Abstract
Background
Very-low-birth-weight (VLBW) preterm neonates are vulnerable to patent ductus arteriosus (PDA), which might be related to high-resistance flow in the superior mesenteric artery (SMA), with decreased diastolic flow in situations of marked intestinal hypoperfusion. No previous studies have evaluated the portal vein and superior mesenteric vein (SMV) parameters to assess the PDA hemodynamic repercussions.
Objective
To assess mesenteric and portal flow in VLBW preterm neonates with or without PDA using serial Doppler ultrasonography (US).
Materials and methods
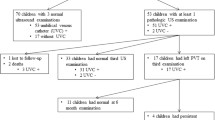
We conducted a prospective longitudinal study on 61 VLBW preterm neonates submitted to 161 Doppler US exams, from 2 days to 20 days of age.
Results
All infants exhibited a progressive daily increase in the mean of the SMA diameter and systolic velocity, the portal vein diameter, the peak velocity, the mean velocity and the flow volume and of SMV diameter (P<0.05). The incidence of PDA was 37.7% (n=23) and infants with the disease revealed a smaller diameter, greater systolic velocity, lower diastolic velocity, and higher resistivity and pulsatility indices on SMA compared to those without PDA (P<0.05). Additionally, 47.8% (n=11) of infants with PDA exhibited absent or reversed end-diastolic flow in the SMA, and its resolution was seen among 54.5% (n=6) of these. Infants with PDA also exhibited lower values of portal vein diameter and flow volume and of SMV diameter (P<0.01).
Conclusion
Doppler US enhances the understanding of mesenteric and portal flow, including the effects of PDA. The study of SMV and portal vein flow is proposed as a new parameter in PDA evaluation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Very-low-birth-weight (VLBW) preterm neonates, considered those born weighing less than 1,500 g, exhibit high morbidity and mortality rates because of organ immaturity and higher vulnerability to diseases and their complications, among them patent ductus arteriosus (PDA) [1, 2].
The ductus arteriosus is a vessel that communicates between the pulmonary artery and the aorta during the fetal period. At this period, the fetal lungs exhibit high vascular resistance and thus receive the smallest part of the right ventricular output. Most of this output is directed to the systemic circulation through the ductus arteriosus. Shortly after birth, with the onset of breathing, the pulmonary vascular resistance decreases and the ductus arteriosus closes. In term newborns, the functional closure of the ductus arteriosus occurs 12–15 h after birth and the permanent closure usually within 5–7 days but sometimes up to 21 days after birth [3,4,5].
PDA occurs when the ductus arteriosus remains open after 72 h. In VLBW neonates, the ductus arteriosus can be closed later and the frequency of the PDA is proportionally higher in this group [5]. Visconti et al. [6] showed that the occurrence of PDA at 72 h of age was 25% among neonates with a birth weight between 1,000 g and 1,500 g, and 65% among those with a birth weight less than 1,000 g. Dominguez et al. [7] found evidence of PDA in 65% and 53% of preterm infants with a gestational age less than 26 weeks and 34 weeks, respectively.
At the beginning of neonatal adaptations, if pulmonary vascular resistance is still high, PDA does not cause symptoms. However, as this resistance decreases, neonates with PDA develop a left-to-right shunt through the arterial duct, which comprises the ductal steal, redirecting up to 30% of the aorta flow to the lungs during diastole. Consequently, pulmonary hyperflow can damage the pulmonary capillaries and cause pulmonary edema/hemorrhage, as well as reduce lung compliance, with a risk of bronchopulmonary dysplasia [5, 8].
The ductal steal is also associated with a reduced diastolic pressure in the aorta, which can lead to a systemic hypoflow and decreased organic perfusion, notably in the intestine, kidneys and brain [9]. When systemic hypotension is refractory, neonatal morbidity increases because of the risk of necrotizing enterocolitis (NEC), renal failure, periventricular leukomalacia and intraventricular hemorrhage [4, 10]. The intestine is usually more affected when self-regulating mechanisms, with splanchnic vasoconstriction and redistribution of intestinal blood flow, are developed to minimize the consequences of hypoflow on vital organs, such as the brain [11]. If the PDA is not treated properly, there is a risk of intestinal ischemia or perforation caused by evolution with NEC; this can even progress to death [12].
Doppler ultrasonography (US) is a noninvasive and widely available method that favors an understanding about the pathophysiological mechanism underlying PDA through the analysis of the descending aorta and its branches [9]. Analyzing the superior mesenteric artery (SMA) flow is considered excellent to characterize the intestinal flow, given that it supplies a significant portion of the intestine [13]. It is known that PDA might be related to high-resistance flow in the SMA, with absent or reversed end-diastolic flow in situations of marked intestinal hypoperfusion from ductal steal [12, 14]. It is questioned whether the context of intestinal hypoperfusion related to PDA could cause any impact on the venous system. Unfortunately, no previous studies have evaluated the portal vein and superior mesenteric vein (SMV) parameters as a means to assess the PDA hemodynamic repercussions.
The purpose of the present study was to assess the mesenteric and portal flow in VLBW preterm neonates with and without PDA using serial Doppler US. Regarding the vascular parameters, we describe the estimated daily variations in all infants and the estimated difference between infants with and without PDA.
Materials and methods
We conducted this longitudinal prospective study between October 2016 and May 2017 at a single teaching hospital with a 50-bed neonatal intensive care unit. The study was approved by the institutional review board. Parents/legal guardians signed informed consent forms before each examination.
We included premature and VLBW newborns (≤1,500 g) in the study, excluding those with complex congenital heart disease (except PDA and patent foramen ovale), gastrointestinal diseases (such as gastroschisis, omphalocele and NEC) and genetic syndromes. We excluded infants with NEC from the study to avoid any confounding effects that NEC alone (and not necessarily related to PDA) could cause under the mesenteric and portal circulation because its pathophysiological mechanism is known to be complex and multifactorial. The diagnosis of PDA was performed through clinical data (e.g., presence of a heart murmur) or echocardiogram (e.g., direct visualization of the ductus arteriosus), and we acquired this information through medical records.
Serial evaluation by Doppler US was used to address the aims of the study. US procedures took place at the incubator-side under the responsibility of a single radiologist who had more than 15 years of dedicated pediatric radiology experience (E.J.C.S.), by using a portable Philips CX50 (Philips Ultrasound, Bothell, WA) with a 12–3-MHz linear transducer. Any influence from postprandial changes of the mesenteric circulation was minimized by performing measurements on infants with a minimum of 3 h of fasting; the infants were fed only after the examination was concluded.
Doppler US sessions were divided into three groups according to the age at which they were performed: Group 1 (US conducted on VLBW neonates age 2–5 days), Group 2 (US conducted on VLBW neonates age 6–9 days) and Group 3 (US conducted on VLBW neonates age 10–20 days). This division was made to facilitate the organization of the US procedures. Infants were not submitted to this examination on their first day of age to minimize any interference in the transition and adaptation from the fetal to the neonatal stage.
The initially proposed procedure was that all infants would undergo at least one Doppler US exam in each group. However, this was not possible for all cases over the course of the study; sometimes the infant was too unstable to be examined, had already been fed enterally, had been discharged from the neonatal intensive care unit, or had died. In case of an unfavorable clinical evolution reported by the assistant neonatologist (e.g., abdominal distension, significant gastric residuals, abdominal pain, leukocytosis, suspected NEC) or evidence of Doppler changes during the examination (e.g., absent or reversed diastolic mesenteric flow), Doppler US was repeated more than once in the same group to better characterize the evolution of the US findings.
The SMA was assessed where it arises from the abdominal aorta, and its diameter and area were calculated in millimeters and millimeters squared, respectively, in the transverse plane in B-mode. The flow of SMA was assessed in the longitudinal plane in spectral Doppler mode, with the correction of angles below 60° and analysis of one cycle after at least three consecutive and similar waveforms, to characterize systolic velocity (peak velocity) and diastolic velocity (end-diastolic flow).
The resistance index (RI) and the pulsatility index (PI) of SMA were also calculated, using the Pourcelot and Gosling-King formula, in which RI = (systolic peak velocity – minimum diastolic velocity) / systolic peak velocity, and PI = (systolic peak velocity – minimum diastolic velocity) / mean velocity. Absent and reversed end-diastolic flow were considered when the SMA diastolic flow was absent (minimum velocity equal to zero) or reversed (minimum velocity exhibiting a negative value), respectively.
As for venous flow, the portal vein was assessed after the confluence of the SMV and the splenic vein, and its diameter, area, peak velocity, minimum velocity, mean velocity and flow velocity were calculated following the abovementioned criteria. The SMV was assessed near its confluence with the splenic vein, and its diameter and area were calculated. The results regarding SMA, portal vein and SMV flow obtained in the present study were disclosed to the assistant neonatologist. Nevertheless, the data were not considered in an isolated manner for clinical management and therapeutic decision-making, including the time and volume of enteral nutrition.
After ensuring the consistency of the data, we performed the statistical analysis with the software Stata 12.1SE (StataCorp, College Station, TX). Categorical variables were summarized by absolute and percentage frequencies, numerical variables by means and standard deviations. We performed the estimated difference between the means of infants with and without PDA using the Student’s t-test. The estimated daily variations regarding the means, intra- and inter-groups were evaluated by adjusting linear mixed models, which consider the dependency structure between the intra-group observations. Tests with P-values less than 0.05 were considered significant.
Results
Sixty-one neonates participated in the study, of whom 52.4% (n=32) were male, with a median weight of 1,150 g (lower quartile = 925 g and upper quartile = 1,320 g) and mean gestational age of 29 weeks (standard deviation ±2.8 weeks). Of all patients, 13.1% (n=8) died, at a median age of 19 days (lower quartile = 16 days and upper quartile = 29 days); 37.5% (n=3) of these deaths were caused by sepsis, 25% (n=2) by respiratory distress syndrome, 12.5% (n=1) by pulmonary hemorrhage, 12.5% (n=1) by bronchopulmonary dysplasia, and 12.5% (n=1) by hypoxia and neurologic disorders.
The PDA incidence in the total cohort was 37.7% (n=23); 39.1% (n=9) of these babies were born weighing less than 1,000 g and 82.6% (n=19) had a heart murmur at the time of the US examination, thus corroborating the diagnosis. Of all the infants with PDA, 65.2% (n=15) received medical treatment (ibuprofen or paracetamol) and 4.3% (n=1) underwent surgical treatment.
A total of 161 Doppler US examinations were performed, among which 53 were in Group 1, 43 in Group 2 and 65 in Group 3 (Table 1). Table 1 shows the number of Doppler US examinations in each age group, according to the presence or absence of the PDA diagnosis.
Table 2 shows the estimated daily variations regarding the mean values in the whole population and the estimated differences between the mean values in neonates with and without PDA (using the mean values of the variables for which the interaction between age and group was not significant at the 5% level). Thus, for each of these variables, the estimated daily variation of the mean was the same in both groups (with and without PDA) and the estimated difference in the means between groups was the same for each age, from 2 days to 20 days (the time period during which measurements were conducted). Figure 1 is a graphic representation of the Doppler velocimetry data shown in Table 2. The SMA, SMV and portal vein parameters are described separately in the next sections.
Graphic representations of the estimated daily variations and estimated differences among neonates with and without patent ductus arteriosus (PDA), considering the mean values of superior mesenteric artery (SMA) systolic velocity (a), SMA diastolic velocity (b), SMA resistivity index (c), SMA pulsatility index (d), portal vein peak velocity (e), portal vein minimum velocity (f) and portal vein flow volume (g), as described in Table 2. CI confidence interval
Superior mesenteric artery
In the analysis of neonates in general, the estimated daily variation was characterized by an increase of 0.02 mm (95% confidence interval [CI]: 0.01–0.03 mm) and 1.52 cm/s (95% CI: 0.22–2.83 cm/s) in mean SMA diameter and systolic velocity, respectively, and this increase was significant (P<0.01 and P=0.02, respectively). SMA diastolic velocity, RI and PI values remained stable, with no significant daily changes.
In the comparative analysis between infants with and without PDA, the estimated daily differences of the means was 0.25 mm (95% CI: −0.38 mm to −0.12 mm) smaller in diameter, 15.5 cm/s (95% CI: 0.3–30.8 cm/s) faster in systolic velocity, 7.7 cm/s (95% CI: −11.5 cm/s to −4.0 cm/s) slower in diastolic velocity, 0.07 (95% CI: 0.04 to 0.10) higher in RI, and 0.72 (95% CI: 0.35 to 1.08) higher in PI of the SMA in neonates diagnosed with PDA, with relevance (P<0.05).
In the present study, 19.7% (n=12) of all the neonates exhibited absent or reversed end-diastolic flow in the SMA (Fig. 2). Of these 12, 91.7% (n=11) were diagnosed with PDA. The evidence of reversed end-diastolic flow exhibited a significant association with the diagnosis of PDA (P=0.01). Absent diastolic flow exhibited no such significant relationship, probably because of its low occurrence. Absent/reversed end-diastolic flow was resolved in 54.5% (n=6) of the infants with PDA after drug treatment. These infants showed a diastolic flow recovery on SMA and a dietary progression, with a daily increase in feeding volumes.
Ultrasonography in a female preterm neonate, born weighing 1,080 g and diagnosed with patent ductus arteriosus (PDA). a, b Doppler US image demonstrates a normal superior mesenteric artery (SMA) (a) and portal vein (b) Doppler US image at 9 days of age demonstrates abnormal flow in the SMA, with reversed end-diastolic flow, when the girl developed abdominal distension. c flow at the first examination, at 5 days of age
Superior mesenteric vein
In the analysis of infants in general, the estimated daily variation was characterized by an increase of 0.05 mm (95% CI: 0.03–0.06 mm) regarding the mean SMV diameter, and this increase was significant (P<0.01). In the comparative analysis between infants with and without PDA, the estimated daily difference of the mean was 0.45 mm (95% CI: −0.62 mm to −0.27 mm) lower in SMV diameter in those diagnosed with PDA, and this was significant (P<0.01).
Portal vein
In the analysis of all of the infants, the estimated daily variation was characterized by an increase of 0.04 mm (95% CI: 0.03–0.06 mm) in mean portal vein diameter, 0.02 mm2 (95% CI: 0.01–0.03 mm2) in area, 0.21 cm/s (95% CI: 0.01–0.40 cm/s) in peak velocity, 0.20 cm/s (95% CI: 0.03–0.37 cm/s) in mean velocity and 3.15 cm3/min (95% CI: 1.97–4.33 cm3/min) in flow volume, and this was significant (P<0.05).
In the comparative analysis of infants with and without PDA, the estimated daily difference was 0.53 mm (95% CI: −0.74 mm to −0.31 mm) less in diameter, 2.7 mm2 (95% CI: −3.7 mm2 to −0.2 mm2) less in area, and 35.2 cm3/min (95% CI: −52.3 cm3/min to −18.1 cm3/min) less in portal vein flow volume in infants diagnosed with PDA, with relevance (P<0.01). The peak and minimum velocity of the portal vein in infants with PDA were also lower, but these results were not significant.
Discussion
Patent ductus arteriosus is a highly common complication among VLBW preterm neonates and the incidence of PDA in the present study was similar to that of previous studies. The ductal steal phenomenon with intestinal hypoperfusion, triggered by PDA, has already been described. Groves et al. [14] associated reversed end-diastolic flow of the descending aorta, with a 35% decrease in flow volume, in the presence of a PDA ductal diameter more than 1.7 mm. Previous studies also noted reduced or reversed diastolic flow in the descending aorta and other abdominal arteries, including the renal and mesenteric arteries, among preterm infants with symptomatic PDA [9, 14,15,16].
In the serial analysis of the present study, the SMA diameter and systolic velocity tended to increase gradually and in a significant manner over time in all infants. Similarly, Martinussen et al. [13] found an increase in the mean flow velocity of the SMA between Days 1 and 7 of age in healthy preterm infants weighing 1,980 g to 2,930 g. Maruyama et al. [17] also found an increase in this parameter between Days 1 and 6 of age among healthy preterm infants weighing 925 g to 1,990 g, and suggested that the increased flow velocity of the SMA might be caused by an increase in blood pressure and in the amount of the enteral diet within this period.
The evolution curve of the SMA diastolic velocity in all participants of the present study exhibited a trend toward remaining stable over the course of time, with no significant daily variations. With a stable diastolic velocity, there were no significant daily repercussions on the RI and PI of the SMA. This finding might be explained by the neonate’s natural adaptation in an attempt to maintain a steady intestinal blood flow despite an increasing demand and flow volume.
Even though the evolution curves showed a similar daily trend in all infants, a different behavior was observed when comparing infants with and without PDA. In the present study, the assessment of the SMA among infants with PDA revealed a high-resistance flow characterized by a smaller diameter, greater systolic velocity, lower diastolic velocity, and higher RI and PI values compared to infants without PDA. These findings agree with the previously proposed mechanism of splanchnic vasoconstriction during the genesis of intestinal hypoperfusion, with an increased systolic flow velocity, not accompanied by an increased diastolic flow velocity, with a resulting increase in the RI [18].
Splanchnic vasoconstriction leads to a redistribution of blood flow to the vital organs as a response to reduced systemic flow triggered by PDA, which results in intestinal hypoperfusion. Meyers et al. [18] showed that PDA causes a reduction in intestinal blood flow. In situations of marked intestinal hypoperfusion caused by PDA, the patient develops more severe changes in the diastolic flow of the SMA [9]. In the present study reversed end-diastolic flow exhibited a significant correlation with a diagnosis of PDA. No significant relation was found regarding absent diastolic flow, which is probably because of its low occurrence.
A case of a preterm neonate with hemodynamically significant PDA and reversed end-diastolic flow in the upper mesenteric vessels has been reported by Arvind et al. [12]. In this case, the diastolic flow was restored and symptoms resolved after medical treatment with ductal closure. Shimada et al. [19] found an increase in the mean blood flow velocity in the SMA after closing the arterial duct in symptomatic patients. In the present study, a resolution of the absent/reversed end-diastolic flow was observed in 54.5% (n=6) of the infants with PDA after medical treatment. These infants showed a diastolic flow recovery on SMA and a dietary progression, with a daily increase in feeding volumes, which is indicative of clinical improvement.
As for venous flow, the evolution curve of the diameter of the SMV tended to increase daily and in a significant manner over time for all infants. Rising curves, with a significant daily increase, were also found for the diameter, peak velocity, mean velocity and flow volume of the portal vein. This finding can also be explained by structural growth and increased dietary needs and demands.
No previous reports have assessed portal vein and SMV parameters as a means to measure the effects of PDA. This procedure was emphasized in the present study, and we found that infants with PDA exhibited smaller SMV and portal vein diameters than those without PDA. In the analysis of the portal vein flow volume, infants with PDA exhibited lower values than those without PDA; this finding agrees with what has been observed regarding intestinal hypoperfusion. The peak velocity, mean velocity and minimum velocity of the portal vein in infants with PDA also exhibited lower values compared to infants without PDA; however, this finding was not significant, probably because of the small sample size.
These findings indicate that systems other than the arterial system can exhibit the effects of ductal steal in PDA. Reduced arterial blood flow in the intestine triggered by PDA can also have an impact on the intestinal venous system, resulting in reduced venous intestinal drainage, thus compromising the infant’s main nutrient entrance route. With the reduced intestinal venous flow, the portal flow is also reduced because it partially depends on the mesenteric flow.
Thus, the evidence of reduced intestinal venous flow and the resulting reduced nutrient supply from what is absorbed in the enterocytes/intestinal capillaries can affect the infant’s development. Knowing that 70% of hepatic flow is derived from the portal vein, one could further examine the functional changes of the liver caused by reduced portal flow among infants with PDA. Future studies should address these questions.
Because the venous system can reflect the hemodynamic repercussions of PDA, the present study proposes assessing the mesenteric and portal veins as novel means to this end. The portal vein stands out as a candidate because it is technically easy to analyze, less influenced by stomach and intestinal gas, and simple to access.
The US parameters are important because they favor the acquisition of further knowledge and signal the need for earlier attention and care for the neonate. However, they must be individualized and cannot be considered as an isolated factor for an etiological definition or a therapeutic choice/modification; rather, they must be considered as complementary information and used in association with other clinical and laboratory data.
Despite the longitudinal and prospective character of the present study, which assessed changes in mesenteric and portal parameters over time, we emphasize that the small evolutive sample is a limitation of the study. The data related to the portal and mesenteric venous flow mentioned in the present study are preliminary and further studies are needed. These data alone cannot confirm the suspicion of PDA, but we believe that these data could be used as a screening to indicate the performance of an electrocardiogram and even help to categorize PDA patients with low or high risk for intestinal complications and thus be useful on therapeutic guidance. However, further studies are needed to incorporate these data into clinical practice.
Conclusion
The examination of the SMA, SMV and portal vein by serial Doppler ultrasonography enhances the understanding of intestinal flow in VLBW preterm neonates, and we observed evolutive curves with a progressive daily increase in the parameters of these vessels. This method can also characterize the hemodynamic repercussions that occur because of PDA, such as an SMA with high-resistance flow, increased PI and RI values, and, eventually, absent or reversed end-diastolic flow. The reduced arterial blood flow also has an impact on intestinal venous drainage, with reduced mesenteric and portal venous flow, and these findings were first evaluated by the present study. Thus, we propose the assessment of superior mesenteric and portal veins as a new parameter for PDA evaluation. However, further studies are needed to incorporate these data into clinical practice.
References
Benitz W (2016) Patent ductus arteriosus in preterm infants. Pediatrics 137:e20153730
Noori S, McCoy M, Friedlich P et al (2009) Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 123:138–144
Miyague N (2005) Persistência do canal arterial em recém-nascidos prematuros [Preterm neonates with patent ductus arteriosus]. J Pediatr 81:429–430
Jain A, Shah PS (2015) Diagnosis, evaluation, and management of patent ductus arteriosus in preterm neonates. JAMA Pediatr 169:863–872
Hamrick SE, Hansmann G (2010) Patent ductus arteriosus of the preterm infant. Pediatrics 125:1020–1030
Visconti L, Morhy S, Deutsch A et al (2013) Características clínicas e ecocardiográficas associadas à evolução do canal arterial em recém-nascidos com peso de nascimento inferior a 1500g [Clinical and echocardiographic characteristics associated with the evolution of ductus arteriosus in neonates with birth weight lower than 1,500g]. Hosp Isr Albert Einstein 11:317–323
Dominguez G, Leboreiro J, Macías M el al (2015) Pesquisa o diagnóstico sintomático por ecocardiografía en la persistencia del conducto arterioso en prematuros [Echocardiographic screening vs. symptomatic diagnosis for patent ductus arteriosus in preterms]. Rev Med Inst Mex Seguro Soc 53:136–141
Antonucci R, Bassareo P, Zaffanello M et al (2010) Patent ductus arteriosus in the preterm infant: new insights into pathogenesis and clinical management. J Matern Fetal Neonatal Med 23:34–37
Hsu KH, Nguyen J, Dekom S et al (2019) Effects of patent ductus arteriosus on organ blood flow in infants born very preterm: a prospective study with serial echocardiography. J Pediatr 216:95–100
Wong SN, Lo RN, Hui PW (1990) Abnormal renal and splanchnic arterial Doppler pattern in premature babies with symptomatic patent ductus arteriosus. J Ultrasound Med 9:125–130
Chock VY, Ramamoorthy C, Van Meurs KP (2012) Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J Pediatr 160:936–942
Arvind S, Tran H, Carse E (2011) Doppler manifestations of ductal steal: role in decision making. Eur J Pediatr 170:795–798
Martinussen M, Brubakk AM, Vik T, Yao AC (1996) Mesenteric blood flow velocity and its relation to transitional circulatory adaptation in appropriate for gestational age preterm infants. Pediatr Res 39:275–280
Groves AM, Kuschel CA, Knight DB, Skinner JR (2008) Does retrograde diastolic flow in the descending aorta signify impaired systemic perfusion in preterm infants? Pediatr Res 63:89–94
Martins FF, Rios DI, Resende MHF et al (2018) Relationship of patent ductus arteriosus size to echocardiographic markers of shunt volume. J Pediatr 202:50–55
Ledo A, Aguar M, Nunez-Ramiro A et al (2017) Abdominal near-infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology 112:238–245
Maruyama K, Koizumi T, Tomomasa T, Morikawa A (1999) Intestinal blood-flow velocity in uncomplicated preterm infants during the early neonatal period. Pediatr Radiol 29:472–477
Meyers RL, Alpan G, Lin E, Clyman RI (1991) Patent ductus arteriosus, indomethacin, and intestinal distension: effects on intestinal blood flow and oxygen consumption. Pediatr Res 29:569–574
Shimada S, Kasai T, Konishi M, Fujiwara T (1994) Effects of patent ductus arteriosus on left ventricular output and organ blood flows in preterm infants with respiratory distress syndrome treated with surfactant. J Pediatr 125:270–277
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cavalcanti, L.S., da Costa e Silva, E.J., Falbo, A.R. et al. Serial Doppler velocimetry of mesenteric and portal flow in very-low-birth-weight preterm neonates with and without patent ductus arteriosus. Pediatr Radiol 50, 1107–1114 (2020). https://doi.org/10.1007/s00247-020-04689-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04689-y