Abstract
Irregular protrusion on optical coherence tomography (OCT) is associated with clinical events and target lesion revascularization. We investigated clinical and procedure characteristics, plaque characteristics, slow flow after stent implantation, and clinical outcomes with irregular protrusion using OCT. Eighty-four lesions in 76 patients undergoing OCT before percutaneous coronary intervention were evaluated. Irregular protrusion was defined as protrusion of material with an irregular surface into the lumen between stent struts with a maximum height of ≥100 μm. Lesions with irregular protrusion were found in 56% (47/84). Compared with lesions without irregular protrusion, those with irregular protrusion had significantly higher low-density lipoprotein cholesterol (LDL-C) levels (108 ± 31 mg/dl vs. 95 ± 25 mg/dl, P = 0.044); a tendency toward decreased use of statins [44% (19/43) vs. 67% (22/33), P = 0.065]; significantly larger reference vessel diameter (3.12 ± 0.53 mm vs. 2.74 ± 0.63 mm, P = 0.004); more frequent slow flow after stent implantation [38% (18/47) vs. 11% (4/37), P = 0.006]; higher incidence of thin-cap fibroatheromas [TCFAs; 49% (23/47) vs. 5% (2/37), P < 0.001]; plaque rupture [40% (19/47) vs. 16% (6/37), P = 0.018]; and a tendency higher incidence of 1-year adverse clinical outcomes (death, acute myocardial infarction, acute coronary syndrome, or target lesion revascularization) [12% (5/43) vs. 0% (0/33), P = 0.075]. In conclusion, irregular protrusion on OCT was associated with high plaque vulnerability, higher LDL-C, less frequent use of statin, larger vessel diameter, slow flow after stent implantation, and 1-year adverse clinical outcomes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
After implantation of drug-eluting stents (DES) for coronary arteries, late stent restenosis and late stent thrombosis remain significant problems. In pathological studies, penetration of the lipid core into the stent strut is associated with neointimal growth and stent thrombosis [1, 2]. In addition, a large amount of protrusion or irregular protrusion detected by optical coherence tomography (OCT) after stent implantation is associated with clinical events and target lesion revascularization [3, 4]. OCT has high resolution [5], and is the only modality able to detect irregular protrusion. However, there are few reports in the literature regarding the relationship between irregular protrusion and plaque characteristics. In addition, the no-reflow phenomenon is another problem observed during percutaneous coronary intervention (PCI) and is associated with cardiac events [6]. Thin-cap fibroatheroma (TCFA) and lipid-rich plaque on OCT are associated with slow flow during PCI [7, 8]. However, the relationship between irregular protrusion and slow flow during PCI is unknown. Clarifying the factors that cause irregular protrusion is important for avoiding cardiac events and slow flow during PCI, and might, therefore, enable clinicians to improve procedural strategy and medical therapy. The aims of this study are (1) to evaluate the clinical, procedure characteristics, and plaque characteristics of irregular protrusion (2) to identify slow flow during PCI of irregular protrusion, and (3) to investigate the clinical outcomes during follow-up period of irregular protrusion.
Methods
Patient population
Between February 2012 and June 2018, we performed PCI for 1443 lesions in 1081 patients for de novo coronary stenosis at Toho University Faculty of Medicine. The present study consecutively enrolled 110 lesions in 100 patients treated by PCI for de novo coronary stenosis that underwent OCT examinations before and after PCI. The exclusion criteria were as follows: (1) left main trunk disease (2) chronic total occlusion (3) cardiogenic shock (4) tortuous or calcified vessels with expected difficulty in advancing the OCT catheter (5) large vessel expected limitation in OCT imaging (6) the presence of large amounts of thrombus (7) congestive heart failure with left ventricular ejection fraction < 40%, and (8) renal insufficiency with baseline serum creatinine > 1.5 mg/dl. This study was performed in accordance with the Code of Federal Regulations and the Declaration of Helsinki. This protocol was approved by the Toho University Omori Medical Center Ethics Committee (institutional review board approval number 25-97). Written informed consent was obtained from each patient before the study.
Non-ST-elevation acute coronary syndromes included non-ST-elevation myocardial infarction (NSTEMI) and unstable angina pectoris (UAP). NSTEMI was defined as ischemic symptoms with elevated troponin level and myocardial ischemia diagnosed by ST-T segment shift [9]. UAP was defined as new-onset angina, progressive angina, or angina at rest within 2 weeks. In patients with stable angina pectoris, the culprit vessel was considered to be the ischemia-related vessel identified by exercise or pharmacologic stress test. Dyslipidemia was defined as low-density lipoprotein cholesterol ≥ 140 mg/dl, high-density lipoprotein cholesterol < 40 mg/dl, triglycerides ≥ 150 mg/dl, or medication use. Follow-up period of clinical outcomes was 12 months. The end of follow-up in the present study was December 2018. Major adverse clinical outcomes were defined as death, acute myocardial infarction, acute coronary syndrome, or target lesion revascularization.
PCI procedure
All patients received aspirin (100 mg/day), and clopidogrel (75 mg/day) or prasugrel (3.75 mg/day, approved daily dose in Japan) before PCI. Intravenous heparin (100 U/kg) and intracoronary nitrates were administered at the beginning of the procedure. After initial angiography, OCT was performed. All patients underwent coronary stent implantation with pre-dilatation. Thrombolysis in myocardial infarction (TIMI) flow grade was assessed as described previously [10]. The angiographic slow flow was defined as a decrease of at least 1 grade in TIMI flow immediately after stent implantation compared with before stent implantation or final TIMI flow grade 0, 1 or 2, with no evidence of thrombus, spasm, or dissection. Platelet glycoprotein IIb/IIIa receptor inhibitors were not used because they are not available in Japan. The stents with a strut thickness < 100 μm were classified as thin, whereas stents with a strut thickness ≥ 100 μm were classified as thick [11].
OCT procedure and analysis
The frequency-domain OCT system (C7-XR, OCT Intravascular Imaging System; St Jude Medical, St Paul, MN, USA) was used. Before PCI, a 2.7F OCT imaging catheter (Dragonfly; LightLab Imaging, Inc, Westford, MA, USA) was advanced distal to the lesion, and automated pullback was performed with blood clearance by injection of contrast medium or dextran. The OCT data were stored digitally and analyzed using the ILUMIEN OCT imaging system (St Jude Medical). Off-line analyzes were performed by two observers blinded to the patients’ clinical data. Any discrepancies between the observers were resolved by consensus. For quantitative evaluation, cross-sectional OCT images were analyzed for the following parameters: the minimal lumen cross-sectional area, reference area (within 5 mm of the proximal and distal edges), and the minimal and maximal stent area.
Qualitative analysis was performed as described previous studies [12, 13]. Lipid plaque was defined as a low-signal region with diffuse border. Lipid-rich plaque was defined as a plaque included in a lipid arc with one or more quadrants. TCFA was defined as lipid-rich plaque with fibrous cap thickness < 65 μm. Plaque rupture was defined as the presence of a fibrous cap discontinuity and a cavity formation in the plaque. Thrombus was defined as a mass attached to the luminal surface or floating within the lumen. Macrophage accumulation was defined as high-intensity and signal-rich linear regions with sharp attenuation (Fig. 1). Intraplaque neovessel was defined as a small black hole or a tubular structure within a plaque. Internal running vasa vasorum was defined as intraplaque neovessels running from the adventitia to plaque without a connection to the vessel lumen recognized on three consecutive cross-sectional OCT images (Fig. 1) [14]. For qualitative analysis of post-PCI, all cross-sectional images within the entire stent length and 5-mm persistent segments were analyzed. Irregular protrusion was defined as protrusion of material with an irregular surface into the lumen between stent struts with a maximum height of ≥ 100 μm (Figs. 1, 2) [3, 15].
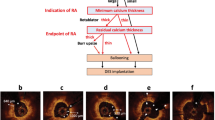
Representative optical coherence tomography images of internal running vasa vasorum, macrophage accumulation and irregular protrusion. Cross-sectional optical coherence tomography images in the right coronary artery (a–d). a–c Pre-stent consecutive images. Multiple internal running vasa vasorum (white arrows) are located at 2 o’clock position. The intraplaque neovessels are running from the adventitia to plaque. d Pre-stent consecutive image. Macrophage accumulation (white arrow) is located at 2 o’clock position. e Post-stent image. Irregular protrusion is seen at 7 o’clock (white arrowhead). The lesion caused slow flow after stent implantation
Representative optical coherence tomography images of ruptured plaque, macrophage accumulation and irregular protrusion. Cross-sectional optical coherence tomography images in the right coronary artery (a, b). a Pre-stent image. The culprit lesion has a ruptured plaque (white arrow). b Pre-stent image. Macrophage accumulation (white arrow) is located at 11 o’clock position. c Post-stent image. Irregular protrusion is seen between 3 and 6 o’clock (white arrowheads). The lesion caused slow flow after stent implantation
Statistical analysis
Statistical analysis was performed using SPSS version 24.0 (SPSS, Inc., Chicago, IL, USA). Continuous data were expressed as mean ± SD. Categorical data were presented as number (percentage). The normality of the data was verified by the Kolmogorov–Smirnov test. Continuous data were compared using unpaired Student’s t test for normally distributed values, or Mann–Whitney U test for non-normally distributed values. Categorical variables were compared with the Chi-square test or Fisher’s exact test. The change of TIMI flow from pre-stenting to post-stenting was evaluated by Wilcoxon’s matched-pair signed-rank test. The Kaplan–Meier method was used for building event curves, and the event-free curves of the groups were compared by the log-rank test. Independent predictors of irregular protrusion were identified by multivariable logistic regression and expressed as odds ratios and their 95% confidence intervals (CI). We selected the variables with a P value < 0.05 in univariate models and included them in multivariable models. P values of < 0.05 were considered statistically significant.
Results
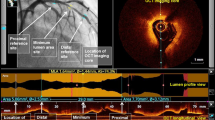
We excluded cases as follows: (1) vein graft lesions (n = 1) (2) large vessel (n = 5), and (3) poor OCT images (n = 20). Finally, we analyzed 84 lesions in 76 patients for this study. Lesions with irregular protrusion were found in 56% (47/84). The clinical and procedure characteristics are shown in Table 1. There were no significant differences between the two groups with regard to age, gender, coronary risk factors, clinical presentation, renal function, hemoglobin A1c, regimen of antiplatelet drug, or distribution of the culprit coronary artery. Compared with patients without irregular protrusion, those with irregular protrusion showed significantly higher low-density lipoprotein cholesterol (LDL-C) levels (108 ± 31 mg/dl vs. 95 ± 25 mg/dl, P = 0.044) and a tendency toward lower use of statins [44% (19/43) vs. 67% (22/33), P = 0.065]. Angiographic and procedure characteristics are shown in Table 2. Compared with lesions without irregular protrusion, those with irregular protrusion showed significantly larger reference vessel diameter (3.12 ± 0.53 mm vs. 2.74 ± 0.63 mm, P = 0.004), significantly larger stent diameter (3.23 ± 0.43 mm vs. 3.00 ± 0.49 mm, P = 0.025), a tendency toward longer total stent length (29.3 ± 14.2 mm vs. 23.7 ± 11.4 mm, P = 0.056), significantly larger maximum balloon diameter (3.56 ± 0.55 mm vs. 3.22 ± 0.63 mm, P = 0.010), significantly more frequent slow flow after stent implantation [38% (18/47) vs. 11% (4/37), P = 0.006]. In lesions with irregular protrusion, TIMI flow was significantly shifted from 3.0 ± 0.2 before stenting to 2.5 ± 0.7 after stenting (P < 0.001; Fig. 3). ΔTIMI flow from pre-stenting to post-stenting was significantly higher in lesions with irregular protrusion than in lesions without irregular protrusion (0.4 ± 0.6 vs. 0.1 ± 0.3, P = 0.009). OCT findings are shown in Table 3. Compared with lesions without irregular protrusion, those with irregular protrusion showed a significantly higher incidence of lipid-rich plaque [70% (33/47) vs. 35% (13/37), P = 0.002], TCFAs [49% (23/47) vs. 5% (2/37), P < 0.001], plaque rupture [40% (19/47) vs. 16% (6/37), P = 0.018], macrophage accumulation [51% (24/47) vs. 24% (9/37), P = 0.015], internal running vasa vasorum [51% (24/47) vs. 11% (4/37), P < 0.001], and thrombus [32% (15/47) vs. 3% (1/37), P < 0.001]. One-year clinical outcomes were shown in Table 4 and Fig. 4. Compared with patients without irregular protrusion, those with irregular protrusion showed a tendency higher incidence of major adverse clinical outcomes [12% (5/43) vs. 0% (0/33), P = 0.075]. Angiographic, procedural and optical coherence tomography predictors of irregular protrusion by univariate and multivariable logistic regression shown in Table 5. The multivariable analysis showed that TCFA was an independent predictor of irregular protrusion (odds ratio 9.00, 95% CI 1.32–61.36, P = 0.025).
(I) Change of TIMI flow from pre-stenting to post-stenting. (1) Irregular protrusion (+). (2) Irregular protrusion (−). (II) Comparison of ΔTIMI flow from pre-stenting to post-stenting according to the presence of irregular protrusion. ΔTIMI flow is significantly higher in lesions with irregular protrusion than in lesions without irregular protrusion (0.4 ± 0.6 vs. 0.1 ± 0.3, P = 0.009)
Kaplan–Meier curves of the major adverse clinical outcomes during the follow-up period according to the presence or absence of irregular protrusion. Compared with patients without irregular protrusion, those with irregular protrusion showed a tendency higher incidence of major adverse clinical outcomes [12% (5/43) vs. 0% (0/33), P = 0.075]
The representative OCT images in a case with irregular protrusion are shown in Figs. 1, 2.
Discussion
Relationship between irregular protrusion and clinical characteristics
Patients who had lesions with irregular protrusion had higher LDL-C levels and lower use of statins. Previous OCT study reported that plaque protrusion is associated with higher LDL-C levels [16]. However, there are few reports regarding the relation between irregular protrusion and use of statins. LDL-C levels are associated with plaque burden, major cardiac events, and plaque vulnerability [17,18,19]. Large plaque burden and vulnerable plaque are associated with a high incidence of plaque protrusion [16, 20]. In our study, patients who had lesions with irregular protrusion had larger reference vessel diameters and more TCFAs; therefore, it is thought that LDL-C was associated with irregular protrusion. Pathological studies reported that stent strut penetration into a lipid core is associated with increased neointimal growth and inflammation of the lesions [1]. Statins improve LDL-C levels and furthermore stabilize lesion inflammation. Intravascular ultrasound (IVUS), OCT, and angioscopy studies reported that the decrease in serum LDL-C levels with statin therapy is associated with de novo coronary plaque volume reduction and stabilization of vulnerable plaques [21,22,23]. Therefore, it is thought that irregular protrusion was associated with the use of statin in our study. Medical therapy with statins before stent implantation may reduce irregular protrusion.
Relationship between irregular protrusion and procedure characteristics
Lesions with irregular protrusion also had larger reference vessel diameter, larger stent diameter, longer total stent length, and larger maximum balloon diameter. It has been reported that a larger reference vessel diameter, larger stent diameter, and longer stenting are associated with plaque protrusion on IVUS and OCT [15, 16, 20]. A larger reference vessel diameter and long lesion contain a higher plaque burden. Lesions with a higher plaque burden need to dilate by larger balloon and stent and hence cause deeper vessel wall injury and thrombus. It was reported that more aggressive stent dilatation is prone to plaque protrusion [24]. In addition, another study reported that long stenting induces uneven distribution of inflation pressure, which leads to plaque protrusion [20]. It was reported that plaque protrusion is associated with stent strut thickness [24]. However, there were no significant differences in strut thickness in our study. Almost all stents used in our study had a thin strut.
Relationship between irregular protrusion and plaque characteristics
In our study, patients with lesions with irregular protrusion showed a higher incidence of lipid-rich plaque, TCFAs, macrophage accumulation, internal running vasa vasorum, thrombus, and plaque rupture. In addition, TCFA was an independent predictor of irregular protrusion. It is thought that the first mechanism of irregular protrusion is thrombus, which is originally present in the lumen and penetrates through the stent strut. The second mechanism of irregular protrusion is penetration of the lipid or necrotic core, and the third mechanism is breakage of the fibrous cap and penetration of the necrotic core leading to thrombus [1, 2, 25]. An OCT study reported that irregular protrusion was associated with lipid-rich plaque, TCFA, and thrombus [15]. Our study is consistent with previous studies. Pathological studies reported that necrotic core protrusion or lipid core penetration is associated with early stent thrombosis or in-stent restenosis [1, 2]. Medial damage or penetration of the stent into a lipid core induces arterial inflammation and hence increases neointimal growth. A strategy to reduce irregular plaque protrusion might be needed to avoid stent thrombosis and restenosis.
Relationship between irregular protrusion and slow flow after stent implantation
Slow flow after stent implantation frequently occurred in lesions with irregular protrusion. It is reported that tissue protrusion on OCT is associated with the no-reflow phenomenon [16]. However, the relationship between irregular protrusion and slow flow during PCI has not been described. Lesions with irregular protrusion showed large reference vessel and stent diameter as well as long stent length; therefore, it is thought that lesions with irregular protrusion have a large volume of plaque. IVUS studies have reported that larger plaque volume and reduction of plaque volume between pre- and post-PCI are associated with slow flow during PCI [26, 27]. Lesions with irregular protrusion had a large number of TCFAs, lipid-rich plaque, and internal running vasa vasorum on OCT. TCFA, large volume of lipid plaque, and internal running vasa vasorum on OCT are frequently observed in lesions with no reflow phenomenon during PCI [7, 8, 28]. It has been reported that TCFA has a large necrotic core, and pathological studies have shown the necrotic core to contain a lipid deposition with foam cells, intramural bleeding, cholesterol crystals, and microcalcifications [29], which easily cause microembolization by mechanical destruction of balloon dilatation. In addition, Virmani et al. reported that ruptured vasa vasorum form intraplaque hemorrhages [30]. Balloon dilatation might cause the iatrogenic creation of vasa vasorum rupture and formation intraplaque hemorrhages. If the plaque characteristics of the protrusion on OCT are evaluated, the material of the embolization might be predicted, allowing us to choose the strategy for the slow flow (e.g., distal protection, antithrombotic agent, or statin before PCI).
Relationship between irregular protrusion and clinical outcome
Patients with irregular protrusion showed a tendency higher incidence of major adverse clinical outcomes. There are several reports about the relation between plaque protrusion and clinical outcomes. In some OCT studies, there are no significant relationships between plaque protrusion and clinical events [16, 31]. The definitions of plaque protrusion in these reports are different from our report. Irregular protrusion shows more severe vessel injury than smooth protrusion. Therefore, irregular protrusion might influence clinical events in our study. An OCT study reports that irregular protrusion is associated with major adverse cardiac events [3]. This finding is consistent with our study. Penetration of the lipid core into the stent strut is associated with neointimal growth and stent thrombosis in pathological studies [1, 2]. Therefore, irregular protrusion might increase the risk for stent restenosis. In addition, lesions with irregular protrusion had more vulnerable plaques in our study. In a virtual histology IVUS report, TCFAs is associated with major adverse cardiac events during follow-up period [32]. TCFAs might occur plaque rupture and develop acute coronary syndrome or acute myocardial infarction. Because irregular protrusion had adverse clinical outcomes, a strategy to reduce irregular protrusion might be needed.
Limitations
There are several limitations of our analysis. First, this was a retrospective and single-center study that enrolled a limited number of patients who were able to undergo OCT evaluation. Second, the treatment strategy and the OCT procedure were performed at the discretion of the physician; therefore, a potential selection bias exists. Third, our classification of irregular protrusion in this study is novel, and we have not investigated the correlation between irregular protrusion on OCT and histopathologic findings. It is necessary to study the consistency between OCT findings and pathology.
Conclusions
Irregular protrusion on OCT was associated with high plaque vulnerability, higher LDL-C, less frequent use of statin, larger vessel diameter, slow flow after stent implantation, and 1-year adverse clinical outcomes.
References
Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R (2002) Morphological predictors of restenosis after coronary stenting in humans. Circulation 105:2974–2980
Nakano M, Yahagi K, Otsuka F, Sakakura K, Finn AV, Kutys R, Ladich E, Fowler DR, Joner M, Virmani R (2014) Causes of early stent thrombosis in patients presenting with acute coronary syndrome: an ex vivo human autopsy study. J Am Coll Cardiol 63:2510–2520
Soeda T, Uemura S, Park SJ, Jang Y, Lee S, Cho JM, Kim SJ, Vergallo R, Minami Y, Ong DS, Gao L, Lee H, Zhang S, Yu B, Saito Y, Jang IK (2015) Incidence and clinical significance of poststent optical coherence tomography findings: one-year follow-up study from a multicenter registry. Circulation 132:1020–1029
Sugiyama T, Kimura S, Ohtani H, Hishikari K, Kojima K, Sagawa Y, Hayasaka K, Mizusawa M, Misawa T, Yamakami Y, Hikita H, Takahashi A, Isobe M (2017) Relationship between quantities of tissue prolapse after percutaneous coronary intervention and neointimal hyperplasia at follow-up on serial optical coherence tomography examination. Int J Cardiol 241:470–477
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA (1991) Optical coherence tomography. Science 254:1178–1181
Niccoli G, Burzotta F, Galiuto L, Crea F (2009) Myocardial no-reflow in humans. J Am Coll Cardiol 54:281–292
Tanaka A, Imanishi T, Kitabata H, Kubo T, Takarada S, Tanimoto T, Kuroi A, Tsujioka H, Ikejima H, Komukai K, Kataiwa H, Okouchi K, Kashiwaghi M, Ishibashi K, Matsumoto H, Takemoto K, Nakamura N, Hirata K, Mizukoshi M, Akasaka T (2009) Lipid-rich plaque and myocardial perfusion after successful stenting in patients with non-ST-segment elevation acute coronary syndrome: an optical coherence tomography study. Eur Heart J 30:1348–1355
Gamou T, Sakata K, Matsubara T, Yasuda T, Miwa K, Inoue M, Kanaya H, Konno T, Hayashi K, Kawashiri M, Yamagishi M (2015) Impact of thin-cap fibroatheroma on predicting deteriorated coronary flow during interventional procedures in acute as well as stable coronary syndromes: insights from optical coherence tomography analysis. Heart Vessels 30:719–727
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J 37:267–315
The TIMI Study Group (1985) The thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med 312:932–936
Kitabata H, Kubo T, Komukai K, Ishibashi K, Tanimoto T, Ino Y, Takarada S, Ozaki Y, Kashiwagi M, Orii M, Shiono Y, Shimamura K, Hirata K, Tanaka A, Kimura K, Mizukoshi M, Imanishi T, Akasaka T (2012) Effect of strut thickness on neointimal atherosclerotic change over an extended follow-up period (≥ 4 years) after bare-metal stent implantation: intracoronary optical coherence tomography examination. Am Heart J 163:608–616
Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW (2010) Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J 31:401–415
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia-Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Räber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G (2012) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 59:1058–1072
Taruya A, Tanaka A, Nishiguchi T, Matsuo Y, Ozaki Y, Kashiwagi M, Shiono Y, Orii M, Yamano T, Ino Y, Hirata K, Kubo T, Akasaka T (2015) Vasa vasorum restructuring in human atherosclerotic plaque vulnerability: a clinical optical coherence tomography study. J Am Coll Cardiol 65:2469–2477
Bryniarski KL, Tahk SJ, Choi SY, Soeda T, Higuma T, Yamamoto E, Xing L, Dai J, Zanchin T, Lee H, Jang IK (2017) Clinical, angiographic, IVUS, and OCT predictors for irregular protrusion after coronary stenting. EuroIntervention 12:e2204–e2211
Sugiyama T, Kimura S, Akiyama D, Hishikari K, Kawaguchi N, Kamiishi T, Hikita H, Takahashi A, Isobe M (2014) Quantitative assessment of tissue prolapse on optical coherence tomography and its relation to underlying plaque morphologies and clinical outcome in patients with elective stent implantation. Int J Cardiol 176:182–190
Dohi T, Maehara A, Moreno PR, Baber U, Kovacic JC, Limaye AM, Ali ZA, Sweeny JM, Mehran R, Dangas GD, Xu K, Sharma SK, Mintz GS, Kini AS (2015) The relationship among extent of lipid-rich plaque, lesion characteristics, and plaque progression/regression in patients with coronary artery disease: a serial nearinfrared spectroscopy and intravascular ultrasound study. Eur Heart J Cardiovasc Imaging 16:81–87
Nasu K, Terashima M, Habara M, Ko E, Ito T, Yokota D, Ishizuka S, Kurita T, Kimura M, Kinoshita Y, Asakura Y, Tsuchikane E, Katoh O, Suzuki T (2013) Impact of cholesterol metabolism on coronary plaque vulnerability of target vessels: a combined analysis of virtual histology intravascular ultrasound and optical coherence tomography. JACC Cardiovasc Interv 6:746–755
Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM (2015) Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 372:2387–2397
Hong YJ, Jeong MH, Ahn Y, Sim DS, Chung JW, Cho JS, Yoon NS, Yoon HJ, Moon JY, Kim KH, Park HW, Kim JH, Cho JG, Park JC, Kang JC (2008) Plaque prolapse after stent implantation in patients with acute myocardial infarction: an intravascular ultrasound analysis. JACC Cardiovasc Imaging 1:489–497
Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, Daida H, Matsuzaki M (2009) Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome. A multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN-ACS [Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome] Study) Clinical Trials. J Am Coll Cardiol 54:293–302
Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, Okumoto Y, Shiono Y, Orii M, Shimamura K, Ueno S, Yamano T, Tanimoto T, Ino Y, Yamaguchi T, Kumiko H, Tanaka A, Imanishi T, Akagi H, Akasaka T (2014) Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY-FIT study. J Am Coll Cardiol 64:2207–2217
Takano M, Mizuno K, Yokoyama S, Seimiya K, Ishibashi F, Okamatsu K, Uemura R (2003) Changes in coronary plaque color and morphology by lipid-lowering therapy with atorvastatin: serial evaluation by coronary angioscopy. J Am Coll Cardiol 42:680–686
Kim SW, Mintz GS, Ohlmann P, Hassani SE, Fernandez S, Lu L, Chu WW, Escolar E, Kuchulakanti PK, Weigold G, Pichard AD, Satler LF, Kent KM, Suddath WO, Waksman R, Weissman NJ (2006) Frequency and severity of plaque prolapse within Cypher and Taxus stents as determined by sequential intravascular ultrasound analysis. Am J Cardiol 98:1206–1211
Farb A, Sangiorgi G, Carter AJ, Walley VM, Edwards WD, Schwartz RS, Virmani R (1999) Pathology of acute and chronic coronary stenting in humans. Circulation 99:44–52
Katayama T, Kubo N, Takagi Y, Funayama H, Ikeda N, Ishida T, Hirahara T, Sugawara Y, Yasu T, Kawakami M, Saito M (2006) Relation of atherothrombosis burden and volume detected by intravascular ultrasound to angiographic no-reflow phenomenon during stent implantation in patients with acute myocardial infarction. Am J Cardiol 97:301–304
Sato H, Iida H, Tanaka A, Tanaka H, Shimodouzono S, Uchida E, Kawarabayashi T, Yoshikawa J (2004) The decrease of plaque volume during percutaneous coronary intervention has a negative impact on coronary flow in acute myocardial infarction: a major role of percutaneous coronary intervention-induced embolization. J Am Coll Cardiol 44:300–304
Amano H, Koizumi M, Okubo R, Yabe T, Watanabe I, Saito D, Toda M, Ikeda T (2017) Comparison of coronary intimal plaques by optical coherence tomography in arteries with versus without internal running vasa vasorum. Am J Cardiol 119:1512–1517
Virmani R, Burke AP, Farb A, Kolodgie FD (2006) Pathology of the vulnerable plaque. J Am Coll Cardiol 47:C13–18
Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25:2054–2061
Kume T, Okura H, Miyamoto Y, Yamada R, Saito K, Tamada T, Koyama T, Neishi Y, Hayashida A, Kawamoto T, Yoshida K (2012) Natural history of stent edge dissection, tissue protrusion and incomplete stent apposition detectable only on optical coherence tomography after stent implantation: preliminary observation. Circ J 76:698–703
Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, Parise H, Templin B, White R, Zhang Z, Serruys PW (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any potential conflicts of interest associated with this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amano, H., Noike, R., Saito, D. et al. Plaque characteristics and slow flow during percutaneous coronary intervention of irregular protrusion by optical coherence tomography. Heart Vessels 34, 1076–1085 (2019). https://doi.org/10.1007/s00380-018-01335-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-018-01335-4