Abstract
Background
Isolated fourth ventricle (IFV) is a challenging entity to manage. In recent years, endoscopic treatment for aqueductoplasty has been on the rise. However, in patients with complex hydrocephalus and distorted ventricular system, its implementation can be complex.
Methods
We present a 3-year-old patient with myelomeningocele and postnatal hydrocephalus treated by ventriculoperitoneal shunt. In follow-up, a progressive IFV and isolated lateral ventricle with symptoms of the posterior fossa developed. An endoscopic aqueductoplasty (EA) with panventricular stent plus septostomy guided with neuronavigation was decided due to the complexity of the ventricular system.
Conclusion
In IFV associated with complex hydrocephalus with distortion of the ventricular system, navigation can be of great help for planning and as a guide for performing EA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Relevant surgical anatomy
In recent decades, the endoscopic treatment of IFV has been on the rise due to improvements in technique, instrumentation, and navigation systems. We present a case of IFV managed by endoscopic aqueductoplasty with panventricular stent placement and septostomy through different approaches.
Before the arrival of endoscopy, the treatment of the IFV was usually performed using microsurgery with fairly poor results [3, 5]. This made this a pathologic condition with high morbidity and mortality. Currently, with new treatments, the management paradigm has changed. The complications associated with the technique have been reduced, and more and more neurosurgeons are recommending early treatment given the good results shown [1, 2, 7, 9, 10].
The main anatomic limitations in the technique are the size and ventricular morphology during endoscopic navigation, the point and intracranial entry angle determined by the size and location of the Monro Foramen and interthalamic mass, and finally the size and thickness of the aqueductal membrane [4]. If a poor technique is performed, there is a risk of damaging vital structures such as the Fornix, interthalamic mass, periaqueductal substance, or basal ganglia.
The standard technique involves positioning the patient in a supine decubitus position. The incision and subsequent craniotomy are performed 4 cm at a pre-coronal level directly behind the hairline [9]. Due to interindividual differences in ventricular anatomy, it is sometimes necessary to modify the trajectory. In order to determine the best trajectory through a straight line that will represent the direction of rigid endoscopy systems towards the surgical target, different groups rely on the use of neuronavigation. This fact allows guiding the approach towards the main objective with less need for traction of neural structures, as well as direct visualization of the main anatomical references: Monro foramen, interthalamic mass, and aqueductal inlet.
In the literature, certain methods to enhance planning and guide EA have been described, with neuro-navigation standing out. However, reports on its correlation with the images observed through the endoscope and its use in complex hydrocephalus are poorly documented in the literature. Our goal is to provide documentation to aid other teams in performing these techniques with the greatest possible safety.
Clinical case and description of the technique
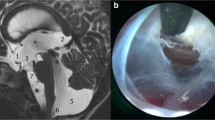
We present a 3-year-old patient with a birth-intervened myelomeningocele and subsequent development of hydrocephalus that required the placement of a ventriculoperitoneal shunt at 2 weeks of age (Fig. 1A–C). During follow-up, the development of IFV, right isolated lateral ventricle along with cervical-dorsal syringomyelia is observed. Clinically, the patient presents with deterioration of motor function in the lower extremities as well as a posterior fossa syndrome.
Preoperative studies. A–C: MRI performed during follow-up after ventricular shunt placement. The catheter entry through the right lateral ventricle is observed with its isolation. The fourth ventricle has a normal morphology. No evidence of syringomyelia. D–F: Follow-up MRI shows greater collapse of the right lateral ventricle along with the development of an isolated fourth ventricle and cervico-dorsal syringomyelia
In the preoperative imaging study, isolated right lateral ventricle and dilated left ventricle were observed. Additionally, a membrane at the level of the Silvian aqueduct was noted causing an obstruction at that level (Fig. 1D–F). Given the findings and clinical evolution, an EA with rigid ventriculoscope (LOTTA, Karl Storz, Tuttlingen, Germany) and guided by neuronavigation was decided. The patient had a good postoperative evolution without severe complications and with optimal recovery. Hospital discharge was performed on the 5th postoperative day, after the performance of the control magnetic resonance imaging that showed radiological improvement, including the syringomyelia (Fig. 5A–E). The patient presented an alteration of the conjugate gaze that completely resolved 2 weeks after discharge.
Patient positioning
Under general anesthesia, the patient was placed in a supine position with the head secured on a circular headrest. Frameless Electromagnetic Neuronavigation system (Medtronic StealthStation S8, Minneapolis USA) was placed on the right frontal region (Fig. 3A).
Planification and surgical technique
After reviewing the patient’s medical history, preoperative images, and existing literature on this type of patients, the following three main procedures were proposed: endoscopic septostomy, endoscopic aqueductoplasty, and external ventricular drain (EVD) placement. Preoperative images were acquired, and trajectory planning was performed using the neuronavigation system.
Endoscopic septostomy
After the placement of the ventricular shunt, the patient developed an isolated right lateral ventricle (Fig. 1A–F). Our primary goal was to communicate both lateral ventricles. To achieve this, we traced various trajectories on preoperative images and then transferred them to the neuronavigation system. Our target was located in the septum, just where the distal region of the ventricular catheter was located. The entry point was fixed along the most favorable trajectory to the target, 1 cm behind the coronal suture and 5 cm from the midline. A linear incision and a high-speed drill were used to create a burr hole (Fig. 3A–C). To guide the procedure in real time, a stylet system protected by a pediatric feeding tube was used (Fig. 2A). The stylet was introduced through the working channel of the endoscope, and its tip was placed on the target region (Fig. 4B). The septum was perforated, and both lateral ventricles were connected.
Material used to perform the procedure. A: We observe the endoscopy system (LOTTA; Karl Storz, Tuttlingen, Germany), a neuronavigation stylet introduced into a pediatric feeding tube for intraoperative guidance, an external ventricular catheter designed as a stent, and finally, hemostasis elements for a hermetic closure of the dural defect. B: Ventricular catheter coated with rifampicin and notches made to act as a stent
Endoscopic aqueductoplasty
The main objective of the surgery was to alleviate the patient’s symptoms related to the IFV (Fig. 1A–F). To achieve this, an endoscopic procedure was planned. Various trajectories were drawn on preoperative images and transferred to the neuronavigation system. Our target was located on the fourth ventricle. The entry point was fixed along the most favorable trajectory, 5 cm in front of the coronal suture on the medio-pupillary line. An incision with C-shaped morphology was planned, and a high-speed motorized burr hole, augmented with a Kerrison, was used to perform it (Fig. 3A–C). This type of incision was chosen to facilitate endoscopic aqueductoplasty with stent placement. A ventricular catheter coated with rifampicin (Bactiseal. Codman-Integra NJ, USA) was notched with scissors to create holes along its entire length (Fig. 2B). This catheter was then connected to an Omayya-type reservoir, which was placed in the subgaleal region. The procedure was performed under neuronavigation guidance (Fig. 4C–F). Using a 3 French Fogarty balloon, the membrane over the Sylvian aqueduct was perforated and expanded. The dilation procedure is performed under endoscopic control, and the balloon is gradually inflated in a controlled manner by the assistant. Our objective, whenever possible, is to create an ostomy with a sufficiently large diameter to accommodate the endoscopy system to visualize the fourth ventricle. The patency of the aqueduct was confirmed, and the previously prepared catheter was left in place as a stent, with the aid of endoscopic visualization.
Intraoperative images. A: Patient positioning. Planning of incisions and burr holes. Placement of the neuronavigation system. B: Bone exposure. C: Anterior burr holes for endoscopic aqueductoplasty and placement of EVD. Noteworthy is the periosteal dissection to act as a sealant at the end of the procedure
Endoscopic images. A: Entry into the left lateral ventricle from a precoronal location. B: Location of the septum pellucidum, distal tip of the ventricular catheter with the neuronavigation system, and septostomy. C–F: Endoscopic aqueductoplasty with stent. SA Sylvian aqueduct, CP choroid plexus, TSV thalamostriate vein, SP septum pellucidum, NV neuronavigation, VC ventricular catheter, S stent, F fornix, IM interthalamic mass
Stent/external ventricular drain (EVD) placement
Finally, during the surgery planning, it was projected to perform a more anterior burr hole to carry out the aqueductoplasty (Fig. 3A–C). The objective is to use this burr hole to introduce the stent under direct endoscopic visualization. Subsequently, it was attached to an Ommaya reservoir to prevent migration. Finally, an external ventricular drainage, which remained closed until its removal, was introduced through the burr hole where the endoscopy system was located. Its use was based on two objectives: (1) To serve as a safety measure in case shunt malfunction. (2) As a continuous measurement of intracranial pressure after the procedure.
To end, two relevant aspects carried out during the closure of the procedure should be noted. As can be seen in Fig. 3, a linear dissection of the periosteum was performed. The aim was to create a hermetic membrane to reduce the risk of cerebrospinal fluid fistula. Additionally, a hemostatic plug (Tachosil®) with an umbrella morphology (Fig. 2A) was created over the trephine.
Indications
The indication for surgery is usually made when the patient exhibits symptoms resulting from fourth ventricle entrapment. However, there is a growing trend to treat those patients who are asymptomatic but show radiological progression during follow-up [7].
The endoscopic approach to the fourth ventricle is highly useful for the treatment of obstructive pathologies of the ventricular system affecting this region. Unlike microsurgical techniques, either via supratentorial or suboccipital approaches, endoscopic procedures are associated with lower surgical risk and offer better long-term outcomes. Over the past few decades, their use has become more widespread, and they are currently considered the first choice, provided there is favorable ventricular anatomy.
In the literature, a high rate of aqueductal stenosis recurrence associated with this technique has been reported [8]. Therefore, the use of stents that maintain the patency of the created ostomy is recommended.
In our case, given that the patient had clinical and radiological progression and had a ventricular anatomy that allowed for an endoscopic procedure, we offered to perform an endoscopic aqueductoplasty. The distorted ventricular anatomy, typical of patients with myelomeningocele, increased the complexity of the technique. To try to address this issue, pre-surgical planning and neuronavigation were used (Fig. 5).
Postoperative images. A–D: Reduction in the size of the fourth ventricle and mass effect on the brainstem can be observed. Stent located in the fourth ventricle. Decrease in ventricular size and apparent communication between both lateral ventricles. Ventricular catheter with parietooccipital entry housed in the right lateral ventricle. Significant reduction in cervicodorsal syringomyelia. E: Postoperative CT showing different entry points used. The red asterisk refers to the Ommaya system to which the stent housed in the fourth ventricle is attached. The yellow asterisk refers to the external ventricular drain used
Limitations
The main limitations associated with this technique can be summarized in three points:
-
1.
Ventricular size
To carry out the endoscopic procedure, it is necessary to have sufficient space for the endoscopic system to fit. Cases have been described in the literature where this has been achieved by progressive dilation using EVD or cranial expansions [11].
-
2.
Ventricular anatomy
The existence of anatomical peculiarities (e.g., wide interthalamic mass/small Monro foramen/ventricular distortion) increases the complexity of the technique.
-
3.
Size and thickness of the aqueductal membrane
An aqueductal membrane <5mm hinders the technique due to the risk of damage to the periaqueductal substance when performing dilation with a balloon or perforation.
How to avoid complications
Pre-surgical planning is essential to study the possible anatomical anomalies of the ventricular system. In the case of a CT scan, it provides information on the bony structures when planning entry points. MRI is fundamental to understanding ventricular anatomy and determining reference points when performing the technique.
In cases such as the one presented here where the technique may be complex due to anatomical peculiarities, the use of neuronavigation systems is essential as they allow for more precise trajectories with a lower risk of injury to vital structures.
Finally, to reduce the risk of cerebrospinal fluid fistulas, a correct closure technique using hermetic methods (e.g., use of periosteum, hemostatic materials) is essential to achieve an adequate post-surgical outcome [12].
Specific perioperative considerations
Preoperative
-
Preoperative CT and MRI to define ventricular anatomy, reference points, and plan the trajectory.
-
Use of neuronavigation to guide the procedure.
Postoperative
-
Hermetic closure of the trajectory to prevent cerebrospinal fluid fistula.
-
Short course of corticosteroids and antiepileptic prophylaxis to prevent neurological deterioration associated with the technique.
Specific information for the patient
If there are no associated complications, this surgery usually has a rapid recovery, and when postoperative neurological deterioration occurs, it tends to be transient. However, patients and their families should be aware that there are comorbidities and risks associated with the surgery. The most common are related to alterations in eye movement or memory, which are usually transient. However, there is a risk of damaging structures involved in consciousness, blood vessels that supply areas that control movements and sensations, and finally a risk of infection of the nervous system and cerebrospinal fluid leak.
The development of the IFV in patients with ventricular shunts and other associated disorders (e.g., myelomeningocele, germinal matrix hemorrhage, meningitis) has been reported in the literature [6]. When it occurs, we must inform about the risk of compression of brainstem structures and spinal cord involvement. The use of endoscopy systems has improved treatment outcomes in this pathology. Furthermore, its association with neuronavigation systems increases its safety and reduces the risk of associated complications.
Abbreviations
- IFV :
-
Isolated fourth ventricle
- EA :
-
Endoscopic aqueductoplasty
- CT :
-
Computer tomography
- MRI :
-
Magnetic resonance imaging
- EVD :
-
External ventricular drain
References
Fritsch MJ, Schroeder HW (2013) Endoscopic aqueductoplasty and stenting. World Neurosurg 79(2 Suppl):S20.e15–S20.e18
Gomar-Alba M, Parrón-Carreño T, Guil-Ibáñez JJ, Vargas-López AJ, Castelló-Ruiz MJ, García-Pérez F, Masegosa-González J (2023) Role of endoscopic aqueductoplasty with panventricular stent in the treatment of isolated fourth ventricle during shunt malfunction: 2-dimensional operative video. Oper Neurosurg (Hagerstown). https://doi.org/10.1227/ons.0000000000000682 Advance online publication
Harter DH (2004) Management strategies for treatment of the trapped fourth ventricle. Childs Nerv Syst 20(10):710–716
Longatti P, Fiorindi A, Perin A, Martinuzzi A (2007) Endoscopic anatomy of the cerebral aqueduct. Neurosurgery 61(3 Suppl):1–6
Panagopoulos D, Karydakis P, Themistocleous M (2021) The entity of the trapped fourth ventricle: a review of its history, pathophysiology, and treatment options. Brain Circ 7(3):147–158
Pomeraniec IJ, Ksendzovsky A, Ellis S, Roberts SE, Jane JA Jr (2016) Frequency and long-term follow-up of trapped fourth ventricle following neonatal posthemorrhagic hydrocephalus. J Neurosurg Pediatr 17(5):552–557
Rekate HL (2005) Endoscopic fourth ventricular aqueductoplasty. J Neurosurg 103(5):773–775
Sagan LM, Kojder I, Poncyljusz W (2006) Endoscopic aqueductal stent placement for the treatment of a trapped fourth ventricle. J Neurosurg 105(4 Suppl):275–280
Schroeder HW, Gaab MR (1999) Endoscopic aqueductoplasty: technique and results. Neurosurgery 45(3):508–518
Schulz M, Goelz L, Spors B, Haberl H, Thomale UW (2012) Endoscopic treatment of isolated fourth ventricle: clinical and radiological outcome. Neurosurgery 70(4):847–859
Tirado-Caballero J, Rivero-Garvia M, Moreno-Madueño G, Gómez-González E, Márquez-Rivas J (2021) Cranial expansion and aqueductoplasty for combined isolated fourth ventricle and slit-ventricle syndrome: a surgical alternative. Childs Nerv Syst 37(3):885–894
Tirado-Caballero J, Herreria-Franco J, Rivero-Garvía M, Moreno-Madueño G, Mayorga-Buiza MJ, Marquez-Rivas J (2021) Technical nuances in neuroendoscopic lavage for germinal matrix hemorrhage in preterm infants: twenty tips and pearls after more than one hundred procedures. Pediatr Neurosurg 56(4):392–400. https://doi.org/10.1159/000516183
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in the studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standard.
Consent to participate and consent for publication
Informed consent to participate and consent for publication was obtained from the patient included in this report.
Conflict of interest
The authors declare no competing interests.
Additional information
Key points
Determine the indications for surgery. Patients with clinical symptoms should undergo surgery. In cases where the patient is asymptomatic but has radiological progression, risk/benefit assessment is necessary.
Establish the approach route. If an endoscopic aqueductoplasty is being considered, the patient must have a ventricular size large enough to accommodate the system. Otherwise, alternatives such as EVD placement or cranial expansion may be considered.
Study preoperative images and develop a surgical plan by identifying limiting structures and the best surgical route.
In complex anatomies, consider the use of neuronavigation.
Explain to the patient and family the possibility of transient neurological alterations, particularly in eye mobility.
Knowledge of ventricular anatomy and experience in intraventricular endoscopy are crucial to obtain good outcomes.
The use of stents appears to show a lower rate of restenosis in the literature.
A proper technique for closing the cortical-subcortical tract is ideal to avoid the risk of cerebrospinal fluid leak.
Periodic follow-up with MRI is recommended for patients. We have protocolized the performance of a control magnetic resonance imaging before discharge, another 1 month after the procedure, at 3 months, 6 months, and then annually. During the interpretation of these images, we investigate possible signs of system failure (e.g., signs of hyperdrainage) and combine it with the information provided by the parents about the patient’s neurological (cognitive, school, motor, etc.) evolution.
In patients with shunts and a history of obstructive hydrocephalus, consideration should be given to endoscopic third ventriculostomy to remove the ventricular shunt.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guil-Ibáñez, J.J., Parrón-Carreño, T., Gomar-Alba, M. et al. Neuronavigated endoscopic aqueductoplasty with panventricular stent plus septostomy for isolated fourth ventricle in complex hydrocephalus and syringomyelia associated with myelomeningocele: how I do it. Acta Neurochir 165, 2333–2338 (2023). https://doi.org/10.1007/s00701-023-05649-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-023-05649-9