Abstract
Purpose
The aim of this study is to describe the symptoms and signs of central nervous system (CNS) tumors in a pediatric population and to assess the time interval between the onset of the disease and the time of the diagnosis.
Methods
A retrospective observational study was conducted at our Oncology Pediatric Unit between January 2000 and November 2011. We included 75 children between 5 months and 16 years (mean age of 7.8 ± 4.7 years), with male to female ratio of 3:2. The tumor localization was supratentorial in 51% of cases, and the most frequent histological type was low-grade astrocytoma (48%).
Results
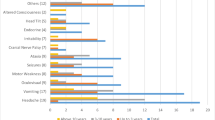
Presenting symptoms were headache (31%), vomiting (31%), seizures (21%), and behavioral change (11%). The most common symptoms at diagnosis were headache (51%), vomiting (51%), visual difficulties (37%), seizures (24%), and behavioral change (21%). By the time of diagnosis, neurologic examination was altered in 68% of our patients. Vomiting (44%) and behavioral change (44%) were the most frequent symptoms in children under 4 years of age, headache (61%) and vomiting (54%) in children older than 4 years. The median interval between symptoms’ onset and diagnosis was 4 weeks (range 0 to 314 weeks). A longer symptom interval was associated with younger age, infratentorial localization and low-grade tumors. The differences in symptom intervals between the different age, location, and grade groups were not statistically significant. Survival probability was influenced by tumor grade but not by diagnostic delay or age of the child.
Conclusions
Headache and vomiting are the earliest and commonest symptoms in children with brain tumors. Visual symptoms and signs and behavioral change are often present. Abnormalities in neurological examination are reported in most of the children. Intracranial hypertension symptoms suggest the need for a neurological clinical examination and an ophthalmological assessment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CNS tumors account for 20% of all childhood cancer and are the largest group of pediatric solid tumors in the developed countries. They represent the second cause of cancer-related death in European children [1]. Clinical manifestations related to CNS neoplasms in children are well known, but at the illness onset, the neurologic and clinical presentation can change, according to tumor localization, children’s age, and tumor’s histology. Furthermore, many symptoms and clinical signs are rather unspecific and can be easily attributed to less severe pediatric disorders, such as gastroenteritis, migraine or behavioral problems, and subtle neurological deficits could be difficult to recognize in younger children [2, 3]. The diagnostic delay for brain tumors is one of the longest of all childhood cancers, with a reported median ranging from 1 to 27 months [2, 4], and seems not to have decreased despite increased availability of neuroimaging during the 1990s [5]. Time before diagnosis represents a very distressing period for the child and his family, and it may affect their adaptation and reaction to the diagnosis [6]. Moreover, long diagnostic delays are associated with a higher risk of developing life threatening complications, irreversible neurological damage, and cognitive deficits in later life [7].
Methods
Case note review of 85 children with brain tumor referred to our Oncology Unit from January 2000 to November 2011 was carried out to obtain information about clinical characteristics at diagnosis, tumor location, histopathology of the tumor, symptoms at onset, symptoms occurring at any moment before the diagnosis, and at diagnosis. The study included children aging between 0 and 16 years. We later excluded 10 patients because of incomplete information. Symptoms were categorized into 13 groups: headache, vomiting, seizures, behavioral change, visual difficulties, vertigo, pain, weight loss/growth retardation, tremor, clumsiness/incoordination, dysphagia, incontinence, and developmental delay. Signs were categorized into 11 groups: visual system abnormalities, cerebellar signs, focal motor weakness, other motor system abnormalities, abnormal muscle tone, sensory abnormalities, endocrine problems, abnormal osteotendineal reflexes, reduced level of consciousness (LOC), macrocephaly, and cranial nerve abnormalities. We defined the symptom interval as the time between the onset of initial symptoms or signs of a CNS tumor and its diagnosis by CT or MR imaging. Information about patient’s survival was obtained from a regional computerized data system.
Statistical analysis
Descriptive analysis was performed to characterize the study population. Mann-Whitney U test and Fisher’s exact test were used for comparison between groups as appropriate. Correlation analysis between continuous variables was performed using Spearman’s correlation as appropriate. Survival curves were calculated using Kaplan-Meier method. For multivariate analysis Cox’s proportional hazards regression model was used to estimate hazard ratio (HR) and 95% confidence intervals (CIs). Predictors were tumor grade and diagnostic delay. All analyses other than decision trees were performed with IBM SPSS 23.0 for Windows (IBM Corp. Armonk, NY, USA), and a p < 0.05 was considered statistically significant for all analyses.
Results
Patient characteristics
The information were available for 75 patients. The median age at presentation was 7.8 years (IQR 25°–75°: 3.3–11.7) with male to female ratio of 3:2 (45 male, 30 female). The tumor was supratentorial in 38 children (51%), infratentorial in 32 (43%), located in the spinal cord in 4 (5%), and diffuse in 1 (1%). In 3 children, more than one location was involved (4%). Pilocytic astrocytoma (31%) was the most frequent histological type, ranked by fibrillar astrocytoma and chraniopharingioma. The histological diagnosis is shown in Table 1.
Symptoms and signs
The most frequent symptoms were headache and vomiting. Less than 3% of the patients presented weight loss, clumsiness/incoordination, tremor, or dysphagia as first symptoms. Table 2 describes frequency of symptoms at the onset of the disease and anywhere before diagnosis in children with CNS tumors. Visual difficulties (32%) were the commonest symptoms developing at a later stage before diagnosis, followed by headache (20%), vomiting (20%), behavioral problems (11%), weight loss (5%), and vertigo (4%). Seizures were an early manifestation in almost all cases and only in 3% of the children developed at a later stage. The combination of vomiting and headache was observed in 10 patients (13%) at symptom onset, and it was more frequent at diagnosis (37%) while isolated vomiting or headache were reported in 20 patients. At diagnosis, only in 4 (5%) of the children papilledema was described in combination with headache and vomiting. In children with pain, this was located in order of decreasing frequency at lumbar spine, neck, limbs, and pelvic girdle.
Neurologic examination at diagnosis was not normal in 51 children (68%). Neurological findings are shown in Table 3. Thirteen (54%) among the 24 patients without detectable neurological alterations presented with seizures. The most frequent neurological signs were visual system abnormalities (20%) and cerebellar signs (7%). Incidence of motor system abnormalities alone was 4%, but they were more frequently associated to other neurological signs (24%). Cranial nerve defects were reported in isolation in 3% of the children, and they were associated to other neurological signs in 9% of the patients. Nobody presented with reduced level of consciousness (including lethargy and coma) as only clinical sign.
Signs of visual system involvement were detected at disease onset in 10 children (13%), and they developed at a later stage in 33 (44%). Five children (7%) presented with ocular signs as the only clinical finding at the onset of the illness; reporting reduction in visual acuity, visual field defects, absence of stereopsis, and repeated episodes of “wide eyes open”. Ocular manifestation observed is reported in Table 4.
Under 4 years of age headache was less common, affecting 26% of children, whereas vomiting (44%) and behavioral change (44%) were the most common symptoms. Headache (62%) and vomiting (54%) were the most frequent symptoms in children older than 4 years old.
Symptom interval
Information about symptom interval was available for 74 patients. The median interval between symptoms onset and diagnosis was 4 weeks (IQR25°–75°: 2–17 weeks). Forty-nine percent of patients were diagnosed within 4 weeks, for 26% of the children between 4 to 16 weeks were needed, and 25% were diagnosed after 16 weeks or later. There was no statistical difference in diagnostic delays related to grade (p = 0.402), localization of tumor (p = 0.197 comparing infratentorial and supratentorial, and p = 0.663 comparing infratentorial and spinal cord localization), and age of the child (Spearman’s correlation coefficient r = 0.625). There was no significant correlation between diagnostic delay and younger or older age group of the child (considering two groups with ages respectively inferior or superior to 4 years) neither (Spearman’s correlation coefficient r = 0.748, p = 0.48).
Survival
Five-year survival information was available for 71 patients as four of the children were lost at follow-up. The estimated overall 5-year survival in our population is 85.9%. The Kaplan-Meier curves of overall survival according to diagnostic delay and tumor grade are shown respectively in Figs. 1 and 2. The group of children with high-grade tumor had a significant worse outcome in terms of 5-year survival probability compared with that of children with low-grade tumors (HR 17.26 95% CI: 4.37–68.37). A diagnostic delay longer than 4 weeks did not result in an increased risk of reduced survival with respect to an earlier diagnosis (HR 0.99 95% CI: 0.79–1.09).
Discussion
Our population included children diagnosed at our hospital, as well as patients coming from other centers, as the Neurosurgery Department of our hospital is a tertiary referring center that collects cases from a wide range of Italian regions.
Over time, a steady increase in the incidence of pediatric brain tumors was reported in several populations [8, 9]. In Europe, between 1978 and 1997, an increase of 1.7% was described [1]. This rise in incidence could be related to three factors: an improvement in the reporting practice in tumor registries, the availability of more advanced diagnostic imaging techniques, or a true increase in incidence rates [3]. Data from AIRTUM Data Bank are in favor of better reporting practice and diagnostic anticipation as the most likely explanations for this increase [10].
Mean age of patients included in this study was 7.8 years, comparable with other bigger studies [3, 11,12,13].
The comparison of the histological diagnosis with other European cohorts, shows a higher percentage of neuronal and mixed neuroglial tumors in our population (15 vs 8 and 5%, respectively in French and Swedish cancer registry), whereas embryonal tumors, in particular medulloblastomas, and ependymal tumors resulted less frequent [14, 15].
In our population, the prevailing symptoms, both at the onset and at diagnosis, were headache and vomiting. They were more frequent in children with posterior fossa tumors, as tumors growing in these sites are responsible of a precocious compression of the cerebral acqueduct, causing symptoms of endocranial hypertension. The association of these two symptoms with papilledema was observed only in 5% of the children at diagnosis, in contrast to 33% of the children reported in previous studies [16]. Headache is the most common symptom in our cohort but it is a common symptom in the general pediatric population as well, with a reported prevalence ranging from 2.7 to 21% [17]. The nature of headache associated with a brain tumor can be very variable [16]. Frequently, it resembles a tension headache or the beginning of a migraine. If not associated to other symptoms suggestive of a brain tumor, it will be difficult to suspect the presence of a cancer, especially in lack of particular features such as a progressive worsening of the pain or a nocturnal or morning prevalent time of presentation. Young age can represent a red flag, as in young children, it is unusual and more likely secondary to an intracranial pathology [18].
In our population, only 1/11 children younger than 2 years complained of headache, and 74% of the children with this symptom were older than 6 years old. Considering that only around 1 of 10 children with a brain tumor will present with headache as only symptom with a normal physical examination, in most cases, history taking and thorough physical and neurologic examination will identify children needing further assessment [17]. Routine neuroimaging in children with headache and a normal neurological examination is generally not appropriate [19].
Vomiting was observed in 31% of the children as first symptom, and it used to be related with headache or cranial nerve abnormalities. Seizures’ incidence at symptom onset was 21%, and in 3% of children, they developed later. The prevalence of this symptom in our cohort is higher compared with other clinical studies, probably reflecting a selection bias, as in our population, glioneuronal and mixed neuroglial tumors were more frequent [2, 11, 20, 21]. Children with hemispherical tumors frequently presented with seizures and were diagnosed at an early stage. NICE guidelines suggest the execution of a MRI in all children who develop epilepsy before the age of 2 years, in those with any suggestion of a focal onset, and in patients in whom seizures continue in spite of first line medication [22].
Behavioral change, already reported as common clinical manifestation in other clinical studies, resulted quite frequent in our population as well, above all in children younger than 4 years old [3, 11, 13, 16, 23]. In older children emotional problems, eating disorders, and declining in school performance were more frequently observed. These clinical manifestations are often subtle and difficult to ascribe to the presence of a CSN tumor, even though they often develop at an early stage [6].
Visual manifestations are very common findings among children with CNS tumors, and in particular, 5 children presented with visual symptoms as the only manifestation at the onset of the disease. At diagnosis, 21% of children presented a squint of new onset or the worsening of a pre-existing squint. Abnormalities of the neurological examination were found in 68% of the children, and most of them with no history of seizures. The association of multiple neurological signs led to neuroimaging. Visual system abnormalities (20%) and cerebellar signs (7%) were the commonest neurological signs observed, highlighting the importance of a neurological and ocular assessment.
Mehta et al., Wilne et al., and Dobrovoljac et al. reported median symptom interval respectively of 12, 13.2, and 8 weeks [3, 5, 11], longer than the median of 4 weeks observed in our population.
Data from the literature show that the duration of symptoms before diagnosis is influenced by age of the child, tumor location, and histology of the tumor. As for the correlation between age and diagnostic delay, we observed an earlier diagnosis in children older than 4 years old (median of 6.0 weeks for children younger than 4 years vs 4.5 weeks for children older than 4 years), but this difference did not result statistical significant. Our data are in contrast with that of many other studies, reporting an earlier diagnosis in younger children compared with older children, suggesting that a more aggressive biology of brain tumors and a greater consideration generally given to nonspecific clinical manifestations in infants could anticipate the time of diagnosis [5, 13, 16, 21, 23,24,25].
As for the relationship between tumor location and symptom interval, supratentorial location was more often associated with an early diagnosis (58% of supratentorial tumors were diagnosed within 4 weeks vs less than 40% of infratentorial tumors). For infratentorial tumors, the diagnosis was made within 4 weeks only in 50% of cases, even when the children had a high-grade tumor, while all high-grade tumors supratentorial or located in the spinal cord were diagnosed within 4 weeks.
In our population, approximately 75% of the children with a high-grade tumor were diagnosed within 4 weeks from the symptoms’ onset suggesting that aggressive tumors have a faster growth pattern and tend to present at an early stage with neurological signs or symptoms due to intracranial hypertension [5, 13, 21, 24,25,26].
The absence of statistical significance in diagnostic delays’ differences with respect to age, location, and grade groups could be related to the small size of our population.
The clinical features were also found to influence the diagnostic delay. Pain, mainly reported in children with posterior fossa or spinal cord tumors, and tremor, led to an early diagnosis in most cases.
On the contrary, long prediagnostic symptomatic intervals were observed for children presenting with vomiting or headache. For more than 40% of the children presenting with these symptoms, the interval was longer than 4 weeks. Diagnostic delay was observed especially when headache was not associated with vomiting or when this association was tardive. Other studies already described long diagnostic delays in children presenting with headache not only when the features of the latter suggested a tension headache or a migraine, but even when alarm signals, such as a progressive or nocturnal pattern of presentation, were present [5, 16, 27].
Visual difficulties were associated with a symptom interval longer than 4 weeks in more than 60% of the cases. Young children could find it difficult to identify and reliably verbalize their visual deficits and these could be misinterpreted by the parents and be mistaken for an increasing clumsiness of the child. Furthermore, even among older children, visual loss can go unrecognized by patients [28,29,30]. Diagnostic delay was also reported in children with clumsiness or incoordination, probably because these manifestations are often subtle and difficult to interpret, above all in young children, and are frequently ignored by parents or overlooked by physicians.
Tumor histopathology resulted the foremost determinant of the child’s survival with a prevailing effect over any possible diagnostic delay. Indeed, aggressive and rapidly growing tumors, with their usually more fully developed pattern of presentation imply not only a shorter prediagnostic symptomatic interval but also a worse prognosis in terms of survival. Kukal et al. and Chen et al. have already described an inverse correlation between diagnostic delay and patient survival. Our study failed to prove the correlation because of the small numbers of our population subgroups. Our results are in accordance with previous studies [24, 25, 31,32,33] showing no negative impact of longer diagnostic delay on survival. Halperin et al. demonstrated that a shorter diagnostic delay was associated with the diagnosis of more advanced medulloblastoma, which represents a poor prognostic sign [34]. Moreover, Arnautovic et al. found a significant correlation between greater diagnostic delay and successive risk of disease progression in children with low grade glioma [33]. Anyway, this study denied any significant association between diagnostic delay and reduced survival or any other negative outcome (among which failure to achieve a gross total resection or need of shunt use). The prognostic value of an incomplete tumor resection is well known [35, 36] but this two last studies contradict the common assumption that an earlier diagnosis, presumably allowing the detection of smaller tumors easier to resect completely, implies a better chance of survival. Ji Hoon et al. described instead a significantly reduced survival in patients with delayed diagnosis of germinoma [37] and Sethi et al. found that patients with intracranial germ cell tumors and delayed diagnosis were more likely to have disseminated disease and receive craniospinal irradiation rather than whole-ventricle or involved-field irradiation radiotherapy, but this did not result in a reduced progression-free survival [32]. Although a longer diagnostic delay seems not to affect survival, it could represent a risk factor for an impaired quality of later life, related to consequences of prolonged hydrocephalus or long-term sequelae of a more aggressive treatment (extensive surgical resection, chemotherapy, and radiotherapy) including neurocognitive deficit, endocrine dysfunction, neurological and vascular late effects, and secondary malignancy [38].
Conclusion
-
1.
Headache and vomiting are the commonest symptoms of CNS tumors, being reported in more than half of the children at diagnosis and affecting about one third of the patients from the onset of the disease.
-
2.
Visual symptoms and signs are common manifestations and are associated with long diagnostic delays. Visual system abnormalities are the commonest neurological sign observed in isolation.
-
3.
Behavioral change is frequently reported, above all among younger children. In almost half of the cases, they represent an early manifestation.
-
4.
Neurological examination is altered in most of the children with CNS tumors, with the exception of patients presenting with seizures.
-
5.
Symptom interval resulted longer for low-grade neoplasms, infratentorial tumor localization, and children younger than 4 years of age.
-
6.
Five-year overall survival probability was influenced by tumor grade but not by diagnostic delay.
Given the heterogeneity of clinical pictures associated with CNS tumors in children, healthcare professionals should be alerted not only by symptoms of raised intracranial pressure, but also by the combination of visual symptoms or behavioral changes along with other nonspecific clinical manifestations. The evaluation of a child presenting with symptoms or signs that could be related with the presence of a CNS tumors should include the collection of an accurate clinical history comprehensive of specific questions about behavioral changes and visual difficulties, physical examination including plotting of growth and head size, and a thorough neurological examination and visual assessment.
References
Peris-Bonet R, Martinez-Garcia C, Lacour B, Petrovich S, Giner-Ripoll B, Navajas A, Steliarova-Foucher E (2006) Childhood central nervous system tumours—incidence and survival in Europe (1978-1997): report from Automated Childhood Cancer Information System project. Eur J Cancer 42:2064–2080
Wilne S, Collier J, Kennedy C, Koller K, Grundy R, Walker D (2007) Presentation of childhood CNS tumours: a systematic review and meta-analysis. Lancet Oncol 8:685–695
Mehta V, Chapman A, McNeely PD, Walling S, Howes WJ (2002) Latency between symptom onset and diagnosis of pediatric brain tumors: an Eastern Canadian geographic study. Neurosurgery 51:365–372 discussion 372-363
Shay V, Fattal-Valevski A, Beni-Adani L, Constantini S (2012) Diagnostic delay of pediatric brain tumors in Israel: a retrospective risk factor analysis. Childs Nerv Syst 28:93–100
Dobrovoljac M, Hengartner H, Boltshauser E, Grotzer MA (2002) Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr 161:663–667
Dixon-Woods M, Findlay M, Young B, Cox H, Heney D (2001) Parents’ accounts of obtaining a diagnosis of childhood cancer. Lancet 357:670–674
Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D (2010) The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child 95:534–539
Smith MA, Freidlin B, Ries LA, Simon R (1998) Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst 90:1269–1277
Dreifaldt AC, Carlberg M, Hardell L (2004) Increasing incidence rates of childhood malignant diseases in Sweden during the period 1960-1998. Eur J Cancer 40:1351–1360
Pisani P, Mosso ML, Buzzoni C, Crosignani P, Michiara M, Tumino R, AIRTUM Working Group (2011) Good news for Italian children. Since 2000 malignant CNS cancer has stopped increasing. Epidemiol Prev 35:245
Wilne S, Collier J, Kennedy C, Jenkins A, Grout J, Mackie S, Koller K, Grundy R, Walker D (2012) Progression from first symptom to diagnosis in childhood brain tumours. Eur J Pediatr 171:87–93
Ansell P, Johnston T, Simpson J, Crouch S, Roman E, Picton S (2010) Brain tumor signs and symptoms: analysis of primary health care records from the UKCCS. Pediatrics 125:112–119
Wilne SH, Ferris RC, Nathwani A, Kennedy CR (2006) The presenting features of brain tumours: a review of 200 cases. Arch Dis Child 91:502–506
Magnani C, Aareleid T, Viscomi S, Pastore G, Berrino F, EUROCARE Working Group (2001) Variation in survival of children with central nervous system (CNS) malignancies diagnosed in Europe between 1978 and 1992: the EUROCARE study. Eur J Cancer 37:711–772
Lannering B, Sandstrom PE, Holm S, Lundgren J, Pfeifer S, Samuelsson U, Stromberg B, Gustafsson G, Swedish Childhood CNSTWG (2009) Classification, incidence and survival analyses of children with CNS tumours diagnosed in Sweden 1984-2005. Acta Paediatr 98:1620–1627
Edgeworth J, Bullock P, Bailey A, Gallagher A, Crouchman M (1996) Why are brain tumours still being missed? Arch Dis Child 74:148–151
Abu-Arefeh I, Russell G (1994) Prevalence of headache and migraine in schoolchildren. BMJ 309:765–769
Raieli V, Eliseo M, Pandolfi E, La Vecchia M, La Franca G, Puma D, Ragusa D (2005) Recurrent and chronic headaches in children below 6 years of age. J Headache Pain 6:135–142
Lewis DW, Dorbad D (2000) The utility of neuroimaging in the evaluation of children with migraine or chronic daily headache who have normal neurological examinations. Headache 40:629–632
Hayashi N, Kidokoro H, Miyajima Y, Fukazawa T, Natsume J, Kubota T, Kojima S (2010) How do the clinical features of brain tumours in childhood progress before diagnosis? Brain and Development 32:636–641
Pinho RS, Andreoni S, Silva NS, Cappellano AM, Masruha MR, Cavalheiro S, Vilanova LC (2011) Pediatric central nervous system tumors: a single-center experience from 1989 to 2009. J Pediatr Hematol Oncol 33:605–609
Nunes VD, Sawyer L, Neilson J, Sarri G, Cross JH (2012) Diagnosis and management of the epilepsies in adults and children: summary of updated NICE guidance. BMJ 344:e281
Coserria Sanchez JF, Garrido Ocana AI, Quiroga Cantero E, Reina Gonzalez AM, Amadeu Da Costa AP, Garcia Zarza N (2007) Presenting signs and symptoms of central nervous system tumors according to age. An Pediatr (Barc) 66:115–120
Saha V, Love S, Eden T, Micallef-Eynaud P, MacKinlay G (1993) Determinants of symptom interval in childhood cancer. Arch Dis Child 68:771–774
Kukal K, Dobrovoljac M, Boltshauser E, Ammann RA, Grotzer MA (2009) Does diagnostic delay result in decreased survival in paediatric brain tumours? Eur J Pediatr 168:303–310
Klitbo DM, Nielsen R, Illum NO, Wehner PS, Carlsen N (2011) Symptoms and time to diagnosis in children with brain tumours. Dan Med Bull 58:A4285
Brasme JF, Chalumeau M, Doz F, Lacour B, Valteau-Couanet D, Gaillard S, Delalande O, Aghakhani N, Sainte-Rose C, Puget S, Grill J (2012) Interval between onset of symptoms and diagnosis of medulloblastoma in children: distribution and determinants in a population-based study. Eur J Pediatr 171:25–32
Suharwardy J, Elston J (1997) The clinical presentation of children with tumours affecting the anterior visual pathways. Eye (Lond) 11(Pt 6):838–844
Feletti A, Marton E, Mazzucco GM, Fang S, Longatti P (2010) Amaurosis in infancy due to craniopharyngioma: a not-exceptional but often misdiagnosed symptom. Neurosurg Focus 28:E7
Harbert MJ, Yeh-Nayre LA, O'Halloran HS, Levy ML, Crawford JR (2012) Unrecognized visual field deficits in children with primary central nervous system brain tumors. J Neuro-Oncol 107:545–549
Chen J, Mullen CA (2017) Patterns of diagnosis and misdiagnosis in pediatric cancer and relationship to survival. J Pediatr Hematol Oncol 39:e110–e115
Sethi RV, Marino R, Niemierko A, Tarbell NJ, Yock TI, MacDonald SM (2013) Delayed diagnosis in children with intracranial germ cell tumors. J Pediatr 163:1448–1453
Arnautovic A, Billups C, Broniscer A, Gajjar A, Boop F, Qaddoumi I (2015) Delayed diagnosis of childhood low-grade glioma: causes, consequences, and potential solutions. Childs Nerv Syst 31:1067–1077
Halperin EC, Friedman HS (1996) Is there a correlation between duration of presenting symptoms and stage of medulloblastoma at the time of diagnosis? Cancer 78:874–880
Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the surveillance, epidemiology, and end results database. Cancer 115:5761–5770
Pogorzala M, Styczynski J, Wysocki M (2014) Survival and prognostic factors in children with brain tumors: long-term follow-up single center study in Poland. Anticancer Res 34:323–326
Phi JH, Kim SK, Lee YA, Shin CH, Cheon JE, Kim IO, Yang SW, Wang KC (2013) Latency of intracranial germ cell tumors and diagnosis delay. Childs Nerv Syst 29:1871–1881
Roddy E, Mueller S (2016) Late effects of treatment of pediatric central nervous system tumors. J Child Neurol 31:237–254
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Stocco, C., Pilotto, C., Passone, E. et al. Presentation and symptom interval in children with central nervous system tumors. A single-center experience. Childs Nerv Syst 33, 2109–2116 (2017). https://doi.org/10.1007/s00381-017-3572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-017-3572-1