Abstract
Purpose
The premature fusion of the sagittal suture (scaphocephaly) may be associated with intracranial cerebrospinal fluid (CSF) dynamics alterations resulting in pericerebral effusion (PE) and/or ventriculomegaly. However, the etiology and development of such collections are not fully elucidated. The aims of this study are to clarify the characteristics of intracranial CSF collection in scaphocephaly, consider the role of surgery, and analyze the prognostic factor on CSF dynamics.
Method
The pre- and postoperative cerebral imaging of 63 children (48 boys, 15 girls) with scaphocephaly who underwent craniotomy with decompression of the superior sagittal sinus (SSS) before 6 months of age between 2008 and 2015 were retrospectively studied. The PE was compared to the estimated value corrected for age (normal control). Evans’ index (EI) was calculated. Correlations among these values and indices were analyzed.
Result
Preoperatively, 36 of 63 patients (57 %) had larger PE than expected, and 36 of 63 patients (57 %) had pathological EI (EI > 0.3), and among them, 26 (46 %) had both. Patients with large PE tended to have pathological EI (p = 0.005). The PE and the EI were significantly decreased postoperatively (p < 0.001). The PE decreased faster than the expected natural course in 59 of 63 patients (92 %) postoperatively.
Conclusion
The precise comparison with estimated PE prevalence corrected for age showed that PE can be found in more than half of the children with scaphocephaly, associated with ventriculomegaly. Surgery has a significant efficacious role in decreasing those CSF collections. The long-term significance of such CSF collections in scaphocephaly is still to be elucidated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with craniosynostosis can present intracranial collections of cerebrospinal fluid (CSF) [1–6], such as enlarged pericerebral spaces or ventricular dilatation, which can be sometimes both associated [7]. The incidence of CSF collections in syndromic craniosynostosis is estimated between 40 and 90 % [8–10]. In contrast, the incidence of CSF collections in non-syndromic craniosynostosis is considered similar to that of the general population [11].
Scaphocephaly is the most common craniosynostosis, accounting for 40–60 % of all craniosynostoses [12–14]. The incidence of the CSF collections in scaphocephaly is classically considered low: 0–10 % [4–6]. However, in previous studies, the CSF collection was determined clinically or radiologically without a proper definition. Specific measurements were not applied. In addition, the postoperative changes in CSF collections were not studied.
The aims of this study are (i) to clarify the characteristics of the CSF collection in scaphocephaly, (ii) to analyze the surgical effect on CSF collection, and (iii) to propose some predictive factors on the postoperative evolution.
Patients and methods
Subjects
There were 315 surgical procedures for scaphocephaly at the Craniofacial Unit of the Department of Pediatric Neurosurgery, Necker-Enfants Malades Hospital, over the period between September 2008 and February 2015. Among those, children who underwent surgery for isolated sagittal suture synostosis before 6 months of age were retrospectively studied. Children with other craniofacial anomalies and intracranial lesions other than CSF collection were excluded. Patients with incomplete data or incomplete pre- and postoperative imaging were excluded. After clinical and radiological data of patients were reviewed, 63 children (48 boys and 15 girls) were included. The mean age at surgery was 21.6 ± 3.1 weeks. The mean age at examination was 18.7 ± 4.5 weeks preoperatively and 34.9 ± 5.8 weeks postoperatively. The mean period between surgery and postoperative examination was 13.5 weeks (5.6 to 40.6 weeks). All children underwent the same cranioplastic surgery—Renier’s H technique, reported previously [14]. In this technique, the sagittal sinus is completely freed from bone from the anterior fontanel to the lambda.
Data collection and analysis
Evaluation of extracerebral CSF collection
The pericerebral effusion (PE) was measured in the frontal region where it is greater. It was defined as the maximum perpendicular distance between the internal wall of the frontal bone and the surface of the frontal cortex.
There have been various definitions of the pathological state of CSF collection [15–17]. Lam et al. investigated the subarachnoid space in infants and children by transcranial ultrasound and derived an approximate curve of the subarachnoid space corrected for age [18]. According to this report, a gradual increase of the subarachnoid spaces is observed from birth up to 7 months of age, and subsequently the arachnoid spaces decline until 1 to 2 years of age. In our study, the craniocortical width described by Lam et al. as the average value of PE in normal control derived from the following formula was adopted:
Two groups were created. Group “pathological PE” is composed of patients with PE greater than the average value expected for the age, and group “normal PE” is composed of those with PE less than the average of normal control. However, this formula has a time limit because the value of y becomes 0 at 65.7 weeks. For such reason, children without a postoperative examination before 65.7 weeks of age were excluded.
Evaluation of intracerebral CSF collection
Maximum bifrontal distance of the lateral ventricles (FV) and maximum inner diameter of the skull (IS) were measured. Evans’ index (EI) was calculated by dividing the FV by the IS. The pathological state of EI was defined as more than 0.3 according to Sari et al. [19].
Postoperative evolution
Preoperative and postoperative values were measured on the same tomographic modality. Otherwise, the patients were excluded from the study. The surgical effect on the PE was evaluated by comparing the “estimated PE without surgery” with the “actual PE” in the postoperative imaging. The postoperative estimated PE (ePE) was calculated as follows:
where prePE is the actual value of the preoperative PE and ΔnPE is the difference between preoperative and postoperative PE of normal control during the same period. (Fig. 1).
Graphical evaluation of PE comparing with estimated normal control. The postoperative estimated PE (ePE) was calculated using the following formula: ePE = prePE + ΔnPE. avENC average curve of the estimated normal control, pre PE actual value of preoperative PE, post PE actual value of postoperative PE, ΔnPE difference between preoperative and postoperative PE of the estimated normal control during the same period, ePE estimated value of PE without surgery in the same period
Other indices
-
1)
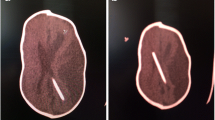
The shape of the cranial bone on the superior sagittal sinus (SSS) was classified into two groups. When the SSS was surrounded at any point of its course by cranial bone in more than half of the circumference of the SSS in association with an arcuate deformity of the outer cranium on the SSS, it was defined as “Ω (omega).” When the bone had no part which fulfilled the condition of the Ω, it was defined as “no Ω” (Fig. 2).
-
2)
The type of PE collection was classified into two groups. When the widest distance was measured at the anterolateral portion, it was defined as type “A.” When measured at the frontal anteromedial portion, it was defined as type “B” (Fig. 3).
Fig. 3 Typical appearance of the type of CSF collection in an axial view of T2-weighted images of MRI (a, b). a Measurement of the PE at the anteromedial portion (a) is smaller than that at the anterolateral portion (b) ( a < b). b Measurement of the PE at the anteromedial portion (a) is larger than that at the anterolateral portion (b) ( a > b)
-
3)
The cranial index was calculated by dividing the maximum fronto-occipital diameter by the maximum biparietal diameter.
Data analysis
The study consisted of a comparison between preoperative and postoperative data. All statistical analyses were performed with JMP Pro 9® software. Student’s t test was adopted to compare between preoperative and postoperative measurements. Wilcoxon’s rank test was adopted to compare the numerical data between the two groups. The chi-square test was adopted to compare categorical data between the two groups. A significance level of p < 0.05 was considered for all analysis.
Results
Preoperative analysis
Patient analysis (Table 1)
The shape of the bone on the SSS was “no Ω” in 38 (60 %) and “Ω” in 25 (40 %). The shape of PE collection was type “A” in 33 (52 %) and type “B” in 30 (48 %). The cranial index was lower in patients with “Ω” than those with “no Ω” (0.68 ± 0.04 vs. 0.70 ± 0.04, p = 0.05), but there was no difference of the cranial index between type “A” and “B” (0.68 ± 0.04 vs. 0.69 ± 0.05, n.s.).
Extracerebral effusion
The overall average of the preoperative PE was 5.6 ± 2.2 mm. Thirty six were categorized as group “pathological PE” (57 %), and 27 were grouped “normal PE” (43 %). The preoperative PE in “Ω” was larger than that in “no Ω” (6.6 ± 2.5 vs. 4.9 ± 1.5 mm, p = 0.0034). The preoperative PE in type “A” was larger than that in type “B” (6.2 ± 2.3 vs. 4.8 ± 1.7 mm, p = 0.0028). The cranial index was lower in group “pathological PE” than in group “normal PE” (0.67 ± 0.04 vs. 0.71 ± 0.03, p < 0.001) (Table 2).
Intracerebral effusion
The overall average of the preoperative EI was 0.31 ± 0.03, in whom 36 were group “pathological EI” (57 %) and 27 were group “normal EI” (43 %). Preoperatively, pathological EI was found in 26 of 36 patients with pathological PE and in 10 of 27 with normal PE (72 vs. 37 %, p = 0.005) (Fig. 4). There was no difference of the preoperative EI between “Ω” and “no Ω” (0.32 ± 0.03 vs. 0.30 ± 0.04, n.s.). The preoperative EI in type “A” was larger than that in type “B” (0.32 ± 0.03 vs. 0.30 ± 0.04, p = 0.039). The cranial index was lower in group “pathological EI” than that in group “normal EI” (0.71 ± 0.04 vs. 0.67 ± 0.04, p < 0.001) (Table 3).
Postoperative evolution
In terms of evolution, after surgery, the PE improved faster than the estimated course in 59 patients (92 %). The actual postoperative PE was smaller than the preoperative PE (4.1 ± 2.2 vs. 5.6 ± 2.2, p < 0.001) and was also smaller than the ePE (4.1 ± 2.2 vs. 5.7 ± 2.3, p < 0.001) (Fig. 5a). Eighteen of 36 patients (50 %) who had the pathological PE preoperatively were normalized, but the other half of patients was still pathological. The proportion of normalized patients was not different between “Ω” and “no Ω” (44 and 56 %, n.s.) and between “A” and “B” (46 and 57 %, n.s.). There was no difference of preoperative cranial index between normalized and still pathological patients (0.71 ± 0.03 vs. 0.67 ± 0.04, n.s.).
In terms of the EI, the actual postoperative EI was smaller than the preoperative EI (0.31 ± 0.03 vs. 0.29 ± 0.03, p < 0.001) (Fig. 5b). Twenty-one of 36 patients (64 %) who had preoperatively a pathological EI were normalized and 15 (36 %) were still pathological. The proportion of normalized patients was not different between “Ω” and “no Ω” (53 and 74 %, n.s.) and between “A” and “B” (62 and 67 %, n.s.). There was no difference of preoperative cranial index between normalized and still pathological patients (0.71 ± 0.04 vs. 0.67 ± 0.04, n.s.).
Discussion
Incidence and severity of CSF collections
Although the incidence of the intracranial CSF collections in scaphocephaly has been considered as rare in the literature, the present study showed that CSF collections in scaphocephaly are more common than those observed in previous reports. Our definition of the limit between normal and pathological state was determined by a precise correction for age. Actual PE was larger than the PE expected for the age in more than half of patients, and the EI was also larger than the classical cutoff value of 0.3. These results reveal that the CSF collection in scaphocephaly is not a rare association.
The cranial index is known as one of the indices of severity in scaphocephaly [20]. In the present study, the cranial index was well correlated with the preoperative PE and EI. This suggests that the more severe scaphocephaly is, the more the CSF accumulates. In addition, the preoperative CSF collection was also correlated with “Ω” morphology and type “A” which we defined. These could also be considered as indices of severity in scaphocephaly. However, functional studies to determine the clinical impact are necessary.
CSF collections significantly reduced after corrective surgery. Particularly, the PE reduced faster than the evolution expected according to previous studies [18]. This finding shows that a relationship between the PE and the sagittal synostosis exists. It confirms that the closure of the sagittal suture may involve certain circulatory disturbance of the CSF. If the circulatory disturbance of the CSF was independent, the surgery would have not modified it. However, there were no differences among indices. This suggests that surgery with the H technique for scaphocephaly reduces the CSF collection in all situations regardless of the severity of scaphocephaly.
Speculated underlying mechanisms
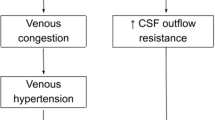
There are two possible mechanisms of extracerebral space expansion. The first mechanism is CSF malabsorption. Resorption classically starts at the arachnoid villi (AV). The AV may be involved indirectly in scaphocephaly due to the deformation on the shape of the bone at the level of the sagittal suture. In fact, the AV exist at the inferolateral aspect of the SSS, and in case of scaphocephaly, a bone deformity as the “Ω” sign may cause a stenosis of the entrance of the AV to the SSS. Nevertheless, since the AV are considered as immature before 1 year of age, their role in PE of scaphocephaly is probably not different from that of the general population. In fact, Barlow et al. described that the possible cause of external hydrocephalus was an immaturity of the AV [21] which cannot catch up to the production of the CSF, resulting in physiological CSF collection in the extracerebral region. The immaturity of the AV is not the main issue in scaphocephaly since the surgery can modify the expected evolution of the PE in this population.
Another possible mechanism of CSF malabsorption is venous hypertension (VHT). In case of scaphocephaly, the bone deformity may constrict locally the sinus, and it may cause a local VHT with upstream CSF malabsorption. Surgery for scaphocephaly which decompresses the bone on the SSS would liberate from local constriction the SSS and normalize the venous congestion, finally improving the absorption of the CSF. In case of bone deformity as the “Ω” sign, the compression could also affect the cortical veins flowing into the SSS, explaining why in these patients the PE is further increased. There are two ways that cortical veins flow into the SSS: (1) flowing through inferolateral aspects of the SSS directly and (2) flowing into the dura mater at a point distant from the SSS, then passing through the dura mater, and finally flowing into the SSS. In both cases, the dura mater is constricted by the bone deformity (“Ω” sign), resulting in the disturbance of the venous flow. Patients with the “Ω” sign in fact had a significant larger PE than those without the “Ω” sign (p = 0.0034) (Table 2). In addition, the PE was well correlated with the EI. Such finding confirms the possible CSF malabsorption in children with scaphocephaly.
The second mechanism of extracerebral space expansion would be to consider the PE as a compensation of abnormal skull growth. Nogueira et al. reported that the skull grew faster than the brain in early infancy, resulting in a transient accumulation of the physiological CSF in the extracerebral region [22]. In case of scaphocephaly, the pathological PE could exist in compensation for the abnormal longitudinal elongation. However, it cannot elucidate the reason why the EI increased preoperatively and why the EI reduced postoperatively.
Nevertheless, probably in scaphocephaly, both mechanisms coexist: local venous hypertension associated in some cases with passive enlargement. In fact, the association of both mechanisms would explain why in a number of cases the decompression of the sagittal sinus would result in a normalization of the PE whereas in others it only resulted in a significant but not complete reduction. In all cases, associating a decompression of the venous sinus and a remodeling of the vault, which can both be achieved with the H technique, would explain why in 92 % of the cases the PE was reduced.
Because of the limited number of cases and short follow-up, the long-term results of distinguishing between normal and pathological PE are still to be determined. Further studies comparing different surgical techniques, for example, surgery with or without decompression of the SSS or suturectomy with or without cranial vault remodeling, are probably necessary to further analyze the role of surgery in the physiopathology of PE.
Conclusion
Intracranial CSF collections, both external pericerebral effusion and internal ventriculomegaly, are common in scaphocephaly when precisely measured. Preoperative CSF collections are well correlated with some indices regarding the severity of scaphocephalic deformity. The CSF collections may be due to hypertension of the superior sagittal sinus secondary to a constriction by a bone deformity associated in some cases with compensation of skull elongation. Surgery for scaphocephaly results in a significant decrease of the CSF collections regardless of scaphocephalic severity.
References
Fishman MA, Hogan GR, Dodge PR (1971) The concurrence of hydrocephalus and craniosynostosis. J Neurosurg 34:621–629
Renier D, Sainte-Rose C, Marchac D, Hirsch JF (1982) Intracranial pressure in craniostenosis. J Neurosurg 57:370–377
Golabi M, Edwards MS, Ousterhout DK (1987) Craniosynostosis and hydrocephalus. Neurosurgery 21:63–67
Collmann H, Sorensen N, Krauss J, Muhling J (1988) Hydrocephalus in craniosynostosis. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 4:279–285
Cinalli G, Sainte-Rose C, Kollar EM, Zerah M, Brunelle F, Chumas P, Arnaud E, Marchac D, Pierre-Kahn A, Renier D (1998) Hydrocephalus and craniosynostosis. J Neurosurg 88:209–214
Chadduck WM, Chadduck JB, Boop FA (1992) The subarachnoid spaces in craniosynostosis. Neurosurgery 30:867–871
Raimondi AJ (1994) A unifying theory for the definition and classification of hydrocephalus. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 10:2–12
Sawin PD, Muhonen MG, Menezes AH (1996) Quantitative analysis of cerebrospinal fluid spaces in children with occipital plagiocephaly. J Neurosurg 85:428–434
Coll G, Arnaud E, Selek L, Brunelle F, Sainte-Rose C, Collet C, Di Rocco F (2012) The growth of the foramen magnum in Crouzon syndrome. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 28:1525–1535
Coll G, Arnaud E, Collet C, Brunelle F, Sainte-Rose C, Di Rocco F (2015) Skull base morphology in fibroblast growth factor receptor type 2-related faciocraniosynostosis: a descriptive analysis. Neurosurgery 76:571–583 discussion 583
Bondurant CP, Jimenez DF (1995) Epidemiology of cerebrospinal fluid shunting. Pediatr Neurosurg 23:254–258 discussion 259
Di Rocco F, Arnaud E, Meyer P, Sainte-Rose C, Renier D (2009) Focus session on the changing “epidemiology” of craniosynostosis (comparing two quinquennia: 1985–1989 and 2003–2007) and its impact on the daily clinical practice: a review from Necker Enfants Malades. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 25:807–811
Di Rocco F, Arnaud E, Renier D (2009) Evolution in the frequency of nonsyndromic craniosynostosis. J Neurosurg Pediatr 4:21–25
Di Rocco F, Knoll BI, Arnaud E, Blanot S, Meyer P, Cuttarree H, Sainte-Rose C, Marchac D (2012) Scaphocephaly correction with retrocoronal and prelambdoid craniotomies (Renier’s “H” technique). Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 28:1327–1332
Prassopoulos P, Cavouras D (1994) CT evaluation of normal CSF spaces in children: relationship to age, gender and cranial size. Eur J Radiol 18:22–25
Fessell DP, Frankel DA, Wolfson WP (2000) Sonography of extraaxial fluid in neurologically normal infants with head circumference greater than or equal to the 95th percentile for age. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine 19:443–447
Fukuyama Y, Miyao M, Ishizu T, Maruyama H (1979) Developmental changes in normal cranial measurements by computed tomography. Dev Med Child Neurol 21:425–432
Lam WW, Ai VH, Wong V, Leong LL (2001) Ultrasonographic measurement of subarachnoid space in normal infants and children. Pediatr Neurol 25:380–384
Sari E, Sari S, Akgun V, Ozcan E, Ince S, Babacan O, Saldir M, Acikel C, Basbozkurt G, Yesilkaya S, Kilic C, Kara K, Vurucu S, Kocaoglu M, Yesilkaya E (2015) Measures of ventricles and Evans’ index: from neonate to adolescent. Pediatr Neurosurg
Massimi L, Caldarelli M, Tamburrini G, Paternoster G, Di Rocco C (2012) Isolated sagittal craniosynostosis: definition, classification, and surgical indications. Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 28:1311–1317
Barlow CF (1984) CSF dynamics in hydrocephalus—with special attention to external hydrocephalus. Brain Dev 6:119–127
Nogueira GJ, Zaglul HF (1991) Hypodense extracerebral images on computed tomography in children. “External hydrocephalus”: a misnomer? Child’s Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 7:336–341
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This non-intervention analysis of imaging data was performed in accordance with the ethical standards of the Institution.
Rights and permissions
About this article
Cite this article
Usami, K., Nicolini, F., Arnaud, E. et al. Cerebrospinal fluid collections in sagittal suture synostosis. Childs Nerv Syst 32, 519–525 (2016). https://doi.org/10.1007/s00381-015-3011-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-015-3011-0