Abstract
Background
Blood flow parameters in the superior mesenteric artery (SMA) change with vasoconstriction or vasodilatation of the intestinal vascular bed. In cases of severe growth retardation as a result of haemodynamic disturbances, the blood flow changes persist into postnatal life.
Objective
To assess early changes of Doppler sonographic blood flow parameters in the SMA for prediction of later intestinal motility disturbances in preterm infants and tolerance of enteral feeding during the first week of life.
Materials and methods
Doppler sonographic blood flow parameters in the SMA were measured on the first day of life and the following 5 days in 478 neonates with a birth weight below 1,500 g. According to the Doppler results, the neonates were divided into two groups—those with pathological parameters and those with normal blood flow parameters. Correlations between blood flow parameters, the development of intestinal dysmotility and the tolerated amount of enteral feeding were calculated.
Results
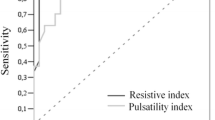
Pathological blood flow parameters were observed in 148 neonates (group 1) and normal blood flow parameters in 330 neonates (group 2). Intestinal motility disturbance occurred in 125 neonates (83%) of group 1 and 47 neonates (15%) of group 2. Neonates in group 2 tolerated significantly more feed by the fifth day of life than neonates in group 1. Postnatal adaptation did not differ between the two groups, although the majority of neonates with intestinal dysmotility were small for gestational age. The predictive value of blood flow parameters for prediction of intestinal motility revealed high sensitivity and specificity by the first postnatal day, 2 or 3 days before development of clinical signs of intestinal dysmotility. There was a strong negative correlation between pathological pulsatility index on day 1 and the quantity of tolerated enteral feeding on day 5.
Conclusions
Pathological blood flow parameters in the SMA can predict problems of intestinal motility and tolerance of enteral feeding. With the early detection of these problems a prompt start of adequate therapy to avoid complications is possible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Adequate perfusion of intestinal organs is essential for the health of the intestinal mucosa. Prenatal uteroplacental insufficiency with chronic fetal hypoxia can lead to fetal growth retardation with a redistribution of blood flow favouring the cerebral circulation and reducing mesenteric perfusion [1–5]. This underlines the importance of chronic or acute hypoxia as the most intensively studied condition associated with disturbances of intestinal motility [6]. Small-for-gestational-age (SGA) neonates, born after prenatal haemodynamic disturbances, are at high risk for developing severe intestinal problems which sometimes require surgical intervention [2, 4, 7–10]. Therefore, early diagnosis of intestinal dysmotility is decisive for a prompt start and success of conservative treatment. The aim of this prospective study was to assess the value of Doppler blood flow parameters of the superior mesenteric artery (SMA) as an early predictor of severe intestinal impairment, possibly before the development of clinical signs and symptoms. Those parameters measured on the first day of life were also assessed for their ability to predict tolerance of enteral feeding during the first week of life.
Materials and methods
This was a prospective study of 478 preterm neonates with a birth weight below 1,500 g. The study was undertaken between 1996 and 2001 in the Department of Obstetrics and Gynaecology of the University of Leipzig. All neonates were admitted to the neonatal intensive care unit of the University Children’s Hospital. Gestational age at delivery was calculated as the best obstetrical estimate according to last menstrual period, combined with the first trimester US examination. Infants were categorized as SGA if their birth weight was at or below the tenth percentile [11]. Neonatal database characteristics included birth weight, gestational age, birth weight percentile, umbilical artery pH, Apgar score and postnatal resuscitation (Table 1).
Intestinal motility disturbance was defined by the presence of at least three of the following clinical signs during the first 5 days of life:
-
Gastro-oesophageal reflux (more than 80% of feed over 3 h).
-
Absence of spontaneous meconium excretion within first 72 h.
-
Sticky meconium, which hindered passage and excretion.
-
Excessive abdominal distension over more than 72 h.
-
Palpable rubbery bowel loops.
-
Bilious vomiting more than four times per day.
Neonates were categorized as having intestinal dysmotility always before feeding and were all examined in the same fashion.
Neonates were assigned to two groups accordingly to the results of Doppler sonographic measurement of the SMA. In all neonates, Doppler sonographic investigation of the SMA was performed between 2 and 12 h of life, after establishing normal physical conditions and before first feeding, and on subsequent days, always before feeding, up to the fifth day. To achieve imaging of the SMA the transducer was placed on the mid-abdomen above the umbilicus. The SMA was identified at its emergence from the aorta and the sample volume was placed a few millimetres from its origin. Peak systolic velocity, end diastolic velocity and pulsatility index were recorded. Blood flow velocities in the SMA were defined as pathological if the measured values were below 2 SD from normal values; the pulsatility index was pathologically increased if more than 2 SD above that of the control group (Table 2). Neonates with at least two pathological blood flow parameters of the SMA on the first day of life were assigned to group 1 and neonates with normal blood flow parameters of the SMA were assigned to group 2.
Evidence of a persistent ductus arteriosus was identified by ECHO at the same time. Heart rate, oscillometrically measured blood pressure, and the need for volume replacement therapy were recorded.
The study was approved by the institutional review board. Parental consent was obtained to carry out Doppler sonographic examinations. The values were assessed using Student’s t-test and Kenndall’s rank-correlation. P values below 0.05 were considered significant. Values are presented as means or medians ±SD
Results
Pathological blood flow parameters of the SMA were observed in 148 neonates (group 1) and normal blood flow parameters in 330 neonates on the first day. The neonates of both groups did not differ as regards birth weight; however, neonates in group 2 had a significantly lower gestational age and higher birth weight centile than neonates in group 1 (Table 2). A total of 125 (83%) neonates in group 1 and 47 (15%) neonates in group 2 developed typical signs of intestinal dysmotility, mostly on day 2 (35%) and day 3 (43%). Neonates in group 2 tolerated significantly more feed than neonates in group l on the fifth day of life (Table 3). The predictive values of blood flow parameters of the SMA on the first day of life for later intestinal problems were calculated, and revealed high sensitivity and specificity (Table 4). A strong negative correlation was also found between pathological pulsatility index on the first day and quantity of tolerated enteral feed on the fifth day (r=−75, P<0.001; Fig. 1). On the fifth day, there was also a significant negative correlation between pulsatility index and amount of tolerated enteral feed on the fifth day of life (r=−0.57, P<0.001).
Heart rate (140±18 vs 143±24 bpm) and mean blood pressure (37±6 vs 33±6 mmHg) were not significantly different between the two groups at the time of first examination or on subsequent days. Infants of both groups did not receive significantly different additional volume replacement (electrolytes or albumin infusion) (11.6±11 vs 15.3±10 ml/kg) on the first day of life. There were significant differences in the need for mechanical ventilation, but no differences in haemodynamic parameters such as patency of the ductus arteriosus or need for catecholamines during the first 5 days (Table 3).
Discussion
Ante- or postnatal hypoxia may cause redistribution of the cardiac output away from non-essential organs, including the intestine, to preserve oxygenation of essential organs [3, 6, 8, 12]. Uteroplacental insufficiency is a major cause of severe growth restriction and increased postnatal morbidity with a high risk for the development of intestinal problems [10, 12–16]. Several studies have indicated that an initial ischaemic insult to the intestine is followed by microbial colonization of damaged gut mucosa [6]. Placental insufficiency, resulting in reduced oxygen delivery to fetal organs, may cause downregulation of neutrophil production in the bone marrow and chronic hypoxia leads to increased production of red cells [17]. Postnatally, high haematocrit leads to hyperviscosity of blood and decreased perfusion. These facts, in combination with persistent vasoconstriction of the intestinal vascular bed after prenatal haemodynamic disturbance, could be the pathophysiological background of intestinal dysmotility, which in protracted cases is followed by infection [17, 18]. With regard to this pathophysiology, an early start of adequate therapy is essential to prevent progression of intestinal impairment and the development of necrotizing enterocolitis. This is the basis for the need to identify early predictive markers for the development of severe intestinal disturbances before there are clinical signs and symptoms, and methods for determining the optimum time to start enteral feeding [4].
Thus we performed a prospective study to examine neonates with a birth weight below 1500 g with respect to the development of typical signs of intestinal dysmotility in relation to the results of Doppler US findings of blood flow in the SMA. We demonstrated pathological blood flow parameters in the SMA in 148 of the 478 neonates examined during the first hours of life and 83% of these developed intestinal dysmotility during the first 5 days of life. As expected, and in accordance with the described pathophysiological mechanism, most of the neonates with intestinal problems were SGA [2, 9, 15–20]. To exclude other haemodynamic influences that could lead to comparable changes of mesenteric blood flow parameters, especially an increased pulsatility index, we evaluated the frequency of a persistent ductus arteriosus in both groups. There were no significant differences at the time of first examination on the first day of life or on the following days [21, 22].
Therefore, we regard the pathologically increased pulsatility index of the SMA and the decreased end diastolic flow velocity in most of the cases with intestinal dysmotility as a sign of a persistent vasoconstriction of the blood vessels to the gut after intrauterine haemodynamic centralization [2, 7]. As stated above, early diagnosis is very important for timely intervention for successful treatment [23]. On the first day of life, usually before the development of typical clinical signs, sodium chloride enemas are very helpful to avoid delay in meconium excretion. When there is early intestinal motility disturbance, pyridostigmine can increase peristalsis and the transport of often sticky meconium. For this reason it is important that the predictive value, sensitivity and specificity of pathological mesenteric blood flow parameters on the first day for the development of intestinal problems during the subsequent days are very high.
Another important point is the finding of a strong correlation between pathologically increased pulsatility index on the first day and the quantity of tolerated feed on day 5. This could lead to a carefully and individually oriented enteral feeding regimen. Most neonates tolerating more than 40 ml/kg per day on the fifth day showed a normal pulsatility index. There are only a few methods available for the early prediction of intestinal problems. A high-risk group of premature infants can be characterized by typical prenatal Doppler US findings [6, 7, 9, 14–16, 19, 23]. To describe postnatal intestinal adaptation, a prospective study was performed to evaluate whether Doppler US measurements of the SMA performed after enteral nutrition could predict tolerance of enteral feeding [24]. Neonates in this study were examined on day 3 or 4 after their first enteral feed. The authors found a correlation between changes of mesenteric blood flow parameters after test feeding and tolerance of feed. Our neonates developed signs of intestinal dysmotility at an earlier age (days 2–3). For that reason, it seems important to demonstrate pathological blood flow parameters on the first day of life and before the start of enteral feeding.
An interesting observational study has been performed to analyse the postnatal intestinal condition. The relationships between gastric pH, mucosal CO2, acid-base balance and gastrointestinal complications after hypoxia were examined in premature neonates. The results showed that abnormalities in pH might predict intestinal complications in neonates below 1,500 g [4]. However, these investigations are too complex for routine clinical routine use. Radiological findings such as distended bowel loops are helpful for the diagnosis, but are nonspecific, and signs such as pneumatosis or portal vein air come too late for early and successful conservative therapy [25–27].
In conclusion, abnormal prenatal and postnatal circulation is an important cause of intestinal dysmotility, which in severe cases might lead to the development of necrotizing enterocolitis. Measurements of blood flow velocities in the SMA could be a useful tool for clinicians to identify neonates at high risk for the development of intestinal problems [28]. Pathological blood flow parameters already present on the first day of life can also predict difficulties in later enteral feeding. In those neonates it is appropriate to delay the safe introduction of enteral feeding. Normalization of mesenteric pulsatility is a positive prognostic sign that the infant will tolerate enteral feeding.
References
Mace JT, Azar GJ, Lee RD, et al (1998) Effects of severe hypoxemia on mesenteric blood flow in neonatal piglets. J Pediatr Res 80:287–294
Robel-Tillig E, Vogtmann C, Bennek J (2002) Prenatal hemodynamic disturbances—pathophysiological background of intestinal motility disturbances in small for gestational age infants. Eur J Pediatr Surg 12:175–179
Osborn DA, Evans N (2001) Early volume expansion for prevention of morbidity and mortality in very preterm infants (Cochrane Review). The Cochrane Library Issue 4
Campbell ME, Costeloe KL (2001) Measuring intramucosal pH in very low birth weight infants. Pediatr Res 50:398–404
Bhatt AB, Tank PD, Barmada KB, et al (2002) Abnormal Doppler flow velocimetry in the growth restricted foetus as a predictor for necrotising enterocolitis. J Postgrad Med 48:182–185
Kirsten GF, van Zyl N, Smith M, et al (1999) Necrotizing enterocolitis in infants born to women with severe early preeclampsia and absent end-diastolic umbilical artery Doppler flow velocity. Am J Perinatol 16:309–314
Reber KM, Nankervis CA, Nowicki PT (2002) Newborn intestinal circulation. Physiology and pathophysiology. Clin Perinatol 29:23–39
Fong KW, Ohlson A, Hannah ME, et al (1999) Prediction of perinatal outcome in fetuses suspected to have intrauterine growth restriction: Doppler US study of fetal cerebral, renal, and umbilical arteries. Radiology 213:681–689
Greenholz SK, Perez C, Wesley JR, et al (1996) Meconium obstruction in markedly premature infant. J Pediatr Surg 31:117–120
Ott WJ (2000) Intrauterine growth restriction and Doppler ultrasonography. J Ultrasound Med 19:661–665
Di Lorenzo M, Bass J, Krantis A (1995) An intraluminal model of necrotizing enterocolitis in the developing neonatal piglet. J Pediatr Surg 30:1138–1142
Rowe MI, Reblock KK, Kurkchubasche AG, et al (1994) Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg 29:987–990
Robel-Tillig E, Knüpfer M, Pulzer F, et al (2002) Doppler sonographic findings in neonates with significant persistent ductus arteriosus (in German). Z Geburtshilfe Neonatol 206:51–56
Achiron R, Mazkereth R, Orvieto R, et al (2002) Echogenic bowel in intrauterine growth restriction fetuses: does this jeopardize the gut? Obstet Gynecol 100:120–125
Fang S, Kempley ST, Gamsu HR (2001) Prediction of early tolerance to enteral feeding in preterm infants by measurement of superior mesenteric artery blood flow velocity. Arch Dis Child Fetal Neonatal Ed 85:42–45
Hoehn T, Stöver B, Bührer C (2001) Colonic pneumatosis intestinalis in preterm infants: different to necrotising enterocolitis with a more benign course? Eur J Pediatr 160:369–371
Maruyama K, Koizumi T, Tomomasa T, et al (1999) Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr Radiol 29:472–477
Ewer AK, McHugo JM, Chapman S, et al (1993) Fetal echogenic gut: a marker of intrauterine gut ischaemia. Arch Dis Child 69:510–513
Kempley ST (1994) Doppler and fetal growth retardation. Arch Dis Child 70:160–164
Saling E (1966) Die sparsehaltung des fetalen kreislaufes. Geburtsh Frauenheilk 26:413–419
Tillig E, Robel R, Vogtmann C, et al (1995) Severe protracted intrauterine impaired perfusion—a cause of enteral motility disorder in premature infants. Z Geburtshilfe Neonatol 199:190–194
Voigt M, Schneider KT, Jahrig K (1996) Analysis of a 1992 birth sample in Germany. 1: new percentile values of the body weight of newborn infants (in German). Geburtshilfe Frauenheilkd 56:550–558
Simchen MJ, Beiner ME, Strauss-Liviathan N, et al (2000) Neonatal outcome in growth-restricted versus appropriately grown preterm infants. Am J Perinatol 17:187–192
Wladimiroff JW, Tonge HM, Stewart PA (1986) Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol 93:471–475
Robel-Tillig E, Vogtmann C, Faber R (2000) Postnatal intestinal disturbances in small-for-gestational-age premature infants after prenatal haemodynamic disturbances. Acta Paediatr 89:324–330
Arbeille P (1997) Fetal arterial Doppler-IUGR and hypoxia. Eur J Obstet Gynecol Reprod Biol 75:51–53
Robel-Tillig E, Mockel A, Vogtmann C (1999) Normal Doppler ultrasound values of the anterior cerebral artery of premature and term infants with reference to cardiac function and intestinal blood flow profile (in German). Z Geburtshilfe Neonatol 203:234–240
Kempley ST, Gamsu H, Vyas S, et al (1991) Effects of fetal growth retardation on visceral and cerebral blood flow velocities. Arch Dis Child 66:805–807
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robel-Tillig, E., Knüpfer, M., Pulzer, F. et al. Blood flow parameters of the superior mesenteric artery as an early predictor of intestinal dysmotility in preterm infants. Pediatr Radiol 34, 958–962 (2004). https://doi.org/10.1007/s00247-004-1285-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-004-1285-6