Abstract
Objects
To characterize the anatomical features of the ventricular regions in patients with myeloschisis and related to successful performance of endoscopic third ventriculostomy (ETV).
Methods
Radiological and endoscopic findings of 21 myeloschisis patients were retrospectively reviewed. Radiological features that could interfere with endoscopic procedures were (1) a huge massa intermedia (12/19), (2) sloping of the third ventricular floor (3/10), (3) narrow anteroposterior length of the third ventricular floor (2/10), and (4) narrow prepontine cistern (8/21). Endoscopic findings were (a) a narrow foramen of Monro (0/3), (b) hypertrophy of the anterior commissure (1/3), (c) sloping of the third ventricle floor (1/3), (d) a huge massa intermedia (3/3), and (e) opaque third ventricular floor (3/3). These endoscopic findings did not interfere with endoscopic procedures by using the Oi-HandyPro neuroendoscope without the above-mentioned radiological features 3 or 4.
Conclusion
Narrow anteroposterior length of the third ventricular floor and narrow prepontine cistern are not infrequently observed. Preoperative evaluation and intraoperative inspection of these findings are very important in successful performance of ETV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endoscopic third ventriculostomy (ETV) has become a first-line treatment of choice for noncommunicating hydrocephalus, though use of ETV in younger infants is still controversial because of its poor success rate [1, 2–4]. Some recent evidence suggests that the rate of success of ETV is not age-dependent but etiology-dependent, and that ETV has a lower rate of success for hydrocephalus associated with myeloschisis than other conditions [5–8]. On the other hand, for patients older than 1 year of age who have already been shunted, ETV has a higher rate of success than for patients undergoing primary treatment, including those with myeloschisis [4, 9–16]. However, patients with myeloschisis are known to have various degrees and types of cerebral and cerebellar malformations other than Chiari II malformation, some of which could be causes of failure of ETV [6, 17–22].
In this study, we retrospectively reviewed the findings of radiological examination of ventricular and paraventricular anatomy of patients with myeloschisis and intraoperative videos of endoscopically treated patients. We then characterized the anatomical features related to success or failure of ETV in patients with myeloschisis.
Materials and methods
Between January 2000 and April 2007, 18 patients underwent V-P shunt placement within 1 month after repair of myeloschisis as primary treatment, and two patients were observed conservatively according to our treatment algorithm (Fig. 1). Only one patient, who was transferred from another hospital at 3 months of age, underwent ETV as a primary procedure after confirmation of both noncommunicating hydrocephalus by CT ventriculography and lack of narrowing of the third ventricular floor and prepontine cistern by MRI. Two patients underwent ETV as a secondary procedure for shunt malfunction at the ages of 15 months and 7 years. Neither had narrowing of the third ventricle floor or prepontine cistern. ETV was performed with a small-diameter, rigid-rod neuroendoscope with a working channel (Oi-HandyPro: Karl Storz, Tuttlingen, Germany) [23].
Radiological and endoscopic findings for these 21 patients with myeloschisis were reviewed retrospectively. In the case of radiological examination, those findings that could interfere with the procedures of endoscopic surgery were evaluated by preoperative MRI or CT scans, such as huge massa intermedia, sloping of the third ventricular floor associated with elongation of the brainstem, narrow anteroposterior length of the third ventricular floor, and narrow prepontine cistern with crowding of the posterior fossa. When evaluation of a finding was difficult due to lack of sagittal view on MRI or CT scans, the case involved was omitted from analysis. In the case of endoscopic examination, the existence of a narrow foramen of Monro, hypertrophy of the anterior commissure, sloping of the third ventricular floor, huge massa intermedia, and opaque third ventricular floor were evaluated by reviewing video tapes of the operations.
Results
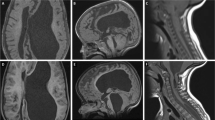
The anatomical features that could interfere with the procedures of endoscopic surgery as evaluated by preoperative MRI or CT scans were as follows (Table 1): (1) huge massa intermedia in 12 of 19 cases (63.2%), (2) steep slope of the third ventricular floor associated with elongation of the brainstem in 3 of 10 cases (30%), (3) narrow anteroposterior length of the floor of the third ventricle in 2 of 10 cases (20%), and (4) narrow prepontine cistern with crowding of the posterior fossa in 8 of 21 cases (38.1%) (Fig. 2). The only patient who underwent ETV as primary treatment had neither a narrow third ventricular floor nor a narrow prepontine cistern, and ETV was safely performed (Figs. 3 and 4).
MR images of a newborn with myeloschisis demonstrate typical findings of fused thalamus (huge massa intermedia), narrow anteroposterior length of the third ventricular floor, and narrow prepontine cistern with crowding of the posterior fossa associated with colpocephalic dilation of the lateral ventricles. The patient underwent V-P shunt placement after repair of myeloschisis
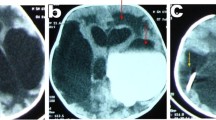
a Endoscopic view of the right foramen of Monro in the same patient as in Fig. 3. b Huge massa intermedia (arrow). c Opaque third ventricular floor
Endoscopic findings were as follows (Table 2): (a) a narrow foramen of Monro was found in none of the patients in our series, (b) hypertrophy of the anterior commissure was found in one of three cases (Fig. 5), (c) sloping of the third ventricular floor was found in one of three cases (Fig. 6), (d) huge massa intermedia were found in all three cases, and (e) opaque third ventricular floors were found in all three cases (Figs. 4 and 6).
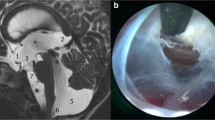
A 7-year-old girl with shunt malfunction. a MRI demonstrates large massa intermedia, though neither the anteroposterior length of the third ventricular floor nor the prepontine cistern is narrow. The anterior commissure is large (arrow), and b endoscopic view reveals a large anterior commissure seen within the foramen of Monro
A huge massa intermedia and opaque third ventricular floor were identified in all ETV cases but did not interfere with the endoscopic procedures with the Oi-HandyPro. None of the patients who underwent ETV exhibited the above-noted radiological findings 3 or 4, and the endoscopic procedures were safely performed in them. ETV was successful in two previously shunted patients but failed in one patient undergoing primary treatment.
Discussion
Shunt surgeries for pediatric hydrocephalus are established methods of treatment but feature high rates of complication, especially in young infants [24]. Although endoscopic third ventriculostomy (ETV) has become a first-line treatment of choice for noncommunicating hydrocephalus [25], use of it in infants is still controversial [1–4]. One reason for the poor rate of success of ETV in infants may involve specific features of CSF dynamics, in which the major CSF pathway has not developed and the minor pathway plays a significant role in transmission of CSF [26]. Another possibility is that altered ventricular and paraventricular anatomy interferes with procedures, especially in patients with myeloschisis [1, 6, 17–22]. Anatomical abnormalities associated with myeloschisis that may interfere with endoscopic procedures have been reported to include the following: (1) narrow foramen of Monro, (2) huge massa intermedia, (3) large anterior commissure, (4) narrow third ventricular floor, (5) opaque third ventricular floor, (6) sloping of the third ventricle floor associated with elongation of the brainstem, (7) narrow prepontine cistern with crowding of the posterior fossa, and (8) thick arachnoid adhesions in the prepontine cistern [6, 17–22]. The small-diameter, rigid-rod Oi-HandyPro neuroendoscope is a useful piece of equipment to perform safe procedures even through small corridors such as narrow foramen of Monro [23], but narrow third ventricular floor and prepontine cistern were considered as contraindications of ETV [1]. Therefore, our treatment strategy for hydrocephalus associated with myeloschisis is to perform shunt surgery as primary treatment if necessary. Then, ETV was performed in patients with shunt malfunction only when narrowing of the third ventricular floor and the prepontine cistern were excluded by the preoperative image (Fig. 1).
In this retrospective study, almost all of the above-noted findings (1 to 8) were identified radiologically and/or endoscopically. Peretta et al. reported that, in 7 patients (3 with myelomeningocele) of 355 undergoing ETV, endoscopic procedures were aborted due to significant alterations in the anatomy of the foramen of Monro and the third ventricular floor [27], though in our small series, no procedures were aborted. This is probably because, as already mentioned, we selected candidate for ETV by preoperative imaging. Although in all our three patients with ETV the foramen of Monro was not narrow, huge massa intermedia and opaque third ventricular floor were identified in all the cases, but the procedures were performed safely by the Oi-HandyPro neuroendoscope [23].
Because ETV for pediatric hydrocephalus has a higher rate of success in patients who have already been shunted than in those undergoing primary treatment [4, 9–16], ETV should be considered when patients have been diagnosed with shunt malfunction. However, in patients with narrowing of the third ventricular floor and/or prepontine cistern, shunt revision should be considered. In addition, if these findings are identified intraoperatively during an attempt at ETV, prompt conversion to shunt surgery is very important to avoid unnecessary injury of vital structures and severe complications.
Conclusions
Huge massa intermedia and opaque third ventricular floor were frequently observed in patients with myeloschisis but did not interfere with endoscopic procedures in our series. The small-diameter, rigid-rod Oi-HandyPro neuroendoscope with high-resolution imaging was useful for this particular population of patients. However, shunt revision should be considered in patients with narrow anteroposterior length of the third ventricular floor and/or narrow prepontine cistern with crowding of the posterior fossa.
References
Di Rocco C, Cinalli G, Massimi L, Spennato P, Cianciulli E, Tamburrini G (2006) Endoscopic third ventricilostomy in the treatment of hydrocephalus in pediatrics patients. In: Pickard JD, Akalan N, Di Rocco C, Dolenc VV, Fahlbusch R, Lobo Antunes J, Mooij JJ, Sindou M, Tulleken CAF (eds) Adv tech stand in neurosurgery. vol. 31. Springer, Wien, pp 121–219
Kadrian D, van Gelder J, Florida D, Jones R, Vonau M, Teo C, Stening W, Kwok B (2005) Long-term reliability of endoscopic third ventriculostomy. Neurosurgery 56:1271–1278
Koch-Wiewrodt D, Wagner W (2006) Success and failure of endoscopic third ventriculostomy in young infants: are there different age distributions. Childs Nerv Syst 22:1537–1541
Teo C, Jones R (1996) Management of hydrocephalus by endoscopic third ventriculostomy in patinets with myelomeningocele. Pediatr Neurosurg 25:57–63
Beems T, Grotenhuis JA (2002) Is the success rate of endoscopic third ventriculostomy age-dependent? An analysis of the results of endoscopic third ventriculostomy in young children. Child’s Nerv Syst 18:605–608
Etus V, Ceylan S (2005) Success of endoscopic third ventriculostomy in children less than 2 years of age. Neurosurg Rev 28:284–288
Fritsch MJ, Kienke S, Ankermann T, Padoin M, Mehdorn HM (2005) Endoscopic third ventriculostomy in infants. J Neurosurg (Pediatr 1) 103:50–53
Gorayeb R, Cavalheiro S, Zymberg S (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg 100:427–429
Bouschert J, Hellwig D, Krauss J (2003) Endoscopic third ventriculostomy for shunt dysfunction. J Neurosurg 98:1032–1039
Buxton N, Macarthur D, Robertson I, Punt J (2003) Neuroendoscopic third ventriculostomy for failed shunts. Surg Neurol 60:193–200
Cinalli G, Spennato P, Ruggiero C, Aliberti F, Zerah M, Trischitta V, Cianciulli E, Maggi G (2006) Intracranial pressure monitoring and lumbar puncture after endoscopic third ventriculostomy in children. Neurosurgery 58:126–136
Mori H, Akiyama K, Nishiyama K, Tanaka R (2000) Endoscopic third ventriculostomy for shunt dependent obstructive hydrocephalus including slit ventricle syndrome. Video J Jpn Neurosurg 18:2
Nishiyama K, Mori H, Tanaka R (2003) Changes in cerebrospinal fluid hydrodynamics following endoscopic third ventriculostomy for shunt-dependent noncommunicating hydrocephalus. J Neurosurg 98:1027–1031
O’Brien D, Javadpour M, Collins DR, Spennato P, Mallucci CL (2005) Endoscopic third ventriculostomy: an outcome analysis of primary cases and procedures performed after ventriculoperitoneal shunt malfunction. J Neurosurg 103:393–400
Sato G, Mori H, Nishiyama K, Tanaka R (1999) Endoscopic third ventriculostomy for malfunction of long-standing VP shunt—case report & review of the literature. Nerv Syst Child 24:461–446
Torisu R, Hitotsumatsu T, Uda K, Abe H, Inoue T, Oka K (2005) Replacement of a 35-year-old ventriculo-peritoneal shunt by endoscopic third ventriculostomy during abodominal surgery: a case report. Jpn J Neurosurg 14:522–526
Kadri H, Mawla AA (2004) Variations of endoscopic ventricular anatomy in children suffering from hydrocephalus associated with myelomeningocele. Minim Invasive Neurosurg 47:339–341
Kawamura T, Morioka T, Nishio S, Mihara F, Fukui M (2001) Cerebral abnormalities in lumbosacral neural tube closure defect: MR imaging evaluation. Childs Nerv Syst 17:405–410
McLone DG, Dias MS (2003) The Chiari II malformation: cause and impact. Childs Nerv Syst 19:540–550
Miyajima M, Horinaka N, Ishii H, Nakanishi H, Hishii M, Arai H (2005) Endoscopic third ventriculostomy for infantile hydrocephalus and its problems. Jpn J Neurosurg 14:449–455
Pavez A, Salazar C, Rivera R, Contreras J, Orellana A, Guzman C, Iribarren O, Hernandez H, Elzo J, Moraga D (2006) Description of endoscopic ventricular anatomy in myelomeningocele. Minim Invasive Neurosurg 49:161–167
Warf B (2005) Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg 102:1–15
Oi S, Samii A, Samii M (2005) Frameless free-hand maneuvering of a small-diameter rigid-rod neuroendoscope with a working channel used during high-resolution imaging. Technical note. J Neurosurg 102:113–118
Di Rocco C, Massimi L, Tamburrini G (2006) Shunts vs endoscopic third ventriculostomy in infants: are there diffrent types and/or rates of complications. Childs Nerv Syst 22:1573–1589
Oi S, Hidaka M, Honda Y, Togo K, Shinoda M, Shimoda M, Tsugane R, Sato O (1999) Neuroendoscopic surgery for specific forms of hydrocephalus. Childs Nerv Syst 15:56–68
Oi S, Di Rocco C (2006) Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Childs Nerv Syst 22:662–669
Peretta P, Ragazzi P, Galarza M, Genitori L, Giordano F, Mussa F, Cinalli G (2006) Complications and pitfalls of neuroendoscopic surgery in children. J Neurosurg 105:187–193
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mori, H., Oi, S., Nonaka, Y. et al. Ventricular anatomy of hydrocephalus associated with myeloschisis and endoscopic third ventriculostomy. Childs Nerv Syst 24, 717–722 (2008). https://doi.org/10.1007/s00381-007-0547-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-007-0547-7