Abstract
Optical coherence tomography (OCT)-angiography coregistration during stent implantation may be useful to avoid geographical mismatch and incomplete lesion coverage. Untreated lipid-rich plaque at stent edge is associated with subsequent stent edge restenosis. The present study sought to compare the frequency of untreated lipid-rich plaque at the stent edge between OCT-guided percutaneous coronary intervention (PCI) with and without OCT-angiography coregistration. We investigated 398 patients who underwent OCT-guided stent implantation (n = 198 in the coregistration group, and n = 200 in the no coregistration group). In OCT after PCI, untreated lipid-lich plaque was identified by the maximum lipid arc > 180˚ in the 5-mm stent edge segment. The PCI-targeted lesion characteristics and stent length were not different between the coregistration group and the no coregistration group. The frequency of untreated lipid-rich plaque in either proximal or distal stent edge segment was significantly lower in the coregistration group than in the no coregistration group (16% vs. 26%, P = 0.015). The frequency of stent-edge dissection (5% vs. 6%, P = 0.516) and untreated stenosis (2% vs. 3%, P = 0.724) was low and without significant differences between the two groups. In OCT-guided PCI, the use of OCT-angiography coregistration was associated with a reduced frequency of untreated lipid-rich plaque at stent edges. OCT-angiography coregistration has a positive impact on PCI results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intravascular optical coherence tomography (OCT) is used to guide percutaneous coronary intervention (PCI). Recently, a novel system that enables real-time coregistration of OCT images with angiography has become available in clinical practice [1]. OCT-angiography coregistration can display the exact localization of the acquired OCT cross-section on the angiogram. Using OCT-angiography coregistration, the operator can accurately recognize the planed stent implantation location by OCT on the angiogram [2]. Therefore, the use of OCT-angiography coregistration in the guidance of PCI may potentially reduce longitudinal geographic mismatch between the stent and the target lesion.
In OCT-guided PCI, one of the key criteria for an appropriate stent edge landing zone is the lipid-free segment. Untreated lipid-rich plaque at the stent edge after stenting is a predictor of subsequent stent edge restenosis [3]. When the stent is placed more proximally or distally than planned, the stent edge may land within the lesion. The longitudinal geographic mismatch may increase the frequency of untreated lipid-rich plaque at the stent edge.
The present study sought to compare the frequency of untreated lipid-rich plaque at the stent edge between OCT-guided PCI with and without OCT-angiography coregistration.
Methods
Study population
This is a retrospective single-center study at Wakayama Medical University. The OCT-angiography coregistration system (Fig. 1) became available in our hospital since November 2015. We screened 300 consecutive patients with PCI during the period when the OCT-angiography coregistration system was routinely used (between October 2018 and December 2019) and 300 consecutive patients with PCI during the period when this system was unavailable (between May 2014 and October 2015). We had a total of 531 patients who underwent OCT-guided stent implantation for de novo native coronary artery lesions. Of these, 133 patients (including 23 inadequate OCT images, 22 low molecular weight dextran use for OCT image acquisition, 8 OCT-angiography coregistration failure, 37 diffuse atherosclerosis without appropriate reference segment for stent edge landing, 7 ultra-long [48 mm] stent use, 16 planned multiple overlapping stents, and 20 stenting for ostial lesions) were excluded. Thus, 398 patients (n = 198 in the coregistration group, and n = 200 in the no coregistration group) constituted the final study population. The present study was approved by the institutional review board, and written informed consent was waived by the institutional review board because of the retrospective design of the study. The present study was performed in accordance with the Declaration of Helsinki.
OCT-angiography coregistration. The OCT imaging was performed before stenting. The location corresponding to the displayed OCT cross-sectional view (i.e. the location corresponding to the OCT imaging core) is projected directly onto the angiogram as a small white marker. Proximal and distal reference sites and minimum lumen area site are determined using lumen profile view, and these locations are shown automatically on the angiogram as blue bars and a yellow bar, respectively. OCT = optical coherence tomography.
Patient clinical characteristics
Patient clinical data were collected by reviewing all the available medical records. Patient clinical data included age, sex, hypertension (defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or antihypertensive medication use), diabetes mellitus (defined as hemoglobin A1c ≥ 6.5% or antidiabetic medication use), dyslipidemia (defined as low-density lipoprotein cholesterol ≥ 140 mg/dL or antidyslipidemic medication use), acute myocardial infarction (defined as ischemic discomfort presenting with new ST-T segment changes on the electrocardiogram and elevated troponin levels), angina pectoris (defined as stable or unstable [e.g. resting, new-onset, or increasing] ischemic chest discomfort), PCI-targeted vessel, PCI-targeted lesion location, bifurcation lesion (defined by angiographically identifiable side-branches greater than 2.0 mm in diameter), severe calcification (identified by radiopacities compromising both sides of the arterial lumen), and long disease (defined by a lesion length of ≥ 28 mm on angiogram) [4].
OCT-guided PCI
OCT was performed using ILUMIEN OPTIS (Abbott Vascular, Santa Clara, California, USA). Intracoronary isosorbide dinitrate (2 to 3 mg) was administered before the OCT procedure. An OCT image catheter was inserted into distal coronary artery over a 0.014-inch conventional angioplasty guidewire. To remove blood from the coronary artery, contrast media was flushed through the guiding catheter at a rate of 2–4 mL/s for approximately 3–6 s using an injector pump. The blood-free OCT images were acquired while the OCT imaging core was withdrawn at a rate of 18 or 36 mm/s using an automated pullback device.
The PCI-targeted lesion was evaluated by OCT before stenting. Pre-dilatation and/or thrombus aspiration before the OCT imaging were performed in the total or subtotal occlusion. OCT-guided stent implantation was performed according to local standard practice [4]. Proximal and distal reference sites adjacent to the lesion were determined at coronary segments with normal-looking lumen and no lipid-rich plaque (defined below). Stent length was determined by measuring distance from distal to proximal reference site. Stent diameter was determined to be 0–0.25 mm larger than distal reference lumen diameter. Goal of stent optimization included minimum stent area greater than 90% of the average (proximal and distal) reference lumen area. All operators were skilled in the OCT-guided stent implantation, and at least one experienced OCT specialist helped operators during the procedure. Stents included 239 XIENCE (Abbott Vascular, Santa Clara, California, USA), 87 SYNERGY (Boston Scientific, Natick, Massachusetts, USA), 36 NOBORI (Terumo, Tokyo, Japan), 26 ULTIMASTER (Terumo, Tokyo, Japan), 3 RESOLUTE (Medtronic, Santa Rosa, California, USA), 2 ORSIRO (Biotronik, Bülach, Switzerland), or 5 VISION (Abbott Vascular, Santa Clara, California, USA).
In the coregistration group, the angiogram during the OCT pullback was obtained from a projection which presented the least foreshortening of the stenosis and minimum overlap of the main vessel and side-branches using an X-ray system (Allura Xper FD10/10, Philips Healthcare, Best, The Netherlands) at 15 frames/s [2]. The angiogram during the OCT pullback was recorded together with the OCT image on the online OCT workstation. OCT-angiography coregistration system semi-automatically captured the location of the OCT imaging core on the angiogram. The operator performed stent implantation using an angiographic roadmap showing markers of the planned proximal and distal stent edge locations determined from the OCT lumen profile view (Fig. 1).
In the no coregistration group, the operator performed stent implantation based on OCT findings using angiography alone.
After stenting, post-dilatation was performed to achieve adequate stent expansion as needed, and the stented segments were evaluated by OCT in both groups.
OCT analysis
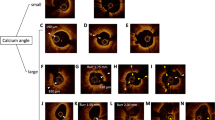
The OCT images were analyzed by an independent investigator (Y.I) using a dedicated off-line review system (Abbott Vascular, Santa Clara, California, USA). Lipidic plaque was defined as a signal-poor region with diffuse border [5]. Lipid-rich plaque was defined as a lipidic plaque with maximum lipid arc > 180˚ in at least one cross-section [3] (Fig. 2).
In OCT before stenting, the analysis was performed on every cross-section in the lesion plus proximal and distal reference segments. The definitions of lesion and reference segments are shown in Fig. 3a. Reference lumen area, minimum lumen area, percent area stenosis, lesion length, and lipid length were measured. The presence of lipid-rich plaque was evaluated in the proximal and distal reference segments and proximal and distal lesion segments.
Definitions of lesion and stent segment. a Lesion. Proximal and distal reference sites were defined as the sites adjacent to the lesion proximally and distally with normal-looking lumen and no lipid-rich plaque. Proximal and distal reference segments were defined as the 5-mm segments adjacent to the reference sites and outside the lesion. Proximal and distal lesion segments were defined as the 5-mm segments adjacent to the reference sites and inside the lesion. b Stent. Stent edges were defined by the most proximal and most distal OCT cross-sections where stent struts were seen in all 4 quadrants of the image. Stent edge segments were defined as the 5-mm segments adjacent to the stent edges and outside the stent. OCT = optical coherence tomography.
In OCT after stenting, the analysis was performed on every cross-section in the stent plus stent edge segments. The definitions of stent edge sites and stent edge segments are shown in Fig. 3b. Lipid length was measured across the stent and edge segments. The presence of untreated lipid-rich plaque was evaluated in the proximal and distal stent edge segments. The presence of dissection (defined as flap angle ≥ 60° of the circumference of the vessel at the site of dissection and/ or ≥ 3 mm in length) and untreated stenosis (defined as percent area stenosis > 40%) were also evaluated in the proximal and distal stent edge segments [6].
Statistical analysis
Statistical analysis was performed using JMP 13.0 (SAS Institute, Cary, North Carolina, USA). Categorical variables were presented as frequencies, with comparison using chi-square statistics or Fisher exact test (if there was an expected cell value < 5). Continuous variables were presented as mean ± 1 standard deviation and were compared using unpaired Student's t test. A p value < 0.05 was considered statistically significant.
Results
Patient and lesion characteristics are shown in Table 1. Age, gender, coronary risk factor, clinical presentation, serum creatinine concentration, PCI-targeted vessel, lesion location and lesion complexity were not different between the coregistration group and the no coregistration group.
OCT findings before stenting are shown in Table 2. Approximately half of patients in either group underwent pre-dilatation and/or thrombus aspiration before OCT imaging (55% vs. 51%, P = 0.418). Reference lumen area, minimum lumen area, percent area stenosis, lesion length, and lipid length were not different between the two groups. There were no lipid-rich plaques in the proximal and distal reference segments in both groups. In the proximal and distal lesion segments, lipid-rich plaques were found to be similar between the two groups (60% vs. 61%, P = 0.854; and 43% vs. 44%, P = 0.989; respectively).
PCI procedural characteristics are shown in Table 3. Direct stenting was rarely performed in either group (5% vs. 6%, P = 0.663). Stent diameter and length were not different between the two groups. The difference between the stent length and the lesion length was similar in the two groups (4 ± 3 mm vs. 4 ± 2 mm, P = 0.744). Post-dilatation was performed frequently in either group (97% vs. 98%, P = 0.552). Maximum balloon diameter, maximum inflation pressure, and amount of contrast media used in PCI were not different between the two groups.
OCT findings after stenting are shown in Table 4. Lipid length in the stent plus stent edge segments was not different between the two groups. Change in lipid length before and after stenting was similar in the two groups. The frequency of untreated lipid-rich plaque in the proximal stent edge segment was significantly lower in the coregistration group than in the no coregistration group (12% vs. 21%, P = 0.016). The frequency of untreated lipid-rich plaque in the distal stent edge segment was not different between the two groups (4% vs. 5%, P = 0.645). The frequency of untreated lipid-rich plaque in either proximal or distal stent edge segment was significantly lower in the coregistration group than in the no coregistration group (16% vs. 26%, P = 0.015) (Fig. 4). The frequency of dissection and untreated stenosis in the stent edge segments was low and without significant differences between the two groups.
Discussion
The major finding of the present study was that the frequency of untreated lipid-rich plaque in the stent edge segments after stenting was significantly lower in the coregistration group than in the no coregistration group. The use of OCT angiography coregistration was associated with accurate stent coverage of lesions with less longitudinal geographic mismatch.
The difficulty of matching cross-sectional OCT images to angiography limits the use of OCT in clinical practice. OCT-angiography coregistration can accurately translate the planed stent landing zone by OCT to angiogram. The use of OCT-angiography coregistration changes PCI strategies such as stent length and stent positioning in almost half of the procedures compared with OCT imaging alone [7]. The benefit of OCT-angiography coregistration is significant in diffuse disease or coronary segment without anatomical landmarks where accurate stent implantation may be hindered [7]. In addition, OCT-angiography coregistration might be especially helpful for less experienced operators in OCT-guided PCI.
OCT-angiography coregistration leads to accurate stent implantation with respect to longitudinal geographic location in coronary arteries. A previous study demonstrated that the distance discrepancy between the planned stent location and the actual implanted stent location was significantly shorter in the coregistration group than in the no coregistration group (1.9 ± 1.6 mm vs. 2.6 ± 2.7 mm, P = 0.03) [8]. In addition, the long-distance discrepancy of ≥ 5 mm tended to be less frequent in the coregistration group than in the no coregistration group (4% vs. 12%, P = 0.07) [8]. These results formed the basis of our study.
OCT-angiography coregistration influences PCI results. Koyama et al. demonstrated that the frequency of dissection (defined as a dissection flap > 60˚) in the distal stent edge segment tended to be lower in the coregistration group than in the no coregistration group (11.1% vs 20.8%, P = 0.07) [8]. The OPTICO‑integration II trial demonstrated that the frequency of untreated stenosis (defined as a minimal lumen area < 4.5mm2) in the stent edge segment was significantly lower in the coregistration group than in the no coregistration group (4.2% vs. 17.0%, P = 0.04) [9]. In addition, the present study revealed that the frequency of untreated lipid-rich plaque in the stent edge segment was significantly lower in the coregistration group than in the no coregistration group. Dissection, untreated stenosis and untreated lipid-rich plaque in the stent edge segment are associated with an increased risk of adverse clinical events such as stent thrombosis and edge restenosis. [10,11,12] Although clinical follow-up data were not available in the present study, the reduction in these inadequate stent edge findings achieved by the use of OCT-angiography coregistration may have a positive impact on long-term outcomes after PCI.
Limitation
There are several limitations that should be acknowledged. First, this was a retrospective study. Hence, there might be a selection bias. Second, since OCT cannot identify vessel boundary of the lipid plaque due to signal attenuation, the lipid angle was measured from the center of the lumen rather than the vessels. This may have caused a bias in the lipid angle due to the lumen area and eccentric plaque. Third, the OCT-angiography coregistration failure occurs when angiography during OCT pullback is not appropriate. However, the failure rate was low (4%) in the present study. Finally, during implantation, stents move longitudinally between systole and diastole by 1.5 mm (mean, range 0.5–5.5 mm) [13]. This is an insurmountable problem that hinders accurate stent implantation.
Conclusion
In OCT-guided PCI, the use of OCT-angiography coregistration was associated with a reduced frequency of untreated lipid-lich plaque at stent edges. OCT-angiography coregistration has a positive impact on PCI results.
Abbreviations
- OCT:
-
Optical coherence tomography
- PCI:
-
Percutaneous coronary intervention
References
Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la Torre Hernandez JM, Escaned J, Hill J, Prati F, Colombo A, di Mario C, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G (2018) Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J 39:3281–3300
van der Sijde JN, Guagliumi G, Sirbu V, Shimamura K, Borghesi M, Karanasos A, Regar E (2016) The OPTIS Integrated System: realtime, co-registration of angiography and optical coherence tomography. EuroIntervention 12:855–860
Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, Katayama Y, Nakamura T, Aoki H, Taruya A, Nishiguchi T, Satogami K, Yamano T, Kameyama T, Orii M, Ota S, Kuroi A, Kitabata H, Tanaka A, Hozumi T, Akasaka T (2016) Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv 9:e004231
Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, Shite J, Fusazaki T, Otake H, Kozuma K, Ioji T, Kaneda H, Serikawa T, Kataoka T, Okada H, Akasaka T (2017) Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): one-year angiographic and clinical results. Eur Heart J 38:3139–3147
Fujii K, Kubo T, Otake H, Nakazawa G, Sonoda S, Hibi K, Shinke T, Kobayashi Y, Ikari Y, Akasaka T (2020) Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography. Cardiovasc Interv Ther 35:13–18
Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, Guagliumi G, Meraj PM, Alfonso F, Samady H, Akasaka T, Carlson EB, Leesar MA, Matsumura M, Ozan MO, Mintz GS, Ben-Yehuda O, Stone GW (2016) Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet 388:2618–2628
Leistner DM, Riedel M, Steinbeck L, Stähli BE, Fröhlich GM, Lauten A, Skurk C, Mochmann HC, Lübking L, Rauch-Kröhnert U, Schnabel RB, Westermann D, Blankenberg S, Landmesser U (2018) Real-time optical coherence tomography coregistration with angiography in percutaneous coronary intervention-impact on physician decision-making: The OPTICO-integration study. Catheter Cardiovasc Interv 92:30–37
Koyama K, Fujino A, Maehara A, Yamamoto MH, Alexandru D, Jennings J, Krug P, Santiago LM, Murray M, Bongiovanni L, Lee T, Kim SY, Wang X, Lin Y, Matsumura M, Ali ZA, Sosa F, Haag E, Mintz GS, Shlofmitz RA (2019) A prospective, single-center, randomized study to assess whether automated coregistration of optical coherence tomography with angiography can reduce geographic miss. Catheter Cardiovasc Interv 93:411–418
Schneider VS, Böhm F, Blum K, Riedel M, Abdelwahed YS, Klotsche J, Steiner JK, Heuberger A, Skurk C, Mochmann HC, Lauten A, Fröhlich G, Rauch-Kröhnert U, Haghikia A, Sinning D, Stähli BE, Landmesser U, Leistner DM (2021) Impact of real-time angiographic co-registered optical coherence tomography on percutaneous coronary intervention: the OPTICO-integration II trial. Clin Res Cardiol 110:249–257
Prati F, Romagnoli E, Burzotta F, Limbruno U, Gatto L, La Manna A, Versaci F, Marco V, Di Vito L, Imola F, Paoletti G, Trani C, Tamburino C, Tavazzi L, Mintz GS (2015) Clinical impact of OCT findings during PCI: the CLI-OPCI II study. JACC Cardiovasc Imaging 8:1297–1305
Wan Ahmad WA, Nakayoshi T, Mahmood Zuhdi AS, Ismail MD, Zainal Abidin I, Ino Y, Kubo T, Akasaka T, Fukumoto Y, Ueno T (2020) Different vascular healing process between bioabsorbable polymer-coated everolimus-eluting stents versus bioresorbable vascular scaffolds via optical coherence tomography and coronary angioscopy (the ENHANCE study: ENdothelial Healing Assessment with Novel Coronary tEchnology). Heart Vessels 35:463–473
Kawamori H, Konishi A, Shinke T, Akahori H, Ishihara M, Tsujita H, Otake H, Toba T, Nakano S, Tanimura K, Tsukiyama Y, Nanba I, Kakei Y, Yasuda T, Omori T, Kubo T, Kozuki A, Shite J, Hirata K (2021) Efficacy of optical frequency domain imaging in detecting peripheral artery disease: the result of a multi-center, open-label, single-arm study. Heart Vessels 36:818–826
Arbab-Zadeh A, DeMaria AN, Penny WF, Russo RJ, Kimura BJ, Bhargava V (1999) Axial movement of the intravascular ultrasound probe during the cardiac cycle: implications for three-dimensional reconstruction and measurements of coronary dimensions. Am Heart J 138:865–872
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
TK: contributed to study conception, data analysis and interpretation, and drafting of the manuscript. YI: performed OCT image analysis and reviewed and revised the manuscript. KT, HE, DH, MT, TW, KS, YS, AKMK, and ST reviewed and revised the manuscript. TA gave the final approval of the manuscript submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kubo, T., Ino, Y., Shiono, Y. et al. Usefulness of optical coherence tomography with angiographic coregistration in the guidance of coronary stent implantation. Heart Vessels 37, 200–207 (2022). https://doi.org/10.1007/s00380-021-01911-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00380-021-01911-1