Abstract
Current literature reveals different opinions about the effectiveness of endoscopic third ventriculostomy in the treatment of hydrocephalus in children less than 2 years of age. Performing a retrospective evaluation of our own experience in this age group, we aimed to contribute to the growing data on the controversial issues related to this procedure in children. In a series of 97 endoscopic third ventriculostomy procedures, 25 were performed in children less than 2 years of age as an initial treatment for hydrocephalus. A retrospective analysis of our data revealed that the overall success rate of endoscopic third ventriculostomy in this age group was 56%. However, analysis of the results in subgroups with different etiologies of hydrocephalus showed that the success rate of the procedure was 83% in patients with defined anatomic obstruction, 66.6% in post-hemorrhagic hydrocephalus, 50% in infection related hydrocephalus and 41.6% in hydrocephalus accompanied by myelomeningocele. This article considers our data and the features of endoscopic third ventriculostomy procedure in this age group, with a detailed review of the literature. In our experience, the success of endoscopic third ventriculostomy is etiology related rather than age-dependent. We suggest that there are no grounds for denying children younger than 2 years this chance for a shunt-free life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus remains one of the greatest challenges in modern neurosurgery. Despite sophisticated advances in shunt systems, numerous revisions for malfunction are still performed and infected shunts are still frequently encountered in neurosurgical practice. Endoscopic third ventriculostomy (ETV) is currently considered the best alternative to shunt systems in the treatment of hydrocephalus. As neurosurgeons continue to gain experience with ETV, the indications for this procedure are constantly being reviewed and expanded. However, different opinions exist in the literature about the effectiveness of ETV in the treatment of hydrocephalus in young children. The vast majority of the authors who reported their experience and results with ETV have mentioned low success rates in patients below the age of 2 years [9, 10, 16, 20, 24], and these results have even led to recommendations by some authors not to perform ETV in patients below the age of 2 years. By contrast, a few studies have indicated that there was no difference in the success rates in very young patients compared with the older age group, or that the rate was only slightly lower [1, 4, 5, 27, 33]. Also, there seems to be growing evidence that the success of ETV depends mainly on the etiology of the hydrocephalus and not on the age of the patient alone [2, 8, 14, 19].
The current study is intended to add to the growing data on the controversial issues related to ETV in children. In this report, we detail our experience with 25 children less than 2 years of age who underwent ETV as an initial treatment for hydrocephalus between 1997 and 2004. A retrospective analysis of the results of the ETV procedures was carried out. ETV success rates in subgroups with different etiologies of the hydrocephalus were analyzed. In addition, the features of endoscopic third ventriculostomy procedure in this age group were discussed with a detailed review of the literature.
Materials and methods
In our department, a series of 97 ETV procedures were performed between years 1997 and 2004. Among those procedures, 29 were performed in patients below the age of 2 years. Out of this group, two patients who had a permanent malfunctioning CSF shunt in place at the time of the ETV procedure were excluded from the study. Two cases in which we had to abandon the ETV procedure due to unsuitable anatomical features were also excluded from the study group. We were therefore able to analyze 25 ETV procedures performed as an initial treatment for hydrocephalus in 25 patients who were less than 2 years of age. These 25 patients were divided into four major groups according to the etiology of hydrocephalus: (a) primary and secondary aqueductal stenosis (late onset and all space-occupying lesions such as tumors and cysts in the third and fourth ventricles or within the cerebellum/ posterior fossa); (b) infection related hydrocephalus; (c) hydrocephalus in meningomyelocele patients (including Chiari type 2); and (d) post-hemorrhagic hydrocephalus, primarily, without ventricular compartmentalization or associate cystic lesions. All ETVs were performed with similar techniques using the freehand method. A 0° rigid rod lens neuroendoscope with an outer diameter of 4.0 mm (Karl Storz GmbH & Co., Tuttlingen, Germany) was used through a guiding tube. All operative procedures were recorded by video imaging (Karl Storz Telecam, SL). Ideally, the perforation in the floor of the third ventricle was made in the tuber cinereum between the infundibular recess of the pituitary stalk and the anterior border of the mammillary bodies. This allows entry into the interpeduncular cistern and avoids injury to the basilar apex. In our technique of performing ETV, we usually used the tip of the monopolar coagulating probe to make a blunt perforation in the floor, and preferred not to make any coagulation unless we encountered a thick floor. The puncture site was dilated by inflating a 4 French balloon catheter. Any thickened and diffuse arachnoidal trabeculations and webs in the interpeduncular cistern were eradicated successfully with blunt dissection until free communication along the basilar artery was visualized. Lactated Ringer’s solution at 37°C was used for irrigation. Postoperatively, patencies of the ventriculostomy sites have been determined with cine phase-contrast magnetic resonance imaging in the majority of the cases.
The definition of “ETV failure” was based on a pragmatic, practical standard that was used in a retrospective international multicenter study in 2002 [31], i.e. a failed case was one in which the patient needed a shunt inserted after attempted ETV.
Results
In our series of 97 ETV procedures, 29 were performed in patients below the age of 2 years. This means that 29.8% of the total group of patients were younger than 2 years. A retrospective analysis was performed in 25 children below the age of 2 years who had undergone ETV as an initial treatment for their hydrocephalus. In this group of patients, the mean age was 6 months and 9 days (range 1 day to 22 months). The mean follow-up was 1 year and 8 months (range 6 months to 4 years and 8 months). The overall success rate in this cohort of 25 patients was 56% (14 patients). The overall success rate in the total group of 97 ETV procedures was 76%. In the group of patients studied, we did not encounter any of the serious complications due to the procedure that have been mentioned by other authors (cranial nerve palsy, traumatic aneurysm of basillary top, hemorrhage, hemiparesis, hypothalamic dysfunction) [17, 30]. Some patients experienced a self-limiting fever up to 38.5°C in the first 2 days.
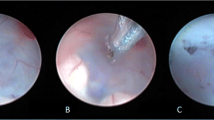
In our experience of ETV with patients below the age of 2 years, we have encountered several intraventricular anatomical variations and abnormalities during the endoscopic procedures. Most of these anatomical variations and abnormalities have been seen in patients with myelomeningocele (Fig. 1). The encountered anatomical features were as follows: narrow and hypervascular entry of the foramen of Monro (in one case), a thick and prominent massa intermedia (in four cases), opaque and/or thick floor of the third ventricle (in two cases), narrow tuber cinereum (in two cases), hollow, steep or vascular floor of the third ventricle (in two cases), small anterior chamber of the third ventricle (in one case), shallow prepontine cistern (in two cases), and partial or total agenesis of interventricular septum (in five cases). We have also experienced two cases in which we had no chance to perform ventriculostomy due to unsuitable anatomical features. Both of these patients had hydrocephalus accompanied with myelomeningocele. In one of these cases, both foramina of Monro were extremely narrow and the entrance to the right foramen of Monro was partially obliterated by a vascular web. In the other case, we had to abandon the procedure because of a very prominent massa intermedia. We placed a CSF shunt under endoscopic guidance in both cases. Because the ETV procedures have not been completed, these two cases were not included in our study cohort. The patients in whom the procedure did not succeed mostly presented with a further increase in head circumference and sometimes a decreased food intake with or without vomiting. Postoperative, cine phase-contrast magnetic resonance imaging studies revealed that the fenestrations were patent in all these patients. These findings led us to diagnose precisely the failure of the ETV procedure and the patients underwent CSF shunt surgery shortly afterwards.
Neuroendoscopic views of various intraventricular anatomical variations and abnormalities encountered in patients with myelomeningocele. a Neuroendoscopic view of the third ventricle floor in a hydrocephalic myelomeningocele patient. Mamillary bodies are not clearly seen. Infundibular recess (i), optic chiasm (oc) and a prominent anterior commissure (a) are seen anterior to the opaque and narrow tuber cinereum (t). b Neuroendoscopic view of the third ventricle floor in another myelomeningocele patient. A non-transparent tuber cinereum (t) and a dilated infundibular recess (i) are seen anterior to the mamillary bodies (m). Note to the vascular structure of the third ventricle floor. c Neuroendoscopic view showing a steep third ventricle floor in a myelomeningocele patient. A narrow tuber cinereum (t) is visible just anterior to the mamillary bodies (m). d Neuroendoscopic view through a very narrow prepontine cistern. Note the close proximity of the basillary artery (ba) and clivus (cl)
Classification of patients according to the etiology of hydrocephalus and the success rates of ETV is outlined in Table 1.
Discussion
Endoscopic third ventriculostomy combines a minimally invasive approach with visual control of manipulations, and is currently considered the best alternative to shunt systems in the treatment of hydrocephalus. Many authors have reported that success with ETV in the treatment of hydrocephalus depends largely on appropriate patient selection. Scarrow et al. stated that for the ETV procedure to be successful, candidates must meet two preoperative criteria: significant obstruction of cerebrospinal fluid (CSF) flow between the ventricles and the subarachnoid space, and preservation of normal CSF absorption from the subarachnoid space into the venous system [28]. Several retrospective case reviews have been performed in an attempt to determine which patient groups have the highest chances for success with ETV [3, 13, 17, 28]. One of the controversial issues is the optimal time to perform ETV in the pediatric population. The question whether infants and very young children have a higher risk of treatment failure after ETV than older children is still being debated. Most authors have stated that ETV is significantly more effective in pediatric patients who are older than 2 years of age [7, 21, 22, 26]. In infants, the underdeveloped subarachnoidal space has been proposed to play a role in the failure of the ETV procedure [4, 11, 19]. In a retrospective chart review of 54 patients who had undergone ETV, Scarrow has reported that children over the age of 3 years with acquired CSF obstruction had a significantly greater probability of success with ETV than those 3 and younger [28]. However, some experts advocate attempting ETV as a first line of therapy in infants, despite only a modest success rate in this group [4, 5]. Javadpour et al. suggested that selective use of ETV as the primary treatment for infants with hydrocephalus is safe, and speculated that this approach could reduce the shunted population of all newly diagnosed hydrocephalic infants by up to 21% [19]. In their study, Cinalli et al. have shown that ETV could be successfully performed even in patients less than 6 months old [8], even though this young age was previously considered a contraindication [23]. In their series, Husain et al. have reported that ETV was successful in 61.5% of patients aged between 6 months and 2 years of age [18]. In a recent study Gorayeb et al. [14] reported a success rate of 64% in children younger than 1 year of age, who have undergone ETV for obstructive hydrocephalus. The authors advocated the use of ETV, when appropriate, regardless of age younger than 1 year.
The overall success rate of ETV in patients under 2 years of age in our series is 56%. The analysis of the results in subgroups with different etiologies of the hydrocephalus showed that the success rates differed remarkably between different etiologies of hydrocephalus (Table 1).
In our series, two of the four patients who had had meningitis/ventriculitis and two of the three patients who had had intraventricular hemorrhage required no shunting or showed improvement after their initial treatment with ETV. Many authorities consider patients with a history of CSF infection or intraventricular/subarachnoid hemorrhage to be prone to ETV failure [12, 26, 34]. Whether ETV is a viable alternative for treating this group of hydrocephalic patients remains a matter of debate. A number of reports have indicated that ETV is, to a certain extent, also successful in this group [15, 32]. In a multicenter retrospective study, Siomin et al. [31] suggested that ETV might play a significant role in the treatment of patients with a history of either infection or hemorrhage, and documented efficacy comparable to more general series of patients with obstructive hydrocephalus.
In patients with posthemorrhagic hydrocephalus, which is usually not even considered as being an indication for ETV, the success rate in our cohort was 66.6%. All the cases of posthemorrhagic hydrocephalus in our study group were diagnosed as non-communicating hydrocephalus because during imaging studies of flow dynamics, the site of obstruction seemed to be the aqueduct. Hydrocephalus due to acquired mechanical obstruction of the Sylvian aqueduct has been reported to occur later in the evolution of intraventricular hemorrhage in preterm infants and ETV has been advocated in such patients [29]. ETV procedure was successful in two of our patients. Based on the satisfactory results in these two cases, our experience seems to support the suggestion of ETV in posthemorrhagic non-communicating hydrocephalus in infants.
In the category of infection related hydrocephalus we had four patients in whom radiological examination showed a triventricular hydrocephalus indicating an obstructive component secondary to the history of CSF infection. The success rate of ETV in this group of patients was 50%.
Although the number of the cases is small, our results seem to support the conclusions of Siomin et al., who have mentioned that ETV might play a significant role in the treatment of patients with a history of either infection or hemorrhage [31]. Several theories have been proposed to explain the success of ETV in such patients [17, 31]. One hypothesis is that a combination of obstruction and impaired CSF reabsorption is responsible for hydrocephalus. If so, ETV might enable access to areas of CSF reabsorption that were previously inaccessible and possibly not impaired. A second theory is that the subarachnoid space is capable of developing and adapting to altered CSF dynamics, ultimately leading to increased CSF reabsorption. A third proposal is that the CSF absorption system either recovers with time, or may remain patent despite insults of bleeding or infection. Another theory is that the sequence of fibrosis and thickening of leptomeninges and consequent obliteration of the subarachnoid may be reversible. These possible mechanisms may constitute an explanation for the sucess of ETV in this group of our patients.
Endoscopic third ventriculostomy was previously thought to be contraindicated in patients with reduced CSF reabsorption capacity, which may be seen in hydrocephalus associated with spinal dysraphism [34]. Numerous authors have reported that ETV was not successful in hydrocephalus accompanied by open neural tube closure defects, and they stated that these results were related to the abnormality in CSF flow pathways and/or the deformed anatomy of the ventricular system in these patients. However, it has been shown that there exists sufficient CSF flow in convexity subarachnoidal area of patients in whom hydrocephalus is associated with myelomeningocele [32]. Also, ETV success rates of more than 70% have been reported in these patients. [23, 32]. Our results showed that ETV was successful in 5 (41.6%) of 12 children with myelomeningocele. Although the success rate obtained in this group is not as high as the rates reported by Teo and Jones [32], it seems more favorable when compared with the results of Beems and Grotenhuis, who reported a success rate of 21% in myelomeningocele patients [2]. We believe that the success rate of 41% in myelomeningocele patients is compromising, and we suggest that offering ETV to these patients is worthwhile. However, the surgeon must keep in mind that myelodysplastic patients frequently exhibit intraventricular anatomical variations.
The technique of ETV procedure is quite safe in experienced hands, if the intraventricular anatomical landmarks are easily identified. However, when anatomical landmarks are not clearly identified, the ETV technique may become quite difficult and full of risks [6, 25]. In hydrocephalus accompanying open spinal dysraphism, the distorted anatomical landmarks may lead to difficulties in navigation during the endoscopic procedure. Also, one may encounter several situations that make the ETV technique demanding and increase the risk of complications. Even the ETV procedure may fail due to these problems at the time of operation. In our experience with these patients, anatomical variations and abnormalities such as narrow and hypervascular entry of the foramen of Monro, thick and prominent massa intermedia, the anomalies of the third ventricle floor, small anterior chamber of the third ventricle or shallow prepontine cystern has led the ETV technique to become difficult and dangerous. We also experienced two cases in which we had no chance to perform ventriculostomy due to anatomical abnormalities. We suggest that one must carefully examine the cranial magnetic resonance imaging studies and should try to observe the possible anatomical variations and abnormalities of the ventricular system and the prepontine cystern before attempting ETV procedure in children with neural tube closure defects. Our experience indicates that ETV procedure in myelodysplastic patients may become a demanding procedure, and thus cannot be recommended to neuroendoscopy beginners.
We encountered no permanent morbidity and no mortality in our ETV procedures. The potential complications of ETV procedure are more serious than those linked to shunt surgery, but a high level of neuroendoscopic expertise minimizes the risk of death and severe morbidity with ETV. Overall, there is much greater potential for serious morbidity and mortality with shunting than with ETV.
The overall success rate is much lower in this group (56%) than in the total group of 97 patients treated using ETV in our department, with an overall success rate of 76%. When the overall success rate is interpreted alone, our results seem to confirm the less favorable outlook for ETV in this age group often cited in literature. However, the success rate in patients with primary and secondary aqueductal stenosis in this age group is as excellent as in older patients. This result is in accordance with the reported data of Beems and Grotenhuis, who have also concluded that the success of TV depends mainly on the etiology of the hydrocephalus [2].
Hydrocephalic pediatric patients benefit the most from ETV because they have the longest time to live with the disease and experience the most complications and shunt revisions [17]. Patient selection seems to be the key in achieving the satisfactory outcome following ETV for this age group. Our data seem to support the results of the recent studies which suggested that success with ETV is etiology-related, not age-dependent [2, 8, 14, 19].
Conclusion
Our experience indicates that there are no grounds for denying children younger than 2 years the chance for a shunt-free life. The results of our study may constitute a support for increasing number of centers which offer ETV to these patients as the first choice of treatment in spite of controversial success rates. We believe that offering ETV to these patients is worthwhile, which means that this group of patients may able to continue life shunt-free when this chance is given.
References
Alvarez JA, Cohen AR (1998) Neonatal applications of neuroendoscopy. Neurosurg Clin N Am 9:405–413
Beems T, Grotenhuis JA (2002) Is the success rate of endoscopic third ventriculostomy age-dependent? An analysis of the results of endoscopic third ventriculostomy in young children. Childs Nerv Syst 18:605–608
Brockmeyer D, Abtin K, Carey L, Walker ML (1998) Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg 28:236–240
Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M (1998) Neuroendoscopic third ventriculostomy in patients less than 1 year old. Pediatr Neurosurg 29:73–76
Buxton N, Macarthur D, Mallucci C, Punt J, Vloeberghs M (1998) Neuroendoscopy in the premature population. Childs Nerv Syst 14:649–652
Cartmill M, Jaspan T, McConachie N, Vloeberghs M (2001) Neuroendoscopic third ventriculostomy in dysmorphic brains. Childs Nerv Syst 17:391–394
Cinalli G (1999) Alternatives to shunting. Childs Nerv Syst 15:718–731
Cinalli G, Sainte-Rose C, Chumas P, Zerah M, Brunelle F, Lot G, Pierre-Khan A, Renier D (1999) Failure of third ventriculostomy in the treatment of aqueductal stenosis in children. J Neurosurg 90:448–454
Cohen AR (1994) Endoscopic ventricular surgery. Pediatr Neurosurg 19:127–134
Cohen AR (1994) Ventriculoscopic surgery. Clin Neurosurg 41:546–562
Fritsch MJ, Mehdorn M (2002) Endoscopic intraventricular surgery for treatment of hydrocephalus and loculated CSF space in children less than one year of age. Pediatr Neurosurg 36:183–188
Fukuhara T, Vorster SJ, Luciano MG (2000) Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery 46:1100–1109
Gangemi M, Donati P, Maiuri F, Longatti P, Godano U, Mascari C (1999) Endoscopic third ventriculostomy for hydrocephalus. Minim Invasive Neurosurg 42:128–132
Gorayeb RP, Cavalheiro S, Zymberg ST (2004) Endoscopic third ventriculostomy in children younger than 1 year of age. J Neurosurg (Pediatrics 5) 100:427–429
Grant JA, McLone DG (1997) Third ventriculostomy: a review. Surg Neurol 47:210–212
Hirsch JF (1982) Percutaneous ventriculocisternostomies in noncommunicating hydrocephalus. Monogr Neurol Sci 8:170–178
Hopf NJ, Grunert P, Fries G, Resch KD, Perneczky A (1999) Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery 44:795–804
Husain M, Jha D, Vatsal DK, Thaman D, Gupta A, Husain N, Gupta RK (2003) Neuro-endoscopic surgery-experience and outcome analysis of 102 consecutive procedures in a busy neurosurgical centre of India. Acta Neurochir 145:369–376
Javadpour M, Mallucci C, Brodbelt A, Golash A, May P (2001) The impact of endoscopic third ventriculostomy on the management of newly diagnosed hydrocephalus in infants. Pediatr Neurosurg 35:131–135
Jones RF, Stening WA, Brydon M (1990) Endoscopic third ventriculostomy. Neurosurgery 26:86–92
Jones RF, Kwok BC, Stening WA, Vonau M (1994) Neuroendoscopic third ventriculostomy: a practical alternative to extracranial shunts in non-communicating hydrocephalus. Acta Neurochir Suppl (Wien) 61:79–83
Jones RF, Kwok BC, Stening WA, Vonau M (1994) The current status of endoscopic third ventriculostomy in the management of non-communicating hydrocephalus. Minim Invasive Neurosurg 37:28–36
Jones RF, Kwok BC, Stening WA, Vonau M (1996) Third ventriculostomy for patients with spinal dysraphism: indications and contraindications. Eur J Pediatr Surg 6(Suppl 1):5–6
Kunz U, Goldman A, Bader C, Waldbauer H, Oldenkott P (1994) Endoscopic fenestration of the 3rd ventricular floor in aqueductal stenosis. Minim Invasive Neurosurg 37:42–47
Morota N, Watabe T, Inukai T, Hongo K, Nakagawa H (2000) Anatomical variants in the floor of the third ventricle; implications for endoscopic third ventriculostomy. J Neurol Neurosurg Psychiatry 69:531–534
Sainte-Rose C (1992) Third ventriculostomy. In: Manwaring KH, Crone KR (eds) Neuroendoscopy, vol 1. Liebert, New York, pp 47–62
Sainte-Rose C, Chumas P (1996) Endoscopic third ventriculostomy. Tech Neurosurg 1:176–184
Scarrow AM, Levy EI, Pascucci L, Albright AL (2000) Outcome analysis of endoscopic III ventriculostomy. Childs Nerv Syst 16:442–445
Scavarda D, Bednarek N, Litre F, Koch C, Lena G, Morville P, Rousseaux P (2003) Acquired aqueductal stenosis in preterm infants: an indication for neuroendoscopic third ventriculostomy. Childs Nerv Syst 19:756–759
Schroeder HW, Niendorf WR, Gaab MR (2002) Complications of endoscopic third ventriculostomy. J Neurosurg 96:1031–1040
Siomin V, Cinalli G, Grotenhuis A, Golash A, Oi S, Kothbauer K, Weiner H, Roth J, Beni-Adani L, Pierre-Kahn A, Takahashi Y, Mallucci C, Abbott R, Wisoff J, Constantini S (2002) Endoscopic third ventriculostomy in patients with cerebrospinal fluid infection and/or hemorrhage. J Neurosurg 97:519–524
Teo C, Jones R (1996) Management of hydrocephalus by endoscopic third ventriculostomy in patients with myelomeningocele. Pediatr Neurosurg 25:57–63
Teo C (1998) Third ventriculostomy in the treatment of hydrocephalus: experience with more than 120 cases. In: Hellwig D, Bauer BL (eds) Minimally invasive techniques for neurosurgery. Springer, Berlin Heidelberg New York, pp 73–76
Vries JK, Friedmann WA (1980) A quantitative assessment of CSF re-absorption in infants with meningomyelocele. Surg Neurol 13:38–40
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Etus, V., Ceylan, S. Success of endoscopic third ventriculostomy in children less than 2 years of age. Neurosurg Rev 28, 284–288 (2005). https://doi.org/10.1007/s10143-005-0407-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-005-0407-4