Abstract
Purpose
Diagnosis of childhood brain tumors is delayed more than diagnosis of other pediatric cancers. However, the contribution of the most common pediatric brain tumors, lowgrade gliomas (LGG), to this delay has never been investigated.
Methods
We retrospectively reviewed cases of childhood LGG diagnosed from January 1995 through December 2005 at our institution. The pre-diagnosis symptom interval (PSI) was conservatively calculated, and its association with race, sex, age, tumor site, tumor grade, and outcome measures (survival, disease progression, shunt use, seizures, extent of resection) was analyzed. Cases of neurofibromatosis type 1 were reported separately.
Results
The 258 children had a median follow-up of 11.1 years, and 226 (88 %) remained alive. Greater pre-diagnosis symptom interval (PSI) was significantly associated with grade I (vs. grade II) tumors (p = 0.03) and age >10 years at diagnosis (p = 0.03). Half of the 16 spinal tumors had a PSI > 6 months. PSI was significantly associated with progression (p = 0.02) in grade I tumors (n = 195) and in grade I tumors outside the posterior fossa (n = 134, p = 0.03). Among children with grade I tumors, median PSI was longer in those who had seizures (10.3 months) than in those who did not (2.5 months) (p = 0.09).
Conclusions
Delayed diagnosis of childhood LGG allows tumor progression. To reduce time to diagnosis, medical curricula should emphasize inclusion of LGG in the differential diagnosis of CNS neoplasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many researchers have attempted to discover why brain tumors remain undetected [13] for much longer than other pediatric neoplasms [14, 32, 37]. Together, available reports identify ten important factors in this delayed diagnosis: (1) deficient history and physical examination [6, 20, 47]; (2) a focus on the triad of headache, vomiting, and papilledema, which are often absent in low-grade glioma (LGG) [13]; (3) inattention to common symptoms and signs such as oculo-visual difficulties [10–12, 14, 17, 20, 42, 47] or behavioral and school problems [6, 11, 13, 18, 30]; (4) disregard of less common symptoms such as seizures [16, 30, 47], torticollis [11, 14, 17, 48], diabetes insipidus [20, 28], growth problems [20, 42, 47], scoliosis [39, 49], and longstanding back pain [39]; (5) neglect of headaches with high-risk features such as long duration, accompanying symptoms, and changing characteristics [6, 8, 20, 25, 29, 41, 47]; (6) disregard of parental reports of their children’s signs and symptoms [10–13, 24, 30]; (7) children’s ability to compensate for even the most severe deficits [11, 46]; (8) inattention to longstanding relapsing and remitting symptoms [30, 38, 39, 47]; (9) pursuit of more familiar diagnoses, such as migraine, gastroenteritis, or psychiatric disorders, that may not even fit the clinical picture [13, 25, 30, 40, 47]; and (10) the adolescent age group, which may have less access to health care than younger children, be affected by pubertal changes, provide a poor history, and/or have limited interaction with parents [10, 23, 24, 32].
Most childhood brain tumors are LGGs [34], whose anatomic site and slow growth may implicate many of the factors described above. Reasoning that the diagnosis of brain tumors, in general, may be expedited by addressing delayed diagnosis of LGG, we investigated the relation of the pre-diagnosis symptom interval (PSI) to clinical features and outcome measures in cases of childhood LGG treated at our institution.
Methods
Patients
All patients diagnosed with LGG between January 20, 1995, through December 28, 2005, were eligible for this retrospective study. Histologic diagnosis of LGG was confirmed by review of pathology reports. For tumors not biopsied, such as optic pathway and tectal tumors, imaging and clinical reports were reviewed. LGG was defined according to the 2007 World Health Organization’s classification [26]. Patients with neurofibromatosis type 1 (NF1) were excluded from statistical comparisons of time to diagnosis, as many received routine surveillance imaging for brain tumors. The data on NF1 patients is presented separately.
Data collection
From each patient’s medical record, we obtained the birth date, sex, race, description of symptoms, duration of symptoms, presence of NF1, initial magnetic resonance imaging or computerized tomography (MRI/CT) report, initial pathologic diagnosis, tumor site, date of surgery, extent of surgical resection, ventriculoperitoneal (VP) shunt placement, chemotherapy and/or radiation therapy (RT), progressive disease (PD) (yes/no), and clinical status at last follow-up. The Institutional Review Board of St. Jude Children’s Research Hospital approved the study and waived the requirement for informed consent.
Duration of symptoms
As symptoms and signs were quite varied, they were grouped into general categories (Table 1). Symptom duration was based on the patient’s or family’s report in the initial history. If a specific date of symptom onset was not reported, the most conservative date of onset was used. For example, if only the month was given, the last day of the month was used as the date of onset. If the patient used a term like “summer,” “winter,” “fall,” or “spring,” the last day of August, February, November, or April, respectively, was used. If a patient provided only a year, then December 31 of that year was used as the symptom start date. The date of diagnosis was defined as the date of the first CT or MRI report after initial presentation. Therefore, the pre-diagnosis symptom interval (PSI) was the period from symptom onset to the first imaging report. In the case of multiple symptoms, the patient’s longest PSI was used in analysis.
Tumor site
Tumor sites comprised five categories: spine, posterior fossa, brainstem, midline and/or optic pathway, and cerebrum. If a tumor involved several locations, the tumor origin from the imaging report was used.
Gross total resection
Tumors were categorized according to extent of resection. Gross total resection (GTR) was defined by the absence of residual tumor as noted on the postoperative MRI, CT scan, or surgical report, within the first 3 months after diagnosis. Sub-total resection was defined as the remainder of any tumor postoperatively.
Statistical analysis
As this was an exploratory study, we investigated the duration of symptoms (PSI) as both a continuous variable and a categorical variable (using cut points of 3, 6, and 12 months). Fisher’s exact test and the chi-square test were used to compare categorical variables. The exact Wilcoxon rank-sum test and the Kruskal-Wallis test were used to compare PSI as a continuous variable. Survival was defined as the interval from the date of diagnosis to the date of death or last contact. Survival distributions were estimated by the method of Kaplan and Meier; the log-rank test was used to compare survival distributions according to PSI (<6 vs. ≥6 months). As we did not have exact dates of tumor progression, we examined the association between PSI and PD by categorical methods (Fisher’s exact test). No adjustment was made for multiple comparisons in this exploratory study. P values were two-sided and were considered to indicate a statistically significant difference if less than 0.05.
Results
Patient characteristics
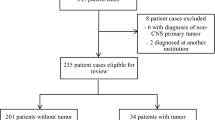
Two hundred and eighty-six (286) patients were diagnosed with LGG between January 20, 1995, and December 28, 2005. Twenty-six of these patients had a diagnosis of NF1 and were excluded from statistical analyses. Of the 260 remaining patients, two who presented only for consultation were excluded. Thus, 258 patients were included in comparisons. Table 2 shows the characteristics of these patients; 51 % were male (n = 131) and the majority were White (n = 201; 78 %). Only six patients had metastatic disease at diagnosis.
Treatment, follow-up, and outcome
All but 9 of the 258 patients had some type of surgery, and 75 (29 %) had a GTR. Eighty-four patients (33 %) received chemotherapy, and 111 (43 %) received RT. Eighty-one patients required a VP shunt. Of the 258 patients, 226 (88 %) were alive at the time of analysis, with a median follow-up of 11.1 years from diagnosis. Ninety-eight patients (38 %) had PD.
Characterization of symptoms and signs
Symptoms and signs were distributed within the categories shown in Table 1. The median number of signs/symptoms observed per patient was 2 (range, 1–5). The total number of symptoms was 546. The most commonly observed symptoms were headache (37 % of patients), ocular-visual symptoms (25 %), vomiting (18 %), seizure (15 %), gait disturbance (15 %), nausea (14 %), and motor deficits (11 %). Note that our categories reflected the symptoms as reported; thus, some (e.g., “nausea,” “vomiting,” and “nausea and vomiting”) overlap.
Duration of symptoms
The last column in Table 1 shows the median PSI in each symptom category. Symptom duration was less than 3 months in most patients (147/258; 57 %), less than 6 months in approximately 70 % of patients (183/258), and less than 12 months in 80 % (205/258). The median duration of symptoms in all patients was 2.1 months (range, 0–131.1 months). Twenty-eight patients had a PSI greater than 2 years and are characterized in Table 3. In this group, 11 patients (39 %) had PD, 6 (21 %) had seizures, 9 (32 %) required a VP shunt, and 9 (32 %) had GTR.
Factors associated with PSI
We found no significant association between delayed diagnosis and race (White vs. Black) or sex (Table 4). Patients with grade I tumors had a significantly longer median duration of symptoms than patients with grade II tumors (median, 2.6 vs. 1.3 months, p = 0.03) (Table 4).
PSI was marginally related to primary tumor location when categorized as <6 vs. ≥6 months (p = 0.06) (Table 4). Patients with spinal tumors had the longest PSI (median, 6.0 months). Of the 16 patients with spinal primary tumors in our cohort, 50 % had symptoms lasting at least 6 months (Table 4) and 31 % had symptoms lasting at least 12 months.
Older age at diagnosis (<3 vs. 3–6 vs. >6 years) was significantly associated with longer PSI (both <6 vs. ≥6 months and <12 vs. ≥12 months (data not shown; p = 0.02 and p = 0.03, respectively). The median duration of symptoms was 4.1 months (range, 0–131.1 months) for patients older than 10 years at diagnosis vs. 1.9 months (range, 0–58.8 months) for patients 10 years and younger (p = 0.03) (Table 4).
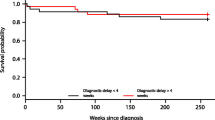
We did not find the duration of symptoms to be significantly associated with the presence of GTR or seizures (Table 4). Further, PSI greater than vs. less than 3 months was not found to be associated with survival (p = 0.64), nor was PSI greater than vs. less than 6 months (p = 0.20) or 12 months (p = 0.33). Duration of symptoms as a continuous variable also showed no association with survival (p = 0.37). Thirty-two of the 258 patients in the study cohort (12.4 %) had died at the time of analysis. Five of the patients who died (16 %) had a PSI greater than 12 months (12.0, 18.1, 24.0, 27.8, and 47.6 months). GTR had been achieved in only 4 (12 %) of the 32 patients who died.
Relation of delayed diagnosis to clinical characteristics, according to tumor grade
In the subset of patients with grade I tumors (n = 195), the median PSI was 3.9 months in patients who had PD, compared to 1.7 months in those who did not (p = 0.02) (Table 5). However, there was no evidence that PSI was significantly associated with survival in patients with grade I tumors (p = 0.48), among whom 20 deaths occurred. Patients with seizures had a substantially longer median PSI (10.3 months) than did other patients (2.5 months) (p = 0.09). Patients in whom GTR could not be achieved also had a longer median PSI than did others (3.0 vs. 1.7 months), but this finding was not statistically significant, nor was PSI significantly related to shunt use (p = 0.28). We further subdivided patients with grade I tumors by tumor site. Of the 61 patients with grade I posterior fossa tumors, only 2 died. Within this subset of patients, the median PSI was longer in patients who had PD (13.0 months) than in those who did not (2.7 months, p = 0.10). Again, we observed longer, although nonsignificant, median PSI in patients with less than GTR and in patients with seizures (p = 0.22 and p = 0.20, respectively). Of the 134 patients with grade I tumors outside the posterior fossa, 18 died. In this subset of patients, the median PSI was significantly longer in those who had PD (3.7 vs. 1.3 months, p = 0.03), and we observed a longer median PSI in patients with less than GTR and in patients with seizures, although these differences were not statistically significant (Table 6).
In the subset of patients with grade II tumors (n = 63), 12 died; all 12 had tumors outside the posterior fossa. The only significant association observed was between PD and PSI as a categorical variable (<6 vs. ≥6 months); only 1 of 12 patients with PSI ≥6 months had PD (8 %), compared to 23 of 51 patients with PSI <6 months (45 %) (p = 0.02). No statistically significant associations were observed between PSI and GTR, seizures, shunt use, PD, or survival in this subset (data not shown).
Patients with NF1
There were 26 patients with NF1; one (a consult patient) was excluded. Of the 25 remaining NF1 cases, most were female (14/25; 56 %) and White (24/25). Most patients (n = 18) had optic pathway tumors. The median age at diagnosis was 4.9 years (range, 0.3–16.4 years). All patients were alive at the time of analysis, with a median follow-up of 10.2 years from diagnosis. None of these patients had metastatic disease at diagnosis. Seven of the 25 NF1 patients (28 %) had no signs or symptoms of LGG, as reported in NF1 surveillance. The remaining 18 patients had a total of 32 symptoms (median, 2 per patient; range, 1–3). Five patients had symptoms lasting more than 1 year.
Discussion
To our knowledge, this is the first study to investigate the impact of delayed diagnosis in children with LGG. We observed that the PSI is significantly associated with lower tumor grade, older age, and disease progression. Patients with grade I tumors had a significantly longer median symptom interval before diagnosis than did patients with grade II tumors. Older patients tended to have a longer PSI than did younger patients, and patients who experienced disease progression had a longer PSI than patients who did not (Table 4). There was no evidence of significant association between PSI and surgical resection (GTR), seizure, shunt use, or survival. PSI was significantly associated with PD in the 195 children with grade I tumors, including tumors outside the posterior fossa.
There was no evidence that the PSI was significantly related to negative outcomes other than PD, in all patients or in patients with grade I tumors, including those outside the posterior fossa. One explanation could be our conservative criteria for defining the interval. For example, if a family or patient could identify only the year when symptoms began, the date of onset was recorded as the last day of that year, regardless of the actual date; this protocol was intended to ensure a judicious analysis but may have underestimated the PSI of many patients. Further, small subsets of cases (e.g., those subdivided according to tumor grade) may have reduced the statistical power of our comparisons. Another possibility is that the PSI is not a significant factor in the outcome of LGGs due to their insidious nature, such that a few months of delay may not reduce the likelihood of survival.
Patients with LGG have an excellent survival rate in general [5, 15]; therefore, factors that can affect their quality of life warrant special attention. Many studies of pediatric LGG have identified such factors, including seizures; shunt use; and the effect of residual tumor on cognition, social skills, and visual function [1–5, 7, 35, 50]. We believe that the low-grade nature of LGGs is a strong argument for efforts to expedite diagnosis, as there may be a definable window of opportunity for GTR, reducing both the risk of progression and the risk of a lower quality of life. In a study by Ater et al. of 274 children with LGG, residual tumor size and young age were the only two factors significantly associated with disease progression [5], which itself means more chemotherapy, more surgery, and RT and the expected long-term sequelae of these therapies. In adults with LGG diagnosed by imaging, patients who had aggressive resection earlier in treatment fared better than those who underwent only biopsy and close monitoring by imaging [21]. If even patients who are followed closely have inferior outcomes, it follows that outcomes are even worse for most patients diagnosed later in the disease course.
Interestingly, several studies have identified delayed diagnosis as a good prognostic factor in childhood brain tumors [16, 17, 19, 24]; this may be explained by their grouping of aggressive tumors with LGGs. The impact of delayed diagnosis should be investigated separately in specific types of brain tumors (e.g., medulloblastoma, high-grade gliomas, ependymomas) rather than in all brain tumors together. Furthermore, with the recent subgrouping of many brain tumors [31, 45], we argue that each tumor subset should be investigated independently with regard to the impact of delayed diagnosis. Halperin and colleagues concluded that a shorter PSI does not reduce survival in medulloblastomas due to the aggressive nature of these tumors [19]. Although some aggressive brain tumors may progress so rapidly that PSI does not influence survival, this conclusion cannot be proved until the effect of PSI is analyzed separately within each tumor subgroup.
Longer PSI was also associated with age greater than 10 years. In fact, 11 of the 17 patients (65 %) with a PSI longer than 3 years were more than 10 years old at diagnosis (Table 3). Other investigators have reported similar observations [12, 14, 23, 24, 32]. Pollock et al. attributed this finding to more regular physician visits and closer parental observation at a young age and perhaps less reliable self-reporting of symptoms by adolescents [32]. Kieran and coworkers attributed it to the difficulty of differentiating true pathology from adolescent behavior and growth changes and to the limited access of many adolescents to health care [23]. Another factor in the duration of PSI at all ages, but especially adolescence, is a low level of suspicion and deficient history taking and physical examination. In fact, many studies have found that parents are more likely than physicians to recognize that their child has a problem [11, 13, 30]. In one study, only 41 % of children had a diagnosis of brain tumor within three visits to their clinician, and 16 % required more than ten visits before diagnosis [30]. Another study found that 50 % of children underwent invasive procedures for other medical conditions before diagnosis of a brain tumor [12]. We have noted that such delays are reported across a variety of nations and socioeconomic settings [6, 8, 10–14, 16–18, 20, 23–25, 28–30, 32, 34, 37–42, 46–49] and therefore are unlikely to be attributable to the level of clinical training or the availability of resources.
We suggest that an underappreciated contributor to delayed diagnosis of brain tumors in children is the fact that most pediatricians are not trained to include LGG in the differential diagnosis, although it comprises the majority of pediatric brain tumors. Despite the intuitive importance of the clinical level of suspicion, this factor has not previously been suggested as a contributor to delayed diagnosis, nor have the duration of symptoms/signs and their waxing and waning nature been seriously considered in the differential diagnosis. Greater awareness of glioblastoma multiforme and other highly malignant tumors makes it less likely that malignancy will be suspected when the symptoms are relapsing and remitting, persist over a long period without threatening life (rarely the case with adult cancers), and lack the familiar triad of signs (vomiting, headache, and papilledema) suggesting higher-grade gliomas [13]. For example, papilledema was documented in only 2.3 % of our study patients. Although this finding could reflect underreporting, others [13] have reported its absence in the majority of children with brain tumors. Another possible factor in delayed diagnosis is children’s ability to adjust to and accommodate these slow-growing tumors [46]. It is well known that the plasticity of the developing brain allows a specific function to be retained within the tumor, redistributed around it, or relocated to a new area or to the opposite hemisphere [9, 43]. For example, switching of right- vs. left-handedness was documented in five of our patients, presumably to compensate for tumor-related weakness. For these reasons, it is not surprising that the symptoms of LGG can persist for many years before diagnosis [22, 36, 44, 48, 50].
Two specific groups in our cohort are worthy of mention. First, spinal tumors had a longer delay of diagnosis than tumors in other locations, although most spinal tumors in children are LGG. This longer delay has been noted previously [39, 49], and we suggest that it may be explained by the nonspecific nature of back pain in children and its attribution to other causes, including scoliosis. The second group of children, those with neurofibromatosis, often had symptoms for years before diagnosis of LGG. As brain tumor is a major diagnostic criterion for NF1 [27], we can offer no explanation for this delay in diagnosis except lack of training to consider brain tumors in children with NF1.
With all of the genetic and biologic advances in pediatric oncology [33], it is crucial not to lose sight of the importance of a thorough history and physical examination. To promote awareness and prompt diagnosis of LGG, particularly when the history and physical examination do not fit the preliminary diagnosis, we propose the acronym “LOW OR PAY (mnemonic for “think LOW-grade glioma OR patient will PAY the price”), further explained in Table 7. This acronym could be incorporated into everyday practice. With such aids, perhaps, we pediatric physicians will eventually outperform parents in the diagnosis of childhood brain tumors [11, 13, 30].
References
Aarsen FK, Paquier PF, Arts WF et al (2009) Cognitive deficits and predictors 3 years after diagnosis of a pilocytic astrocytoma in childhood. J Clin Oncol 27(21):3526–3532
Aarsen FK, Paquier PF, Reddingius RE et al (2006) Functional outcome after low-grade astrocytoma treatment in childhood. Cancer 106(2):396–402
Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE (2004) Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology 62(8):1311–1316
Armstrong GT, Conklin HM, Huang S et al (2011) Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol 13(2):223–234
Ater JL, Zhou T, Holmes E et al (2012) Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol 30(21):2641–2647
Barlow CF (1982) Headaches and brain tumors. Am J Dis Child 136(2):99–100
Chou SY, Digre KB (1999) Neuro-ophthalmic complications of raised intracranial pressure, hydrocephalus, and shunt malfunction. Neurosurg Clin N Am 10(4):587–608
Consortium TCBT (1991) The epidemiology of headache among children with brain tumor. Headache in children with brain tumors. J Neurooncol 10(1):31–46
Desmurget M, Bonnetblanc F, Duffau H (2007) Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain 130(Pt 4):898–914
Dixon-Woods M, Findlay M, Young B, Cox H, Heney D (2001) Parents’ accounts of obtaining a diagnosis of childhood cancer. Lancet 357(9257):670–674
Dobrovoljac M, Hengartner H, Boltshauser E, Grotzer MA (2002) Delay in the diagnosis of paediatric brain tumours. Eur J Pediatr 161(12):663–667
Dörner L, Fritsch MJ, Stark AM, Mehdorn HM (2007) Posterior fossa tumors in children: how long does it take to establish the diagnosis? Childs Nerv Syst 23(8):887–890
Edgeworth J, Bullock P, Bailey A, Gallagher A, Crouchman M (1996) Why are brain tumours still being missed? Arch Dis Child 74(2):148–151
Flores LE, Williams DL, Bell BA, O’Brien M, Ragab AH (1986) Delay in the diagnosis of pediatric brain tumors. Am J Dis Child 140(7):684–686
Gajjar A, Sanford RA, Heideman R et al (1997) Low-grade astrocytoma: a decade of experience at St. Jude Children’s Research Hospital. J Clin Oncol 15(8):2792–2799
Gjerris F (1976) Clinical aspects and long-term prognosis of intracranial tumours in infancy and childhood. Dev Med Child Neurol 18(2):145–159
Gjerris F (1978) Clinical aspects and long-term prognosis in supratentorial tumors of infancy and childhood. Acta Neurol Scand 57(6):445–470
Grant GA, Avellino AM, Loeser JD, Ellenbogen RG, Berger MS, Roberts TS (1999) Management of intrinsic gliomas of the tectal plate in children. A ten-year review. Pediatr Neurosurg 31(4):170–176
Halperin EC, Friedman HS (1996) Is there a correlation between duration of presenting symptoms and stage of medulloblastoma at the time of diagnosis? Cancer 78(4):874–880
Honig PJ, Charney EB (1982) Children with brain tumor headaches. Distinguishing features. Am J Dis Child 136(2):121–124
Jakola AS, Myrmel KS, Kloster R et al (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308(18):1881–1888
Johnson JH Jr, Hariharan S, Berman J et al (1997) Clinical outcome of pediatric gangliogliomas: ninety-nine cases over 20 years. Pediatr Neurosurg 27(4):203–207
Kieran MW, Walker D, Frappaz D, Prados M (2010) Brain tumors: from childhood through adolescence into adulthood. J Clin Oncol 28(32):4783–4789
Kukal K, Dobrovoljac M, Boltshauser E, Ammann RA, Grotzer MA (2009) Does diagnostic delay result in decreased survival in paediatric brain tumours? Eur J Pediatr 168(3):303–310
Lewis DW, Ashwal S, Dahl G et al (2002) Practice parameter: evaluation of children and adolescents with recurrent headaches: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology 59(4):490–498
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109
Lund AM, Skovby F (1991) Optic gliomas in children with neurofibromatosis type 1. Eur J Pediatr 150(12):835–838
Maghnie M, Cosi G, Genovese E et al (2000) Central diabetes insipidus in children and young adults. N Engl J Med 343(14):998–1007
Medina LS, Kuntz KM, Pomeroy S (2001) Children with headache suspected of having a brain tumor: a cost-effectiveness analysis of diagnostic strategies. Pediatrics 108(2):255–263
Mehta V, Chapman A, McNeely PD, Walling S, Howes WJ (2002) Latency between symptom onset and diagnosis of pediatric brain tumors: an Eastern Canadian geographic study. Neurosurgery 51(2):365–372
Northcott PA, Korshunov A, Witt H et al (2011) Medulloblastoma comprises four distinct molecular variants. J Clin Oncol 29(11):1408–1414
Pollock BH, Krischer JP, Vietti TJ (1991) Interval between symptom onset and diagnosis of pediatric solid tumors. J Pediatr 119(5):725–732
Pui CH, Gajjar AJ, Kane JR, Qaddoumi IA, Pappo AS (2011) Challenging issues in pediatric oncology. Nat Rev Clin Oncol 8(9):540–549
Qaddoumi I, Sultan I, Gajjar A (2009) Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the Surveillance, Epidemiology, and End Results database. Cancer 115(24):5761–5770
Reimers TS, Ehrenfels S, Mortensen EL et al (2003) Cognitive deficits in long-term survivors of childhood brain tumors: identification of predictive factors. Med Pediatr Oncol 40(1):26–34
Robertson PL, Muraszko KM, Brunberg JA, Axtell RA, Dauser RC, Turrisi AT (1995) Pediatric midbrain tumors: a benign subgroup of brainstem gliomas. Pediatr Neurosurg 22(2):65–73
Saha V, Love S, Eden T, Micallef-Eynaud P, MacKinlay G (1993) Determinants of symptom interval in childhood cancer. Arch Dis Child 68(6):771–774
Sarkari NB, Bickerstaff ER (1969) Relapses and remissions in brain stem tumours. Br Med J 2(5648):21–23
Segal D, Lidar Z, Corn A, Constantini S (2012) Delay in diagnosis of primary intradural spinal cord tumors. Surg Neurol Int 3:52
Shemie S, Jay V, Rutka J, Armstrong D (1997) Acute obstructive hydrocephalus and sudden death in children. Ann Emerg Med 29(4):524–528
Sobri M, Lamont AC, Alias NA, Win MN (2003) Red flags in patients presenting with headache: clinical indications for neuroimaging. Br J Radiol 76(908):532–535
Suharwardy J, Elston J (1997) The clinical presentation of children with tumours affecting the anterior visual pathways. Eye 11:838–844
Takahashi S, Jussen D, Vajkoczy P, Picht T (2012) Plastic relocation of motor cortex in a patient with LGG (low grade glioma) confirmed by NBS (navigated brain stimulation). Acta Neurochir (Wien) 154(11):2003–2008
Walker DG, Kaye AH (2003) Low grade glial neoplasms. J Clin Neurosci 10(1):1–13
Wani K, Armstrong TS, Vera-Bolanos E et al (2012) A prognostic gene expression signature in infratentorial ependymoma. Acta Neuropathol 123(5):727–738
Wilne SH, Dineen RA, Dommett RM, Chu TP, Walker DA (2013) Identifying brain tumours in children and young adults. BMJ347:f5844
Wilne SH, Ferris RC, Nathwani A, Kennedy CR (2006) The presenting features of brain tumours: a review of 200 cases. Arch Dis Child 91(6):502–506
Wilne S, Koller K, Collier J, Kennedy C, Grundy R, Walker D (2010) The diagnosis of brain tumours in children: a guideline to assist healthcare professionals in the assessment of children who may have a brain tumour. Arch Dis Child 95(7):534–549
Wilne S, Walker D (2010) Spine and spinal cord tumours in children: a diagnostic and therapeutic challenge to healthcare systems. Arch Dis Child Educ Pract Ed 95(2):47–54
Yule SM, Hide TA, Cranney M, Simpson E, Barrett A (2001) Low grade astrocytomas in the West of Scotland 1987-96: treatment, outcome, and cognitive functioning. Arch Dis Child 84(1):61–64
Acknowledgments
This work was supported by Cancer Center Support Grant CA21765 and Pediatric Oncology Program Support Grant CA02394 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC). The authors thank Sharon Naron for editing the manuscript.
Conflict of interest
The authors state that they have no conflicts of interest to disclose, financial or otherwise. This retrospective study was approved by the St. Jude Children’s Research Hospital Institutional Review Board, which waived the requirement for informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arnautovic, A., Billups, C., Broniscer, A. et al. Delayed diagnosis of childhood low-grade glioma: causes, consequences, and potential solutions. Childs Nerv Syst 31, 1067–1077 (2015). https://doi.org/10.1007/s00381-015-2670-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-2670-1