammatory response in the gut. Schietroma et al. [30] found that initial abdominal exploration, when major intestinal handling occurs, coincides with increased intestinal permeability. Surgical trauma, manipulation of the intestine, and mesentery traction are generally less prominent in laparoscopic procedures because of the ample space created by the pneumoperitoneum and the amplified view achieved with the laparoscope. Therefore, it is likely that minor trauma and minor intestinal handling results in lower oxidative stress in laparoscopic procedures. The Trendelenburg position, from the point of view of hemodynamics, may not provoke significant changes in the portal blood flow during surgery [31]. Therefore, injuries from ischemia/reperfusion may be attenuated and oxidative damage could be minor. Indeed, higher MDA levels were found in patients who underwent laparoscopic surgery in the head-up position compared to those who were placed in the head-down position [26].
Exposure of the peritoneal cavity to room air in laparotomy may be another reason for the discrepancy in oxidative stress between the procedures. Oxygen concentration in the peritoneal cavity is lower than in the atmosphere (< 40 mmHg). In laparotomy, the viscera are exposed to a conspicuous concentration of oxygen for a long period in contrast with laparoscopy [17].
Oxidative stress is implicated in inflammatory response. During inflammation, mast cells and leukocytes are recruited to the site of damage, which leads to a “respiratory burst” due to an increased uptake of oxygen, and, thus, an increased release and accumulation of RS at the site of damage. On the other hand, inflammatory cells also produce soluble mediators, such as metabolites of arachidonic acid, cytokines, and chemokines, which act by further recruiting inflammatory cells to the site of damage and producing more RS. These key mediators can activate signal transduction cascades as well as induce changes in transcription factors such as nuclear factor kappa B (NF-κB). Induction of COX-2 and inducible nitric oxide synthase (iNOS), aberrant expression of inflammatory cytokines such as TNFα, IL-1, IL-6, and IL-8, and alterations in the expression of specific microRNAs have also been reported to play a role in oxidative stress-induced inflammation [32]. In our previous studies we have found lower levels of α-defensins, TLR-2, and TLR-4 in patients who underwent laparoscopic surgery for colorectal cancer 24 h after the procedure [33, 34]. Since oxidative stress is implicated in inflammatory response and vice versa [32, 35–39], it could be hypothesized that the short-term benefits of laparoscopic surgery are associated to some degree with lower oxidative stress during this procedure. Moreover, since oxidative stress is implicated in carcinogenesis, tumor diffusion, and metastasis [8, 32], it cannot be excluded that lower oxidative stress may be beneficial from an oncological point of view. Indeed, some authors reported lower tumor recurrence and longer overall survival in patients with stage III cancer who underwent laparoscopic resection compared with the patients underwent open resection [40–42].
In this study, 8-epiPGF2α was shown to be more sensitive than the other markers to the variances of oxidative stress. The lipids of the cellular membrane are the primary macromolecules involved in the reactions with the RS, while the proteins and the nucleic acids are involved at a later time. Perhaps this is the reason for the prompt detection of the 8-epiPGF2α variations.
This study has revealed the short-term impact of laparoscopic surgery on the markers of oxidative stress. Although most of the oxidative stress markers are short-lived, as oxidative stress persists and the redox status is imbalanced, the markers are produced continuously, and even in the earliest periods their variations could be representative of damage or benefit. Studies that investigate oxidative stress in open and laparoscopic surgery for colorectal cancer with later time points and focused on the oncologic outcome are needed in order to better elucidate and confirm this hypothesis.
Certain limitations of the study should be pointed out. Later measurements in the postoperative period until the markers returned to normal levels would have given further details on the course of oxidative stress in each patient group. Control arms with benign colon disease would have showed more clearly the effect of surgical methods on oxidative stress since oncological patients have high baseline levels of oxidative stress. Finally, a longer follow-up of the patients would have revealed whether the observed differences are associated with oncological benefits to the patients.
In conclusion, this study provides evidence that laparoscopic surgery for colorectal cancer is associated with significantly lower oxidative stress compared to open surgery. 8-epiPGF2α represents the most suitable marker of oxidative stress that is able to define readily the oxidative status of patients who underwent surgery for colorectal cancer.
References
Collard CD, Gelman S (2001) Pathophysiology, clinical manifestations, and prevention of ischemia reperfusion injury. Anesthesiology 94:1133–1138
Eleftheriadis E, Kotzampassi K, Papanotas K, Heliadis N, Sarris K (1996) Gut ischemia, oxidative stress, and bacterial translocation in elevated abdominal pressure in rats. World J Surg 20:11–16
Bentes de Souza AM, Wang CC, Chu CY, Lam PM, Rogers MS (2003) The effect of intra-abdominal pressure on the generation of 8-iso prostaglandin F2alpha during laparoscopy in rabbits. Hum Reprod 18:2181–2188
Sare M, Yilmaz I, Hamamci D, Birincioglu M, Ozmen M, Yesilada O (2000) The effect of carbon dioxide pneumoperitoneum on free radicals. Surg Endosc 14:649–652
Bentes de Souza AM, Rogers MS, Wang CC, Yuen PM, Ng PS (2003) Comparison of peritoneal oxidative stress during laparoscopy and laparotomy. J Am Assoc Gynecol Laparosc 10:65–74
Mittal A, Phillips AR, Loveday B, Windsor JA (2008) The potential role for xanthine oxidase inhibition in major intra-abdominal surgery. World J Surg 32:288–295
Anup R, Balasubramanian KA (2000) Surgical stress and the gastrointestinal tract. J Surg Res 92:291–300
Klaunig JE, Kamendulis LM, Hocevar BA (2010) Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol 38:96–109
Otamiri T, Sjodahl R (1989) Increased lipid peroxidation in malignant tissues of patients with colorectal cancer. Cancer 64:422–425
Hendrickse CW, Kelly RW, Radley S, Donovan IA, Keighley MR, Neoptolemos JP (1994) Lipid peroxidation and prostaglandins in colorectal cancer. Br J Surg 81:1219–1223
Gackowski D, Banaszkiewicz Z, Rozalski R, Jawien A, Olinski R (2002) Persistent oxidative stress in colorectal carcinoma patients. Int J Cancer 101:395–397
Chang D, Wang F, Zhao YS, Pan HZ (2008) Evaluation of oxidative stress in colorectal cancer patients. Biomed Environ Sci 21:286–289
Lauschke H, Tolba R, Burger B, Minor T, Hirner A (2002) Lipid peroxidation as additional marker in patients with colorectal cancer. Results of a preliminary study. Eur Surg Res 34:346–350
Hiki N, Shimizu N, Yamaguchi H, Imamura K, Kami K, Kubota K, Kaminishi M (2006) Manipulation of the small intestine as a cause of the increased inflammatory response after open compared with laparoscopic surgery. Br J Surg 93:195–204
Di Giacomo C, Acquaviva R, Lanteri R, Licata F, Licata A, Vanella A (2003) Nonproteic antioxidant status in plasma of subjects with colon cancer. Exp Biol Med (Maywood) 228:525–528
Arsalani-Zadeh R, Ullah S, Khan S, MacFie J (2011) Oxidative stress in laparoscopic versus open abdominal surgery: a systematic review. J Surg Res 169:e59–e68
Tsuchiya M, Sato EF, Inoue M, Asada A (2008) Open abdominal surgery increases intraoperative oxidative stress: can it be prevented? Anesth Analg 107:1946–1952
Braga M, Vignali A, Gianotti L, Zuliani W, Radaelli G, Gruarin P, Dellabona P, Di Carlo V (2002) Laparoscopic versus open colorectal surgery: a randomized trial on short-term outcome. Ann Surg 236:759–766 disscussion 767
Fleischmann E, Kugener A, Kabon B, Kimberger O, Herbst F, Kurz A (2007) Laparoscopic surgery impairs tissue oxygen tension more than open surgery. Br J Surg 94:362–368
Montuschi P, Barnes PJ, Roberts LJ 2nd (2004) Isoprostanes: markers and mediators of oxidative stress. FASEB J 18:1791–1800
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38
Yeo WS, Lee SJ, Lee JR, Kim KP (2008) Nitrosative protein tyrosine modifications: biochemistry and functional significance. BMB Rep 41:194–203
Li Z, Wu J, Deleo CJ (2006) RNA damage and surveillance under oxidative stress. IUBMB Life 58:581–588
Valavanidis A, Vlachogianni T, Fiotakis C (2008) 8-Hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C 27:120–139
Kong Q, Lin CL (2010) Oxidative damage to RNA: mechanisms, consequences, and diseases. Cell Mol Life Sci 67:1817–1829
Glantzounis GK, Tsimaris I, Tselepis AD, Thomas C, Galaris DA, Tsimoyiannis EC (2005) Alterations in plasma oxidative stress markers after laparoscopic operations of the upper and lower abdomen. Angiology 56:459–465
Yeh CC, Lai CY, Hsieh LL, Tang R, Wu FY, Sung FC (2010) Protein carbonyl levels, glutathione S-transferase polymorphisms and risk of colorectal cancer. Carcinogenesis 31:228–233
Cianchi F, Cortesini C, Fantappie O, Messerini L, Schiavone N, Vannacci A, Nistri S, Sardi I, Baroni G, Marzocca C, Perna F, Mazzanti R, Bechi P, Masini E (2003) Inducible nitric oxide synthase expression in human colorectal cancer: correlation with tumor angiogenesis. Am J Pathol 162:793–801
Szaleczky E, Pronai L, Nakazawa H, Tulassay Z (2000) Evidence of in vivo peroxynitrite formation in patients with colorectal carcinoma, higher plasma nitrate/nitrite levels, and lower protection against oxygen free radicals. J Clin Gastroenterol 30:47–51
Schietroma M, Carlei F, Cappelli S, Amicucci G (2006) Intestinal permeability and systemic endotoxemia after laparotomic or laparoscopic cholecystectomy. Ann Surg 243:359–363
Kotake Y, Takeda J, Matsumoto M, Tagawa M, Kikuchi H (2001) Subclinical hepatic dysfunction in laparoscopic cholecystectomy and laparoscopic colectomy. Br J Anaesth 87:774–777
Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB (2010) Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49:1603–1616
Tsimogiannis KE, Tellis CC, Tselepis AD, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G (2012) Toll-like receptors in the inflammatory response during open and laparoscopic colectomy for colorectal cancer. Surg Endosc 26:330–336
Tsimogiannis KE, Tellis K, Tselepis A, Pappas-Gogos GK, Tsimoyiannis EC, Basdanis G (2011) α-Defensin expression of inflammatory response in open and laparoscopic colectomy for colorectal cancer. World J Surg 35:1911–1917
Christen S, Finckh B, Lykkesfeldt J, Gessler P, Frese-Schaper M, Nielsen P, Schmid ER, Schmitt B (2005) Oxidative stress precedes peak systemic inflammatory response in pediatric patients undergoing cardiopulmonary bypass operation. Free Radic Biol Med 38:1323–1332
Crapo JD (2003) Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J 22(Suppl 44):4s–6s
Closa D, Folch-Puy E (2004) Oxygen free radicals and the systemic inflammatory response. IUBMB Life 56:185–191
Gloire G, Legrand-Poels S, Piette J (2006) NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol 72:1493–1505
Asehnoune K, Strassheim D, Mitra S, Kim Yeol J, Abraham E (2004) Involvement of reactive oxygen species in toll-like receptor 4-dependent activation of NF-κΒ. J Immunol 172:2522–2529
Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359:2224–2229
Lourenco T, Murray A, Grant A, McKinley A, Krukowski Z, Vale L (2008) Laparoscopic surgery for colorectal cancer: safe and effective? - A systematic review. Surg Endosc 22:1146–1160
Laurent C, Leblanc F, Wutrich P, Scheffler M, Rullier E (2009) Laparoscopic versus open surgery for rectal cancer: long-term oncologic results. Ann Surg 250:54–61
Acknowledgments
We thank Dr. Grigorios Tripsianis, Professor of Statistics at Democritus University of Thrace, for his work with the statistics in this study [Clinical Trials.gov identifier: NCT00928928 (www.clinicaltrials.gov)].
Disclosures
Drs. George Pappas-Gogos, Constantinos Tellis, Konstantinos Lasithiotakis, Konstantinos Tsimogiannis, Evangelos Tsimoyiannis, Alexandros D. Tselepis, George Chalkiadakis, and Emmanuel Chrysos have no conflicts of interest or financial ties to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pappas-Gogos, G., Tellis, C., Lasithiotakis, K. et al. Oxidative stress markers in laparoscopic versus open colectomy for cancer: a double-blind randomized study. Surg Endosc 27, 2357–2365 (2013). https://doi.org/10.1007/s00464-013-2788-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-013-2788-8