Abstract
Purpose
Ventricular shunt-induced craniosynostosis is a widely recognised cause of secondary craniosynostosis. We reviewed the management and long-term outcome of the cases of cranial deformity post cerebrospinal fluid shunting in our unit and compared these with previously published series.
Methods
The Australian Craniofacial Unit and Department of Neurosurgery database was searched to identify cases of ventricular shunt-induced cranial deformity and a case note review was undertaken.
Results
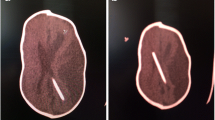
Eight cases were identified, and all were shunted within 6 months of birth. Our patients required shunting with a low pressure valve for hydrocephalus secondary to either aqueduct stenosis or intraventricular haemorrhage. The diagnosis was made following computed tomography (CT) three-dimensional surface reconstruction of the skull. Two cases of confirmed suture fusion were treated with cranial vault remodelling and programmable shunt insertion. In six cases, the sutures were not completely fused on the CT images despite a scaphocephalic head shape. These patients were managed conservatively with close monitoring.
Conclusion
Cranial vault remodelling together with insertion of programmable shunt valve is indicated in CT confirmed cases of secondary craniosynostosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ventricular shunting is a well-established treatment for hydrocephalus in infancy [1]. However, it has been recognised that decompression of the cerebral ventricles may interfere with the cranial vault growth, and in some cases, this leads to premature fusion of the sutures. This was first reported by Strenger [2] in 1963, and since then, ventricular shunting has been a widely recognised cause of secondary craniosynostosis [3–7]. The published incidence of this complication varies between 1.0% and 12.4% [4, 5, 8]. Surprisingly, however, there is a marked paucity of information in the literature about this condition especially in relation to its management. We wish to review the cases of secondary synostosis from our unit to assess management and long-term outcome of this rare condition.
Method
The Australian Craniofacial Unit and Department of Neurosurgery database was searched to identify cases of ventricular shunt-induced craniosynostosis. Inclusion in the study required confirmation of scaphocephaly according to March of Dimes cranial index of scaphocephaly and ventricular shunting in the first 12 months of life. Cases of syndrome-associated scaphocephaly were excluded. A case note review was undertaken to confirm that the inclusion criteria were met and to assess the varying management and progress of these children.
Results
Eight cases of scaphocephaly resulting from ventricular shunting were identified, and no one was excluded. There were three girls and five boys. All were shunted within 6 months of birth (Table 1). Two cases required shunting for hydrocephalus secondary to aqueduct stenosis, five were shunted for hydrocephalus following intraventricular haemorrhage and one was shunted after meningitis. Low pressure shunt valves were inserted in all cases. The children were followed up at a minimum of three monthly in their first year of life and during this routine appointment, the abnormal head shape was noted which prompted a computed tomography (CT) three-dimensional surface reconstruction of the skull for suspicion of secondary craniosynostosis. Two cases were treated with cranial vault remodelling and programmable shunt valve insertion (Figs. 1, 2 and 3). In five cases, the radiological appearances of the sagittal suture appeared to be patent, although thickened bone was found adjacent to the sutures. These cases were managed conservatively with close follow-up and serial imaging. In one case, the sagittal suture was only partially fused; therefore, an attempt was made to expand the cranial vault by inserting a programmable shunt valve and then progressively increasing the valve opening pressure. This was abandoned when the child started exhibiting signs of raised intracranial pressure. He has since been managed conservatively as well. One child died at the age of 7 years old following acute hydrocephalus secondary to shunt obstruction. The other seven children have varying degrees of developmental delay on formal neuropsychology assessment without clinical signs and symptoms of raised intracranial pressure. In two of the cases managed conservatively, there has been a marked improvement in the head shape without any radiological evidence of progressive suture fusion.
Discussion
The prevalence of congenital and infantile hydrocephalus has been estimated as 0.48 to 0.81 per 1,000 live and still births [9–11]. Hydrocephalus occurs in approximately 35% of infants with intraventricular haemorrhage and 15–20% of those will require cerebrospinal fluid diversion by means of ventricular shunting procedures [12]. We could find no significant series published describing the management of shunt induced craniosynostosis despite it being a well-acknowledged complication of shunting. To date, we believe that this will be the largest case series reviewing the management of shunt-induced cranial deformity.
The major complications associated with uncorrected craniosynostosis include increased intracranial pressure, altered cranial base resulting in facial asymmetry, malocclusion as well as potential adverse effect on the psychological well-being [13, 14]. There has been limited data published specifically in relation to the repair of secondary craniosynostosis. Some studies have recognised the increased complexity involved in the repair of the cranial vault abnormality in these patients and various methods have been explored to maintain the skull shape and prevent recurrence [3, 4, 7, 8, 15]. There are, however, also those that strongly discourage surgical correction in these patients arguing that the operative risks are unacceptable in these patients who are not at risk for developing raised intracranial pressure [3, 4].
Parasagittal and linear craniectomies were described as a method to treat secondary synostosis by Roberts et al. [5] and Kloss [6]. Alternatively, Schendel et al. [7] reported good results with at a minimum sagittal strip craniectomy and biparietal osteotomies as well as the addition of an occipital and frontal remodelling in those cases with severe protrusion. The bone flaps were rigidly secured with transverse microplates to maintain the expanded shape and prevent recurrent collapse [7]. More recently, expansile springs were utilised to treat patients with scaphocephaly secondary to ventricular shunting [16]. This method was advocated as an advantage over previous craniofacial reshaping techniques in terms of reducing morbidity and blood loss through limited dissection and shorter operative time.
Six patients did not have complete fusion of the cranial sutures on CT despite their scaphocephalic head shape. Davis and Lauritzen suggested in their paper published in May 2008 [16] that in such cases, craniosynostosis would have inevitably resulted and, therefore, advocated intervention before onset. We opted to manage these patients conservatively with close clinical and radiological follow-up. As reported above, two patients achieved a remarkable improvement in their head shape without any intervention; therefore, it is unnecessary to place all these patients through the risks of an operation based solely on their clinical head shape. The close monitoring is specifically directed towards early detection and prompt intervention in those patients who subsequently develop radiological evidence of fused suture.
The cranial deformity seen in our cohort was a scaphocephalic shape as are in several other reported cases. We utilised the same calvarial remodelling technique as that used in primary sagittal synostosis in our unit to achieve a near normal cranial contour. This is a safe and appropriate procedure in those with CT-proven craniosynostosis. Additionally, we believe it is imperative to use a programmable shunt valve to dilate the ventricles postoperatively to prevent the development of a subdural fluid collection which can be expected given the expanded cranial vault. It is prudent to be cautious despite published series of Shuster et al. [15] reporting that this was not an observed complication because the small cohort did not reach statistical significance and the development of a subdural collection is potentially a consequential even though as yet a theoretical one.
Our experience has led to a change in our practice. We now only insert programmable shunt valves in patients less than 12 months old, the rationale being to maintain physiological tension across the cranial sutures and conceivably prevent premature fusion by careful regulation of ventricular decompression. Alternatively, an endoscopic third ventriculostomy is conducted in appropriate cases. This restores physiological cerebrospinal fluid circulation circumventing the problems associated with ventricular over-drainage.
We conclude that cranial vault remodelling should only be performed in those cases with radiological evidence of total single or multiple cranial suture synostosis and that post remodelling insertion of a programmable shunt valve is an important part of the management strategy. Furthermore, there is no role for a prophylactic corrective procedure in patients with cranial deformity without complete fusion on CT imaging.
References
Drake JM (2008) The surgical management of pediatric hydrocephalus. Neurosurgery 62(suppl. 2):633
Strenger L (1963) Complications of ventriculovenous shunts. J Neurosurg 20:219
Andersson H (1966) Craniosynostosis as a complication after operation for hydrocephalus. Acta Paediatr Scand 55:192
Faulhauer K, Schmitz P (1978) Overdrainage phenomena in shunt treated hydrocephalus. Acta Neurochirur 45:89
Roberts JR, Rickham PP (1970) Craniostenosis following Holter valve operations. Dev Med Child Neurol 12(suppl. 22):145
Kloss JL (1968) Craniosynostosis secondary to ventriculoatrial shunt. Am J Dis Child 116:315
Schendel SA, Shuer LM (1994) Multiple-suture synostosis subsequent to ventricular shunting. Plast Reconstr Surg 93:1073
Pudenz RH, Foltz EL (1991) Hydrocephalus: overdrainage by ventricular shunts: a review and recommendations. Surg Neurol 35:200
Chumas P, Tyagi A, Livingston J (2001) Hydrocephalus–what’s new? Arch Dis Child Fetal Neonatal Ed 85(3):F149
Blackburn BL, Fineman RM (1994) Epidemiology of congenital hydrocephalus in Utah, 1940–1979: report of an iatrogenically related “epidemic”. Am J Med Genet 52(2):123
Fernell E, Hagberg G, Hagberg B (1994) Infantile hydrocephalus epidemiology: an indicator of enhanced survival. Arch Dis Child Fetal Neonatal Ed 70(2):F123
Volpe JJ (2001) Intracranial hemorrhage: germinal matrix-intraventricular hemorrhage. In: Volpe JJ (ed) Neurology of the Newborn, 4th edn. WB Saunders, Philadelphia, p 428
Barrit J, Brooksbank M, Simpson D (1981) Scaphocephaly: aesthetic and psychosocial considerations. Dev Med Child Neurol 23:183
Virtanen R, Korhonen T, Fagerholm J, Viljanto J (1999) Neurocognitive sequela of scaphocephaly. Pediatrics 103(4):791
Shuster BA, Norbash AM, Schendel SA (1995) Correction of scaphocephaly secondary to ventricular shunting procedures. Plast Reconstr Surg 96:1012
Davis C, Lauritzen C (2008) Spring-assisted remodeling for ventricular shunt-induced cranial deformity. J Craniofac Surg 19(3):588
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doorenbosch, X., Molloy, C.J., David, D.J. et al. Management of cranial deformity following ventricular shunting. Childs Nerv Syst 25, 871–874 (2009). https://doi.org/10.1007/s00381-009-0842-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-009-0842-6