Abstract
Background
There is little in the literature regarding the use of gray-scale and Doppler sonography of the bowel in necrotizing enterocolitis (NEC) and how findings depicted by this modality might assist in predicting outcome and influence management.
Objective
To correlate sonographic findings with outcome in NEC.
Materials and methods
This was a retrospective analysis of clinical and abdominal ultrasonography (AUS) findings in NEC from January 2003 to December 2005. AUS findings were evaluated for portal venous gas, free gas, peritoneal fluid, bowel wall thickness, echogenicity, perfusion and intramural gas. Patients were categorized into two groups, according to their outcome.
Results
A total of 40 infants were identified who had AUS for NEC prior to any surgical intervention. Group A comprised 18 neonates treated medically and recovered fully, and group B comprised 22 neonates who required surgery or died. Free gas (six patients) and focal fluid collections (three patients) were only found in group B. Increased bowel wall echogenicity, absent bowel perfusion, portal venous gas, bowel wall thinning, bowel wall thickening, free fluid with echoes and intramural gas were seen in both groups, but more frequently in group B. Anechoic free fluid was seen more frequently in group A. Increased bowel perfusion was seen equally in both groups.
Conclusion
An adverse outcome was associated with the sonographic findings of free gas, focal fluid collections or three or more of the following: increased bowel wall echogenicity, absent bowel perfusion, portal venous gas, bowel wall thinning, bowel wall thickening, free fluid with echoes and intramural gas. Sonographic findings are useful in predicting outcome and therefore might help guide management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is little in the literature regarding the use of gray-scale and Doppler sonography of the bowel in necrotizing enterocolitis (NEC) and how findings depicted by this modality might assist in predicting outcome and influence management [1, 2]. We retrospectively analyzed the clinical and abdominal ultrasonography (AUS) findings in a large series of neonates with NEC in an attempt to establish the role that AUS might play in predicting outcome in these patients.
Materials and methods
We retrospectively analyzed all neonates with NEC in our neonatal intensive care unit during the 3-year period January 2003 to December 2005. The diagnosis of NEC was based on a combination of clinical and abdominal radiographic (AXR) findings. The study was approved by the Research Ethics Board of our institution.
The patients were identified from the neonatal intensive care unit database; their clinical, AXR and AUS findings were reviewed. The AUS and AXR findings were reviewed by consensus by two radiologists blinded to the findings of the other modality and to patient outcome. The findings of AXR performed prior to and after the AUS were reviewed specifically for the presence of free gas or a mass effect caused by fluid collections.
In our institution, AUS is used as a problem-solving modality only in those neonates suspected or known to have NEC and in whom it is thought that more information provided by AUS might facilitate management decisions. We followed the sonographic technique described by Faingold et al. [1]. This includes an initial evaluation of the entire abdomen with vector and curved-array transducers. In addition, high-resolution images with high-frequency (8–15 MHz) linear-array transducers are also obtained for evaluation of the abdomen and are specifically required for evaluation of the bowel wall and the presence of free gas. Gray-scale images are used to assess the bowel wall for echogenicity, thickness, and intramural gas; color Doppler sonography is used to evaluate bowel wall perfusion.
The sonographic equipment used was an Acuson Sequoia (Siemens Medical Solutions, Malvern, Pa.) and the examinations were performed by experienced pediatric radiology fellows or technologists.
The AUS features were categorized into 11 findings:
-
1.
Bowel echogenicity was considered abnormal when there was loss of the hypoechoic muscle layer (“gut signature”) with overall increase in wall echogenicity. Increased echogenicity can reflect wall edema, inflammation or hemorrhage.
-
2.
Bowel wall thickening was considered to be present if the thickness measured by calipers was 2.7 mm or more [1].
-
3.
Bowel wall thinning was considered to be present if the thickness measured by calipers was 1 mm or less [1].
-
4.
Increased bowel perfusion was recognized when there was increased color flow signal (more than nine dots of color Doppler signal per square centimeter) in a loop of bowel when the standard technique described by Faingold et al. [1] was used with a velocity setting of 0.086 m/s. Increased perfusion was also recognized by the presence of the “circular”, “Y” or “zebra” patterns of flow [1].
-
5.
Bowel perfusion was considered to be absent when no flow was evident within the bowel wall on color Doppler sonography, initially utilizing the same setting as above (0.086 m/s), and confirmed if flow was still absent after decreasing the velocity setting to 0.029 m/s in an attempt to detect the slowest possible velocities.
-
6.
Intramural gas was considered to be present when there were punctate or granular increased echogenicities within the bowel wall [3–5]. Posterior dirty acoustic shadowing was not considered necessary for this diagnosis.
-
7.
Portal venous gas was considered to be present when punctate or linear echogenicities were noted within the main portal vein or its intrahepatic branches on gray-scale sonography.
-
8.
Free gas was considered to be present when echogenic lines or foci with posterior ring-down or comet-tail artifacts were evident outside the bowel and posterior to the abdominal wall [6].
-
9.
Anechoic free intraperitoneal fluid.
-
10.
Free intraperitoneal fluid with echoes.
-
11.
Focal fluid collections. These represented focal loculations of fluid with complex echogenicity.
Patients were categorized into two groups based on outcome. Group A included neonates who were treated medically and who recovered fully. Group B included neonates who required surgery for perforation in the acute setting (laparotomy or placement of a peritoneal drain) and surgery for late strictures, as well as those who died as a result of NEC.
We determined the number of patients in groups A and B in whom the above sonographic features were depicted by AUS. We then calculated the risk ratios and 95% confidence intervals (CI) for those features that occurred in both groups. Those features seen in both groups and that also had a risk ratio predictive of a poor outcome were then subjected to a receiver-operator curve (ROC) evaluation. The SAS 9.1 (SAS Institute, Cary, N.C.) statistical package was used for data manipulations and analysis.
Results
In the 3-year period of our review there were 107 neonates diagnosed with NEC. Of the 54 neonates evaluated with AUS, 14 were excluded because the AUS was performed postoperatively. There were, therefore, 40 neonates in whom the sonographic correlation with outcome could be appropriately evaluated. The AUS was performed within the first 3 days after the onset of symptoms in 38 neonates, and on days 7 and 13 after the onset of symptoms in the final 2 patients. Of the 40 patients included in this correlation, 8 had more than one AUS examination during a single episode of NEC, and in these only the first AUS examination was reviewed. Three other patients had recurrent NEC, and in these only the first AUS examination of the first episode was reviewed.
Group A included 18 neonates who responded well to medical management. Their mean gestational age was 31 weeks (range 24–40 weeks) and mean birth weight was 1,546 g (range 680–3774 g). Group B included 22 patients who did not respond well to medical management. Their mean gestational age was 29 weeks (range 24–40 weeks) and mean birth weight was 1,361 g (range 540–3500 g). Of these 22, 17 required surgery during the acute phase of NEC (8 of whom died) and 2 others died as a result of NEC with no surgery. The remaining three required surgery for strictures that were diagnosed 4 to 5 weeks after the acute phase. There was no statistically significant difference in the birth weights and gestational ages between groups A and B (P = 0.45 and P = 0.17, respectively).
Table 1 shows the correlation between the 11 sonographic features that were evaluated and patient outcome. These are arranged in descending order of their correlation with a poor outcome. These features can be divided into three categories.
The first category includes two sonographic features that were seen only in group B: free intraperitoneal gas (six patients) and focal fluid collections (three patients; Table 1). Of the six neonates with free gas evident on AUS, the AXR showed free gas in only four and was negative for free gas in two (Figs. 1 and 2). There was a seventh patient who had free gas on AXR that was not depicted on AUS. Bowel perforation was confirmed at surgery in all seven. In these seven patients with free gas on either AXR or AUS the time interval between examinations with the two modalities ranged from 10 min up to 9 h, and in five the interval was under 2 h. AUS was the first of the two modalities to depict free gas in four of the seven and AXR the first in the other three.
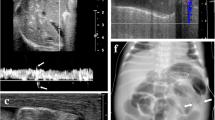
Neonate with NEC and bowel perforation. Sonography was performed because of clinical deterioration. The previous abdominal radiograph showed no evidence of free gas. a Longitudinal sonogram in the midline of the upper abdomen shows hyperechoic foci (arrows) representing bubbles of free gas between the abdominal wall and the anterior surface of the left lobe of the liver. There is a small amount of reverberation artifact. b Longitudinal sonogram in the left flank shows a linear tract of hyperechoic foci caused by free gas bubbling out of a region of bowel perforation (black arrow). The bubbles track anteriorly through the echogenic free peritoneal fluid and come to float just beneath the anterior abdominal wall on top of the echogenic fluid (between white arrows). c Lateral shoot-through abdominal radiograph acquired after the sonogram confirms the presence of free gas anteriorly (arrow)
Longitudinal abdominal sonograms in the midline show very small amounts of free gas in two neonates with NEC and proven bowel perforation at surgery. a A single hyperechoic focus (arrow) is seen between the anterior abdominal wall and the anterior surface of the liver. b A hyperechoic focus (arrow) with posterior reverberation artifact is seen between the anterior abdominal wall and free peritoneal fluid. These images illustrate that very small amounts of free gas can be depicted with sonography
All three patients with focal fluid collections had multiple small (largest 2.5 cm diameter) collections with complex echogenicity (Figs. 3 and 4). It was impossible to predict the presence of these collections on AXR in any of these three patients, as there was no mass effect. In one the bowel showed marked diffuse distention with gas and in the other two the AXR showed relatively gasless areas that did not correlate with the collections depicted by AUS.
Premature neonate with NEC and bowel perforation proved at surgery. Supine (a) and lateral shoot-through (b) abdominal radiographs show some distended bowel limited to the left hemiabdomen in a. There is no evidence of free gas. The right hemiabdomen and pelvis are gasless. Longitudinal sonograms of the right hemiabdomen and pelvis 10 hours later (c, d) show two focal fluid collections (arrows) as well as several collapsed loops of echogenic bowel. The sonogram depicts the size of the focal fluid collections relative to the amount of collapsed gasless bowel, neither of which could be appreciated on the radiographs
Neonate with NEC and bowel perforation. a Supine abdominal radiograph shows diffuse distention of the bowel with gas and intramural gas. b Transverse sonogram of the left lower quadrant shows a focal fluid collection with complex echogenicity consistent with an abscess (between electronic calipers). The presence of the abscess could not be appreciated on the radiograph
The second category included seven sonographic findings that, although seen in both groups A and B, were more common in group B (Table 1; Figs. 5, 6 and 7). The risk ratio and 95% CI for these seven sonographic findings were calculated and are shown in Table 1, where they are arranged in a descending order of risk ratio. The ROC evaluation of these seven sonographic features (Fig. 8) showed that if one chooses a cut-off point when three of these seven parameters are present there is a sensitivity of 0.82 (95% CI 0.60–0.95) and specificity of 0.78 (95% CI 0.52–0.94) for predicting an adverse outcome. If one aims to have more than three of these seven parameters present the specificity for a poor outcome increases but the sensitivity decreases (Fig. 8).
Neonate with NEC and clinical deterioration. a Supine abdominal radiograph shows diffuse distention of the bowel with gas. b Longitudinal sonogram of the right upper quadrant 1 hour later shows distended fluid-filled loops of bowel with increased echogenicity of the wall. c Transverse sonogram of the right lower quadrant shows free fluid with low-level echoes. d Longitudinal color Doppler sonogram of the left flank shows two loops of bowel (A, B). Loop A is hyperemic, whereas loop B shows absence of flow. The area of increased flow indicated by the arrow is part of the mesenteric flow and not in the wall of loop B. The sonographic examination in this patient depicts three of the sonographic features in category 2 (increased bowel wall echogenicity, free fluid with echoes, and absence of bowel wall flow) which, when seen together, are predictive of a poor outcome. At surgery a hemicolectomy was performed because of diffuse necrosis
Neonate with NEC and necrotic bowel. a Supine abdominal radiograph shows nonspecific distention of the bowel with gas and intramural gas. There is no free gas. b Transverse sonogram of the right lower quadrant shows free fluid with echoes and bowel with increased wall echogenicity. c Transverse sonogram of the right flank shows collapsed loops of bowel with thickened echogenic wall. d Transverse sonogram shows a loop of bowel with multiple confluent hyperechoic foci in the dependent part of the wall and along its sides consistent with intramural gas. e Color Doppler sonogram of the left flank shows a loop of bowel that has absence of flow in most of its circumference. The abdominal sonogram in this patient depicts five sonographic features of category 2 (increased bowel wall echogenicity, free fluid with echoes, thickened bowel wall, intramural gas, and absence of bowel wall flow) which, when seen together, are predictive of a poor outcome. Laparotomy revealed pan-necrosis, and the patient died
Bowel wall thinning (a category 2 sonographic feature) in two neonates with NEC. a, b Neonate with NEC with clinical deterioration. a Supine abdominal radiograph shows a non-specific bowel gas pattern with some distention in the left upper quadrant. b Transverse sonogram of the left lower quadrant 1 hour later shows a loop of bowel in which the wall is increased in echogenicity and thin. c Longitudinal sonogram of the right paraumbilical area in another patient shows a loop of bowel with marked thinning of the wall and also exhibits multiple hyperechoic foci caused by intramural gas. There is some surrounding free fluid with echoes. The bowel loops with thinning of the wall in these two patients reflects ischemic bowel wall with marked sloughing of the mucosa and submucosa. In both of these patients laparotomy revealed extensive necrosis, and both patients died
Receiver operating curve of seven sonographic features seen in both groups A and B but more commonly in group B. The curve illustrates that if one chooses a cut-off point when three of these seven parameters are present there is a sensitivity of 0.82 (95% CI 0.60–0.95) and specificity of 0.78 (95% CI 0.52–0.94) for predicting an adverse outcome. If greater numbers of these parameters are required to be present concomitantly, the specificity increases but the sensitivity decreases
The third category included the last two of the 11 sonographic findings (Table 1). Increased bowel wall perfusion was seen in 12/18 (67%) of group A and in 14/22 (64%) of group B. Anechoic peritoneal fluid was seen in 5/18 (28%) of group A and in only 1/22 (5%) of group B. These findings had a risk ratio of less than 1.0 and were not predictive of an adverse outcome.
Discussion
This study showed that in NEC the sonographic findings can be categorized into three groups relative to their value in predicting outcome. The first category included the findings of free peritoneal gas (Figs. 1 and 2) and focal fluid collections (Figs. 3 and 4), which were found only in those patients who did poorly (group B), indicating that these are significant sonographic findings for predicting an adverse outcome. This is not surprising, as both findings are a direct consequence of bowel perforation.
Traditionally, the detection of free gas has relied on the use of AXR. In this study we showed that in the neonate AUS appears to be at least as sensitive as AXR for depicting free gas and, in certain circumstances, might even be more sensitive, as illustrated by our two patients in whom the AUS depicted free gas that was not detected by AXR (Figs. 1 and 2). Furthermore, the free gas was noted initially on AUS in four of our patients, and this prompted the performance of the AXR and was thus instrumental in guiding management. Although these numbers are small, the findings are in accordance with those of Faingold et al. [1], who noted that free gas was present in 4 of 22 patients with NEC and that this was depicted by both AXR and AUS. Very small amounts of free gas can be most easily appreciated as hyperechoic foci with dirty shadowing between the anterior surface of the liver and the abdominal wall [2] (Figs. 1 and 2). Furthermore, in adults AUS has also been considered to be a reliable alternate imaging technique for the detection of free intraperitoneal gas in the postsurgical abdomen and in patients with bowel perforation [6, 7].
Focal fluid collections can occur after bowel perforation without evidence of free gas on AXR or AUS [1, 8]. The finding of focal fluid collections on AUS underlines the importance of this modality in this clinical setting, as it can easily depict such collections, which are rarely accurately demonstrated on AXR [9]. This was evident in our three patients with focal collections, as the AXR showed no evidence of a mass effect in any of the three (Figs. 3 and 4).
The second category of sonographic features includes seven findings that were seen in both groups A and B but were much more commonly seen in patients with an adverse outcome (group B), and their risk ratio varied from 1.6 to 3.7 (Table 1; Figs. 5, 6 and 7). A risk ratio above 1 indicates that the finding is a clinically significant risk factor, and if it is over 3, it is a highly significant risk factor. The fact that these findings also occurred in patients who did well (group A) indicates that individually they are less reliable in predicting outcome than the first category of sonographic findings. However, the ROC showed that, when evaluated in combination, these findings have a high sensitivity (82%) and specificity (78%) for predicting outcome (Fig. 8). Therefore, in clinical practice, the presence of three or more of these seven findings enables one to confidently predict an adverse outcome.
In contrast to the above, the third category includes two AUS findings that were found to be unhelpful in predicting an adverse outcome because both had a risk ratio of less than 1 (Table 1). These included increased bowel wall perfusion, which is considered to reflect the presence of viable bowel in NEC [1], and the presence of anechoic peritoneal fluid.
The above categorization of the AUS findings is, therefore, helpful in determining which patients will do poorly and, as such, AUS might be helpful in guiding management. However, we believe that AUS is not required for appropriate management decisions in many patients with NEC, particularly those who have typical clinical and/or AXR findings and those who are responding well to therapy. This is illustrated by the fact that in the 3-year period of our study we used AUS as a problem-solving modality in only 50% of patients (54/107) with NEC.
We found that AUS was helpful in two clinical situations in NEC. The first of these was in those patients in whom both the clinical and AXR findings were nonspecific and the diagnosis was indeterminate. However, this aspect is not the topic of this paper.
The second clinical situation in which we used AUS was in those patients in whom the diagnosis of NEC had been established but who were not responding to medical therapy, particularly if the AXR was non-specific. The findings of this study confirmed that AUS is extremely important in this clinical situation. AUS is invaluable for depicting the presence of free gas and focal fluid collections (category 1 features) in those patients who might have already perforated but have no evidence of free gas on AXR, and AUS is also important for determining which patients might have necrotic bowel prior to perforation, as these patients might also have a poor outcome. This latter situation might be suggested by the presence of three or more of the sonographic features in category 2.
NEC is an ischemic process of the bowel that leads to inflammation and necrosis and includes the development of intramural gas. As necrosis progresses, the mucosa, submucosa and muscularis can slough, leaving a markedly thinned wall. More markedly affected loops of bowel might perforate, and this is associated with an increased mortality [10]. Documentation of the presence of bowel necrosis by AUS prior to perforation might well influence surgical management and allow the surgical team to intervene in those patients with necrosis prior to the development of free gas on AXR or AUS [1].
In 2005 Faingold et al. [1] reported their findings on the use of Doppler sonography for assessment of bowel viability in 22 neonates with NEC. These authors were the first to show that thinning of the bowel wall and absence of perfusion could be documented by AUS. These findings correlated at surgery with bowel necrosis, some with perforation, even before there was evidence of free gas on AXR. However, in the present larger series, bowel wall thinning and absent bowel perfusion were found to be in category two of the sonographic findings (see Table 1), as they were present in both those patients who did poorly and those who did well. The fact that we found these sonographic features in both groups indicates that individually these findings may not be as helpful in predicting outcome and, therefore, guiding management as initially suggested by Faingold et al. [1]. We should emphasize, however, that the present study was a retrospective analysis of sonographic examinations done by several operators, in contrast to the series reported by Faingold et al. [1] in which all examinations were done by one experienced operator in order to standardize the technique. Sonography is highly operator-dependent and there has been no study that has assessed interobserver variability in NEC.
There might be other reasons for these differences. The finding of these two sonographic features in both groups might simply reflect a limitation of this modality. On the other hand, no study has evaluated possible fluctuations in bowel perfusion in NEC in a serial manner, and we might not have done some of our AUS examinations at the most appropriate time to detect absent perfusion or possible reperfusion. Furthermore, evaluation of bowel wall thinning is difficult and we might have to reassess the way in which we evaluate this parameter in NEC and how we differentiate this from stretching of the bowel wall in loops that are markedly distended with no wall sloughing.
Conclusion
We attempted to correlate 11 sonographic findings with outcome in NEC. The presence of free gas and focal collections were indicative of a poor outcome. Increased perfusion and anechoic fluid were unhelpful findings. The presence of three or more of the remaining findings was highly predictive of a poor outcome. This study suggests that these sonographic findings are useful in guiding the clinical management of this difficult patient population.
The present challenge is to demonstrate prospectively the role of these sonographic findings in guiding management and further studies are required to directly compare AXR and AUS in NEC. Finally, it is important for other institutions to validate our findings.
References
Faingold R, Daneman A, Tomlinson G et al (2005) Necrotizing enterocolitis: assessment of bowel viability with color Doppler US. Radiology 235:587–594
Epelman M, Daneman A, Navarro OM et al (2007) Necrotizing enterocolitis (NEC): a review of state-of-the-art imaging findings with pathologic correlation. Radiographics (in press)
Kim WY, Kim WS, Kim IO et al (2005) Sonographic evaluation of neonates with early-stage necrotizing enterocolitis. Pediatr Radiol 35:1056–1061
Kohzaki S, Hayashi K, Fukuda T et al (1994) Case report: the “aurora sign” – a new sonographic sign of pneumatosis cystoides intestinalis. Br J Radiol 67:1275–1277
Goske MJ, Goldblum JR, Applegate KE et al (1999) The “circle sign”: a new sonographic sign of pneumatosis intestinalis – clinical, pathologic and experimental findings. Pediatr Radiol 29:530–535
Braccini G, Lamacchia M, Boraschi P et al (1996) Ultrasound versus plain film in the detection of pneumoperitoneum. Abdom Imaging 21:404–412
Seitz K, Reising KD (1982) Sonographic detection of free air in the abdominal cavity. Ultraschall Med 3:4–6
Rabinowitz JG, Siegle RL (1976) Changing clinical roentgenographic patterns of necrotizing enterocolitis. AJR 126:560–566
Miller SF, Seibert JJ, Kinder DL et al (1993) Use of ultrasound in detection of occult bowel perforation in neonates. J Ultrasound Med 12:531–535
Buonomo C (1999) The radiology of necrotizing enterocolitis. Radiol Clin North Am 37:1187–1198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, C.T., Daneman, A., Navarro, O.M. et al. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 37, 274–282 (2007). https://doi.org/10.1007/s00247-006-0393-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-006-0393-x