Abstract
Purpose
Necrotizing enterocolitis (NEC) is a serious gastrointestinal disorder in newborns. Early diagnosis and rapid treatment is essential for seeking good outcome for neonates. The aim of our study was to evaluate intestinal blood flow in superior mesenteric artery (SMA) and portal vein (PV) in neonates with suspected or confirmed NEC and investigate the prognostic cut-off values to develop NEC.
Methods
Doppler flowmetry of SMA and PV was performed for 62 newborns. Resistive (RI) and pulsatility (PI) indexes were measured in SMA as well as Volumetric blood flow (Vflow) in PV. ROC curves were applied to estimate sensitivity and specificity and to identify cut-off values.
Results
There were 93.5 % preterm neonates. 29 patients (46.8 %) were diagnosed with NEC and 33 (53.2 %) formed a control group. 96.3 % NEC patients had RI >0.75 with sensitivity of 96.3 % and specificity of 90.9 % (OR 260). 88.9 % NEC patients had PI >1.85 with sensitivity of 88.9 % and specificity of 78.8 % (OR 29). Portal Vflow lower than 37 ml/min was present in 89.7 % patients with NEC (OR 11.7).
Conclusion
Intestinal blood flow Dopplerography can be a useful tool for diagnosing and predicting NEC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Necrotizing enterocolitis (NEC) is the most common serious gastrointestinal disorder affecting preterm infants [1]. It affects 1–8 % of all infants admitted to the neonatal intensive care unit and its mortality rate can reach 10–50 % [2]. Early and precise diagnosis as well as rapid treatment is essential for seeking good outcome for neonates. However, no standard abdominal imagining examination techniques are currently available to identify NEC. Pneumoperitoneum is an indication for surgery, but when it occurs it might be too late to rescue the patient [3]. Earlier diagnosis of severely ischemic bowels loops before perforation could potentially reduce the incidence and mortality of NEC [4]. Recently there has been growing number of studies showing that abdominal ultrasound can be a more effective diagnostic tool for earlier detection of NEC than X-ray. Multiple data demonstrate that neonates with NEC have a significant increase in flow velocity in the superior mesenteric artery (SMA) in comparison with the healthy ones [4–7]. Moreover, to our knowledge there are only two recently published articles on the neonatal portal venous (PV) blood flowmetry that showed a significant decline in portal blood flow volume may be useful for the early diagnosis of NEC [3, 8]. However, data regarding the diagnostic validity and prognostic value of abdominal ultrasound are limited and often focused on a single finding rather than a combination of findings. Furthermore, until now ultrasound findings seem to have little impact on clinical decisions [9].
In this study, we aimed to evaluate and compare the intestinal blood flow parameters in both, superior mesenteric artery and portal vein in neonates; investigate if the Doppler ultrasound can be used as a key method for the prediction and diagnosis of NEC; and discover the prognostic cut-off values of NEC.
Patients and methods
This prospective clinical study was carried out during the period 2010–2014 in Pediatric Surgery department of Hospital of Lithuanian University of Health Sciences Kaunas Clinics with the permission of Kaunas Regional Committee for Biomedical Research Ethics (No. BE 2–17).
Patients with radiological NEC symptoms (fixed intestine loop, segmental distension of intestine, intestine wall pneumatosis, pneumoperitoneum) formed “NEC group”. The “Control group” consisted of newborns treated in the neonatal unit who were consulted by pediatric surgeon due to one of the following symptoms: changes in the blood test, regurgitation, distended abdomen, blood in the stools. In every case it was more a singular symptom due to other reasons (pneumonia, anal fissure, meteorism, uncomplicated regurgitation) and it was not clarified as NEC.
Modified Bell scoring system was used for the newborns with NEC to determine the stage of NEC [10]. All the patients underwent surgery in the same Pediatric Surgery department. Indications for surgery treatment were: pneumoperitoneum, symptoms of intestinal obstruction, intestinal pneumatosis. Gestational age and weight at birth were estimated prior to the surgery. The following data were analyzed and assessed in regard to any impact on the mode of surgery and prediction of their significance for patients’ survival.
From 62 enrolled newborns, 29 patients (46.8 %) were diagnosed with NEC (NEC group) and 33 (53.2 %) formed control group. Their gestational age ranged from 23 to 40 weeks (mean gestational age 27 weeks). 58 (93.5 %) patients were preterm while remaining 4 (6.5 %) newborns were full term (all in NEC group). Birth weight ranged from 462 to 3610 g (average weight 1152 g). There were 13 patients (21 %) with Stage 1 NEC, 12 patients (19.4 %) with Stage 2 NEC, and 4 patients (6.5 %) with Stage 3 NEC according to the Bell scoring system. Stage 3 NEC patients underwent surgery due to intestinal perforation, but unfortunately two of them died. Baseline characteristics of the groups are featured in Table 1.
Doppler flowmetry
We measured the blood flow of the portal vein and superior mesenteric artery in enrolled newborns. All ultrasound (US) examinations were performed by US scanner GE VIVID6 with linear 7 and 8 MHz research sensors. The testing was performed by the same pediatric radiologist. Patients were tested in the supine position. Color Doppler US in combination with Grey scales US were applied for all the subjects. Flow measurements were repeated three times for each vessel to minimize the errors at least 1 h after their last feeding.
When testing with linear 7 MHz sensor, the superior mesenteric artery was found from branching of the aorta. Its diameter was measured and by applying B regime, anatomic vascular condition was assessed (possible thrombus, its radius). Afterwards, more detailed spectrum blood circulation analysis was performed choosing color Doppler window position in the center of SMA and absolute and derived blood circulation indexes were measured. Absolute indexes were the following: peak systolic velocity (PSV) and diastolic velocity. Mean flow velocity (MFV) was measured based on these indexes and volumetric blood flow (Vflow) was calculated. Two derivative values were calculated during this test. Pulsatility index (PI) was calculated with Gosling and King formula: PI = (Vsystole − Vdiastole)/Vmean [11]. Resistive index (RI) was calculated with Pourcelot formula: RI = (Vsystole − Vdiastole)/Vsystole [12].
We used warm gel to reduce the patient’s movement, trying to minimize discomfort during the procedure to keep his breathing and heart rate steady. These factors could cause changes of blood circulation in portal vein. Absolute indexes in the central part of PV were registered 1 cm from mesenteric superior vein and v.lienalis junction by applying Doppler US. Portal PSV, MFV and Vflow were registered. Assuring indexes reliability, the measurements were performed covering no less than 5 complexes of registered in test vascular wave pulsations.
Statistical analysis
In data processing, p < 0.05 was considered the threshold for statistical significance. Kolmogorov–Smirnov test was applied for the evaluation of distribution of the data. Parametric student t test was applied for two independent groups and for more than two groups—parametric dispersion analysis (ANOVA). Chi square (χ2) criteria were applied for quantitative symptoms dependency evaluation. Binary logistic regression analysis was applied for predicting significant differences of comparative analysis. ROC (receiver operating characteristics) curves measuring the area under the curve (AUC) were applied for estimating the sensitivity and specificity and to identify cut-off values for predicting NEC.
Results
Doppler indices in SMA
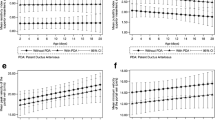
The differences of the Doppler indices of SMA including PSV, MFV, Vflow and diameter of artery were not statistically significant between NEC and control group (Table 2). However, the values of resistive index and the pulsatility index of SMA were significantly different between NEC and control groups (Table 2, p < 0.001). The mean value of RI was 0.75. RI of >0.75 was found in 3 (9.1 %) patients in control group and 26 (96.3 %) patients in NEC group (p < 0.001, Table 2). RI of >0.75 had a sensitivity of 96.3 % and a specificity of 90.9 % in predicting NEC (Fig. 1) odds ratio (OR) 260.0 [95 % CI 25.46–2654.31] (Table 3).
The mean value of PI was 1.85. PI of >1.85 was found in 7 (21.2 %) patients in control group and 24 (88.9 %) patients in NEC group (p < 0.001, Table 2). PI of >1.85 had a sensitivity of 88.9 % and a specificity of 78.8 % in predicting NEC (Fig. 1) OR 29.7 [95 % CI 6.89–128.19] (Table 3).
Doppler indices in portal vein
The Doppler indices of PV including PSV, MFV and Vflow were significantly higher in newborns who developed NEC compared to the control group (p < 0.001, Table 4).
PSV in portal vein—17.5 cm/s (sensitivity 75.9 %, specificity 66.7 %), MFV—10.8 cm/s (sensitivity 69 %, specificity 63.6 %) and portal Vflow—37 ml/min (sensitivity 89.7 %, specificity 57.6 %) (Fig. 2). All of these parameters of portal flow were significantly lower in NEC group compared with control group (p < 0.001, Table 3). Portal Vflow <37 ml/min was presented in 14 (42.4 %) healthy newborns and in 26 (89.7 %) patients with NEC, OR 11.76 [95 % CI 2.96–46.76] (Table 3).
Conjointly
The distribution of blood flowmetry indices were significantly different between different NEC stages newborns with Stage 1 NEC had lower RI and PI values than in Stage 2 NEC (p < 0.001, Table 5). However, we cannot compare RI and PI of newborns with Stage 3 NEC, because arterial flow was not detected in 2 of 4 patients (50 %) from this group due to extensive intestinal necrosis and septic shock. Additionally, portal volumetric blood flow of <37 ml/min was seen in all newborns with Stage 2 and Stage 3 NEC (100 %, p < 0.001) (Table 5).
Discussion
Necrotizing enterocolitis is very severe disease that affects neonates with very unspecific early symptoms, that makes it difficult to diagnose and treat properly on time [13]. According to literature about 90 % of newborns with NEC are preterm and that corresponds to our data [14–16]. Prevention and early diagnosis of NEC remains the most important goal. There is growing interest of the value of Doppler ultrasound in detecting NEC [9]. Safe, inexpensive and without radiation diagnostic tool as well as real time imaging makes it prior to simple plain x-ray [3, 17]. The key of our study was to detect the early changes in intestinal blood flow and to find out the prognostic factors of NEC using Doppler ultrasound.
Pathological findings of NEC associated with ischemic events and the fact that NEC most commonly occurs in the terminal ileum and proximal colon, which makes watershed area of the superior mesenteric artery, suggests that derangement of the circulatory system is involved [18]. Recently several studies found that neonates with NEC have a significant increase in flow velocity in the SMA in comparison with the healthy ones during the first day of life [1, 5]. However, our results did not reveal a significant difference in SMA flowmetry parameters. The same studies have shown that infants with risk factors for NEC tend to have higher resistance pattern [1, 5]. This supports the role of the decreased intestinal blood flow in the pathogenesis of NEC. Furthermore, one study of experimental rabbit model concluded that the increase in the RI constitutes a very strong argument for splanchnic vascular constriction and also suggests that ischemia may be the early trigger of NEC pathogenesis [19]. The results of our research strongly support this theory. As you can see in Table 2, RI and PI are significantly higher in newborns with NEC then in healthy ones. E. Robe-Tilling did large prospective study where they found strong correlation between pathologically increased RI and PI and food intolerance for preterm babies [6]. They suggest that decreased PI is a good prognostic sign and lets to identify when the patient will start tolerating enteric nutrition. We could strongly agree with previous hypothesis that increased resistive and pulsatility indexes in NEC group demonstrate evident risk of developing NEC. These data suggest that abnormalities in splanchnic circulation have a role in the etiology of NEC in newborns. Some authors have suggested that Doppler ultrasound findings in SMA may be used to predict NEC [19, 20]. Our data are in concordance and have assessed and presented the changes in blood flow velocity in SMA with cut-off values having relatively high sensitivity and specificity rates. We found when RI >0.75 can predict the risk to develop NEC significantly (OR 260) with high sensitivity (96.3 %) and specificity (90.9 %) rates. PI of more than 1.85 (OR 29) can be a good prognosticator as well with sensitivity 88.9 % and specificity of 78.8 %, respectively.
In recent years, it has become possible to evaluate hepatic blood flow of newborns. However, to our knowledge there are only two recently published papers by Kobayashi et al. and Mustafa et al. on the neonatal portal venous blood flowmetry by Doppler. Both authors agree that significant decline in portal blood flow volume may be useful for the early diagnosis of NEC [3, 8]. Even though Kobayashi et al. group reported that a significant decrease in portal blood flow volume may be useful for the early diagnosis of NEC, they emphasized that the absolute cut-off value for portal vein blood flow is unknown [3]. As well as previous study, we showed the significant lower portal venous flow in newborns with NEC, but additionally we found out the the cut-off values of intestinal flow parameters causing the risk to develop NEC. Volumetric blood flow was the most important parameter in portal vein measurements. Portal Vflow lower than 37 ml/min increases the risk to develop NEC (OR 11.7) with relatively high sensitivity 89.7 % and specificity 57.6 %.
Timing of the bowel ischemia and its relationship with NEC severity has not been clearly defined [10, 21]. However, it is essential to diagnose NEC as soon as possible to start the adequate treatment to prevent further progression of NEC. Still it remains challenging for clinicians to distinguish between NEC stages and the appropriate treatment. Our study group demonstrated that newborns with Stage 1 NEC had lower RI and PI values than in Stage 2 NEC while portal volumetric blood flow of <37 ml/min was registered in all newborns with Stage 2 and Stage 3 NEC, respectively. We think that introducing new diagnostic modalities could contribute to early diagnosis and timely treatment of NEC.
Conclusion
The key findings of the presented study were that infants who developed necrotizing enterocolitis had a higher-resistance pattern of flow in the superior mesenteric artery and lower blood flow in the portal vein.
This indicates that Doppler flow velocimetry of superior mesenteric artery and portal vein might be clinically useful to identify infants at increased risk of NEC. Furthermore, Doppler sonography could be an effective diagnostic tool to find out the severity of NEC and might be beneficial for choosing the appropriate management of the disease.
References
Ahmad Khodair S (2014) Color Doppler blood flow indices of the superior mesenteric artery as an early predictor of necrotizing enterocolitis in preterm neonates. Int J Med Imaging 2:39. doi:10.11648/j.ijmi.20140202.17
Jesse N, Neu J (2006) Necrotizing enterocolitis: relationship to innate immunity, clinical features, and strategies for prevention. Neoreviews 7:e143–e150. doi:10.1542/neo.7-3-e143
Kobayashi M, Mizuno M, Matsumoto A, Wakabayashi G (2015) Neonatal portal venous blood flowmetry by Doppler ultrasound for early diagnosis of ischemia in intestinal tract. Eur J Pediatr Surg 25:292–298. doi:10.1055/s-0034-1374820
Epelman M, Daneman A, Navarro OM et al (2007) Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics 27:285–305. doi:10.1148/rg.272055098
Murdoch EM, Sinha AK, Shanmugalingam ST et al (2006) Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics 118:1999–2003. doi:10.1542/peds.2006-0272
Robel-Tillig E, Knüpfer M, Pulzer F, Vogtmann C (2004) Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radiol 34:958–962. doi:10.1007/s00247-004-1285-6
Louis D, Mukhopadhyay K, Sodhi KS et al (2013) Superior mesenteric artery Doppler is poor at predicting feed intolerance and NEC in preterm small for gestational age neonates. J Matern Fetal Neonatal Med 26:1855–1859. doi:10.3109/14767058.2013.799649
Akin MA, Yikilmaz A, Gunes T et al (2014) Quantitative assessment of hepatic blood flow in the diagnosis and management of necrotizing enterocolitis. J Matern Fetal Neonatal Med. doi:10.3109/14767058.2014.980230
Bohnhorst B (2013) Usefulness of abdominal ultrasound in diagnosing necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 98:F445–F450. doi:10.1136/archdischild-2012-302848
Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33:179–201
Baschat AA, Gembruch U, Reiss I et al (2000) Relationship between arterial and venous Doppler and perinatal outcome in fetal growth restriction. Ultrasound Obstet Gynecol 16:407–413. doi:10.1046/j.1469-0705.2000.00284.x
Johnson JN, Ansong AK, Li JS et al (2011) Celiac artery flow pattern in infants with single right ventricle following the Norwood procedure with a modified Blalock-Taussig or right ventricle to pulmonary artery shunt. Pediatr Cardiol 32:479–486. doi:10.1007/s00246-011-9906-y
Lambert DK, Christensen RD, Henry E et al (2007) Necrotizing enterocolitis in term neonates: data from a multihospital health-care system. J Perinatol 27:437–443. doi:10.1038/sj.jp.7211738
Llanos AR, Moss ME, Pinzòn MC et al (2002) Epidemiology of neonatal necrotising enterocolitis: a population-based study. Paediatr Perinat Epidemiol 16:342–349
Guthrie SO, Gordon PV, Thomas V et al (2003) Necrotizing enterocolitis among neonates in the United States. J Perinatol 23:278–285. doi:10.1038/sj.jp.7210892
Stoll BJ (1994) Epidemiology of necrotizing enterocolitis. Clin Perinatol 21:205–218
Campbell S, Vyas S, Nicolaides KH (1991) Doppler investigation of the fetal circulation. J Perinat Med 19:21–26
Schnabl K-L, Van Aerde J-E, Thomson A-B, Clandinin M-T (2008) Necrotizing enterocolitis: a multifactorial disease with no cure. World J Gastroenterol 14:2142–2161
Choi Y-H, Kim I-O, Cheon J-E et al (2010) Doppler sonographic findings in an experimental rabbit model of necrotizing enterocolitis. J Ultrasound Med 29:379–386
Silva CT, Daneman A, Navarro OM et al (2007) Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 37:274–282. doi:10.1007/s00247-006-0393-x
Dimmitt RA, Moss RL (2001) Clinical Management of Necrotizing Enterocolitis. Neoreviews 2:110e–117e. doi:10.1542/neo.2-5-e110
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Kaunas Regional Committee for Biomedical Research Ethics No. BE 2–17).
Informed consent was obtained from all individual participants’ parents included in the study.
Rights and permissions
About this article
Cite this article
Urboniene, A., Palepsaitis, A., Uktveris, R. et al. Doppler flowmetry of the superior mesenteric artery and portal vein: impact for the early prediction of necrotizing enterocolitis in neonates. Pediatr Surg Int 31, 1061–1066 (2015). https://doi.org/10.1007/s00383-015-3792-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-015-3792-y