Abstract
Biological nitrification inhibitors (BNIs) are released from plant roots as exudates to repress nitrifier activity in agricultural soils, and this can improve nitrogen (N) recovery from fertilizer and enhance the N-use-efficiency (NUE). This review summarizes the current understanding of the regulatory mechanisms of BNIs release from roots of plants, such as Brachiaria humidicola (pasture grasses), Sorghum bicolor (hybrid sorghum) and Oryza sativa (paddy rice). BNIs can be categorized as hydrophilic- and hydrophobic-BNIs. Root systems can rapidly release hydrophilic-BNIs when NH4+ is present in rhizosphere in combination with low pH, which is associated with the activation of plasma membrane H+-ATPase. Since plasma membrane H+-ATPase is responsible for the establishment of membrane potential and generation of proton motive force for the secondary transport of various substances. The BNIs release may probably occur through the voltage-gated anion channels by the membrane potential variation or via secondary transporters, most likely MATE transporters, powered by the proton motive force. In addition, ATP-binding cassette (ABC) transporters may be also involved in the active efflux of hydrophilic-BNIs. On the contrary, the release of the hydrophobic BNIs, such as sorgoleone, from plant roots may be mediated by the vesicle traffic process and/or exocytosis. In addition, the possible effects of various environmental factors on the BNIs release in soils have been discussed. Future research should focus on the identification of the corresponding BNIs transporters in plants, and this may be helpful for the application of BNI crops in the agricultural practice via breeding and genetic modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ammonium form (NH4+-N) of N fertilizers are easily transformed to the nitrate form (NO3−-N) due to the nitrification process in aerobic soils (Daims et al. 2015). But unlike NH4+, anionic NO3− cannot be adsorbed by negatively charged soil particles and thus is very mobile in soil. Therefore, NO3− in soil can be leached into water system and cause environmental pollution like water eutrophication, whereas denitrification produces various gaseous N forms (e.g., N2O, NO, and N2), among which, N2O is one of the greenhouse gases responsible for the global warming (Meinshausen et al. 2009). Both of nitrification and denitrification result in the reduction of NUE in agricultural systems (Gooding et al. 2012; Fowler et al. 2013; Subbarao et al. 2012). Thus, keeping soil N as NH4+ for an extended period may improve N recovery and maintain the ecosystem’s sustainability.
Application of synthetic nitrification inhibitors is one of the ongoing practices to control soil nitrification in agricultural soils. Nitrapyrin, 3,4-dimethylpyrazole phosphate (DMPP), and dicyadiamide (DCD) are well-known commercial synthetic nitrification inhibitors (Slangen and Kerkhoff 1984; Zerulla et al. 2001). However, synthetic nitrification inhibitors are not widely used by farmers due to their relatively high cost, associated environmental safety issue, and additional labor costs (Lam et al. 2017). Various nitrification inhibitory substances have been detected in the rhizosphere of plants and are termed biological nitrification inhibitors (BNIs) being responsible for biological nitrification inhibition (Subbarao et al. 2009, 2015, 2017). The presence and function of BNIs have been demonstrated in Sorghum bicolor (sorghum) (Zakir et al. 2008), Brachiaria humidicola (pasture grasses) (Subbarao et al. 2009), Oryza sativa (rice) (Sun et al. 2016), Triticum aestivum (wheat) (O’Sullivan et al. 2016), and Leymus racemosus (a wild relative of wheat) (Subbarao et al. 2007c). BNIs released from roots of these plant species can reduce the abundance of ammonium oxidation bacteria and archaea in soil (Nardi et al. 2013, 2020; Lu et al. 2018) and/or inhibit the ammonium oxidation activity of Nitrosomonas europaea in situ (Zakir et al. 2008; Sun et al. 2016; Subbarao et al. 2006a, b). Brachialactone secreted by roots of Brachiaria humidicola, a tropical pasture grass, can inhibit AMO (ammonia monooxygenase) and HAO (hydroxyl amino oxidoreductase) enzymes (Subbarao et al. 2009). Methyl 3-(4-hydroxyphenyl) propionate (MHPP) secreted by sorghum roots inhibits AMO pathway (Zakir et al. 2008; Subbarao et al. 2007a, 2013). Sorgoleone and sakuranetin are also secreted by sorghum roots, and inhibit the AMO and HAO, but sakuranetin did not show effective BNI function in soil assay (Alsaadawi et al. 1986; Subbarao et al. 2013). The recent research confirmed that 1, 9-decanediol from rice had a significant inhibitory effect on nitrification in alkaline tidal soil, neutral paddy soil, and acid red soil (Lu et al. 2018).
Interestingly, some BNIs also exhibited other functions besides inhibiting nitrification in soil. MHPP from sorghum roots could modify root system architecture by inhibiting primary root elongation and improving lateral root growth in Arabidopsis thaliana, and this may increase the total roots volume for the uptake of mineral nutrients in soil (Liu et al. 2016). Sakuranetin was found to act as flavanone phytoalexin against plant pathogen in rice leaves (Kodama et al. 1992). In addition, sorgoleone can function as a herbicide because it can inhibit photosynthesis of competitor plants by binding to the D1 protein and disturb mitochondrial electron transport, root H+-ATPase activity, and water uptake (Dayan et al. 2010).
Hydrophilic and hydrophobic BNIs
As root exudates, BNIs are synthesized and secreted by plant roots in the rhizosphere (Bais et al. 2001; Walker et al. 2003), and thus, they can modify the soil microbial community (Nardi et al. 2013, 2020). Usually, they are low molecular weight compounds, such as phenolics or other secondary metabolites. The BNIs are in general divided into two different categories. One is water soluble, hereafter referred as hydrophilic-BNIs, such as MHPP (Zakir et al. 2008). The other category is soluble in acidified-DCM (dichloromethane), hereafter referred as hydrophobic-BNIs, which could be obtained by washing roots with acidified-DCM (Subbarao et al. 2013). Due to their differential solubility in water, it is expected that hydrophobic BNIs may remain close to the root as they could be strongly adsorbed by soil particles within the rhizosphere (Subbarao et al. 2012). In contrast, the hydrophilic-BNIs may move farther away from the point of release due to their solubility in water, and this may amplify their capacity to control nitrification beyond the rhizosphere. Sorgoleone, a benzoquinone is a major component of the root-DCM wash and accounts for 80% of the hydrophobic-BNIs activity in sorghum roots (Subbarao et al. 2013). In contrast, sakuranetin, a flavanone, and MHPP, a phenylpropanoid, are hydrophilic, and both are released from sorghum roots (Zakir et al. 2008). In addition, brachialactone and its isomers and derivatives, belonging to the cyclic diterpenes, released by Brachiaria humidicola (Subbarao et al. 2009; Egenolf et al. 2020), and 1,9-decanediol, a fatty alcohol, released by paddy rice (Sun et al. 2016), are also hydrophilic-BNIs. The BNIs identified from plant root exudates are listed in Table 1.
Processes of BNIs release from roots
For a long time, secretion of compounds from plant roots was primarily thought to be a passive process of diffusion across the plasma membrane, because small uncharged low molecular weight compounds were thought to pass through the lipid membranes freely (Guern et al. 1987). It is well-known that passive diffusion process depends on membrane permeability and concentration gradient of exudates between the cytoplasm and apoplast (Marschner 2012). In addition, many diffusion processes have been indicated to be mediated by aquaporins (aquaglyceroporins), which are integral membrane proteins that facilitate the transport of water and many other neutral molecules, such as glycerol, ammonia, and urea across cell membrane (Assmann and Haubrick 1996). However, most root exudates are electrically charged molecules or polarized ions (Bertin et al. 2003), and thus, simple diffusion across the plasma membrane bilayer cannot occur due to their low solubility in the lipid membrane. In some cases, the release of root exudates does not depend on its concentration in the root cells (Zhang et al. 2004; Zhu et al. 2005), suggesting that root exudation could be a tightly regulated process rather than simple diffusion (Pariasca-Tanaka et al. 2010). Further, some BNIs, such as sorgoleone, might be toxic alleochemicals, which cannot freely exist in the cytoplasm, but need to be sequestered in subcellular vesicles and released by exocytosis (Czarnota et al. 2003). Therefore, the release of BNIs from roots into the rhizosphere should be facilitated by membrane transport systems such as channels, pumps, carriers, and exocytosis. So far, the transporter of BNIs has not been identified by molecular and genetic evidence.
Involvement of plasma membrane H+-ATPase in the release of hydrophilic-BNIs under NH4 + nutrition
However, the recent two decades of research on BNIs provides us many clues to find possible transporter of BNIs. It has been suggested that NH4+ might act as a signal to trigger the release of BNIs (Subbarao et al. 2007b). Indeed the presence of NH4+ but not of NO3− in root medium could induce BNIs release from roots of various plants, such as Brachiaria humidicola (Subbarao et al. 2009), sorghum (Zakir et al. 2008; Subbarao et al. 2007b, c), wheat (Subbarao et al. 2007c), and rice (Zhang et al. 2019). NH4+ uptake in plant roots is coupled with H+ release and acidification of the rhizosphere, whereas NO3− cotransport with H+ across the plasma membrane results in the increase of rhizosphere pH (Marschner 2012). Moreover, NH4+ nutrition causes a stronger depolarization of plasma membrane as compared with NO3− nutrition (Schubert and Yan 1997). We found that the plasma membrane H+-ATPase activity is induced by NH4+ nutrition, which is associated with the acidification of rhizosphere (Zhu et al. 2009). Plasma membrane H+-ATPase is a universal electrogenic H+ pump, which generates H+ electrochemical gradient to provide driving force for the secondary influx or efflux of ions and metabolites across the plasma membrane (Palmgren 2001). In this way, it could facilitate the transport of hydrophilic-BNIs being putative anionic substances across the plasma membrane. In addition, the metabolism of NH4+ is coupled with H+ generation in the cytoplasm (Lewis et al. 1982; Marschner 2012), and thus, the enhanced activity of plasma membrane H+-ATPase can pump the excessive H+ out of the cell to maintain intracellular pH homeostasis (Schubert and Yan 1997; Zhang et al. 2021). At the same time, the released H+ could also be used as counterions for BNIs release.
It has been hypothesized that BNIs release is linked to the plasma membrane H+-ATPase activity by considering that pharmacological agents can stimulate (fusicoccin) or suppress (vanadate) plasma membrane H+-ATPase activities and this can affect the releases of BNIs from sorghum roots without NH4+ (under the treatment of fusicoccin) or with NH4+ (under the treatment of vanadate) (Zhu et al. 2012). This hypothesis was supported by the recent finding that H+ release and hydrophilic-BNIs release are stoichiometrically linked under various conditions, e.g., different rhizosphere pH, and a range of NH4+ concentrations in root medium (Di et al. 2018). Low rhizosphere pH caused by the uptake of cations, such as NH4+ or even K+, could trigger the activity of plasma membrane H+-ATPase. Recently, it was reported that 3-eip-brachialactone, one kind of BNIs from Brachiaria humidicola roots, is released via secondary transport depending on plasma membrane H+-ATPase generated proton motive force (Egenolf et al. 2021).
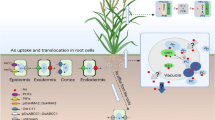
Further, the effect of methyl-ammonium, a non-metabolizable analogue of NH4+, was used to investigate whether NH4+ assimilation is also responsible for the stimulation of BNIs release under NH4+ nutrition. There was no significant effect of methyl-ammonium on plasma membrane H+-ATPase activity and BNIs release, suggesting the stimulatory effect of NH4+ on hydrophilic-BNIs release is functionally linked with NH4+ assimilation (Zeng et al. 2016). Apart from NH4+ uptake, NH4+ assimilation in root cells is also a critical factor to induce hydrophilic-BNIs release by triggering plasma membrane H+-ATPase activity to pump out excessive H+ generated by ammonium assimilation and avoid cytosolic acidosis (Zeng et al. 2016). This is also true that transcriptional and post-translational regulation of plasma membrane H+-ATPases was found to be involved in the stimulation of BNIs release under NH4+ nutrition (Zeng et al. 2016; Afzal et al. 2020). At least five plasma membrane H+-ATPase genes responded to NH4+ with a similar expression pattern (Zeng et al. 2016). Thus, the activation of plasma membrane H+-ATPase under NH4+ nutrition is at least partly due to transcriptional regulation of H+-ATPase genes. Recently, we found that NH4+ and low rhizosphere pH (pH 3.0) brought activation of plasma membrane H+-ATPase, based not only on transcriptional regulatory level that resulted in higher steady-state protein concentration of this enzyme, but also on the strongly up-regulated phosphorylation level of plasma membrane H+-ATPase, which further boosted up the H+ pumping activity that ultimately facilitated hydrophilic-BNIs release (Afzal et al. 2020) (Fig. 1).
Prospective pathways for BNIs transport in plant root cells. The uptake of NH4+ acidifies rhizosphere and assimilation of NH4+ in the cytoplasm of root cells produces H+, which in turn induces activation of plasma membrane H+-ATPase activity. The enhanced H+ gradient across the plasma membrane provides the driving force for hydrophilic-BNIs release through anion channels. If the anion channels were blocked, BNIs could also be possibly released by ABC transporters, while hydrophobic BNIs, such as sorgoleone, can be transported by vesicles and released out of cell via exocytosis
Because hydrophilic-BNIs are putative anionic substances (Subbarao et al. 2006b; 2007a), and most of the membrane anchored anion channels are voltage gated and depend on membrane potential (Tyerman 1992), the release of the most of hydrophilic-BNIs may be mediated by the cooperation between corresponding anion channels and plasma membrane H+-ATPases that generate the electrochemical gradient (Fig. 1).
Transporters potentially involved in hydrophilic-BNIs release
Because two commonly used anion channel blockers, anthracene-9-carboxylate and niflumic acid, could not inhibit (on the contrary, enhanced) the release of hydrophilic-BNIs from roots of three sorghum genotypes (Di et al. 2018), and these two anion channel blockers cannot inhibit the plasma membrane H+-ATPase activity (Zhu et al. 2005), we hypothesized that hydrophilic-BNIs were also transported through other transporters besides anion channels, such as ATP-binding cassette (ABC) transporters, and the multi-drug and toxic compound extrusion (MATE) transporters. It is well-known that ABC transporters mediate diverse cellular transport processes, such as the excretion of potentially toxic compounds, lipid translocation, and flavonoids secretion (Balzi and Goffeau 1994; Higgins 1995; Maathuis et al. 2003; Buer et al. 2007). ABC transporters are widely distributed in plant species (Martinoia et al. 2002). The model plant Arabidopsis contains 129 ABC transporter genes, seven of which spanning four subfamilies (MRP, PDR, ATH, and PGP) have been identified to participate in the secretion of phytochemicals from plant roots (Badri et al. 2008). More than one ABC transporters are involved in the secretion of a given phytochemical, and one ABC transporter can secrete more than one kind of secondary metabolites (Badri et al. 2008). Because BNIs release is totally suppressed in sorghum roots by vanadate, an inhibitor also of ABC transporters in plants (Rea 2007; Coskun et al. 2017), it is possible that BNIs could be released through one or more ABC transporters located in the plasma membrane of root cells (Fig. 1).
Transporters belonging to the multi-drug and toxic compound extrusion (MATE) family have been indicated to be responsible for citrate exudation under aluminum toxicity (Furukawa et al. 2007). The transportation of anions mediated by MATE depends on the gradient of counter ion, which is usually H+ in plant cells (Shen et al. 2005; Doshi et al. 2017). In addition, citrate released from proteoid roots of white lupin is also related to the activity of plasma membrane H+-ATPase (Yan et al. 2002; Zhu et al. 2005). The citrate efflux proteins in white lupin are characterized by electronic patch clamp (Zhang et al. 2004). Although the exact transporters involved in citrate release from proteoid roots of white lupin have not been identified, they were considered to be similar to MATE proteins (Zhang et al. 2004). MATE proteins can also transport benzoxazinoids, artemisinin, juglone, phenolics, alkaloids, and flavonoids (Zhao and Dixon 2009). MHPP and sakuranetin, two BNIs identified from sorghum, are phenolic and flavonoid, respectively (Subbarao et al. 2013). The BNIs release from sorghum roots depends on the plasma membrane H+-ATPase (Zhu et al. 2012; Zeng et al. 2016). Taken together, MATE transporters may be also involved in BNIs release, although further studies are required to verify this hypothesis (Sivaguru et al. 2013; Doshi et al. 2017).
Release of hydrophobic-BNIs
Unlike hydrophilic-BNIs, hydrophobic-BNIs release does not depend on rhizosphere pH in several sorghum varieties (Di et al. 2018). In addition, the relationship between the plasma membrane H+-ATPase activity and hydrophobic-BNI activity is not correlated (Di et al. 2018). Since most of the hydrophobic-BNI activity is attributed to sorgoleone (Subbarao et al. 2013), the release of this compound seems independent of plasma membrane H+-ATPase generated proton motive force or membrane potential. In addition, due to the toxic property of sorgoleone, its excretion by roots might involve vesicular transport (Battey and Blackbourn 1993). Like many phenylpropane and flavonoids, sorgoleone may be synthesized on the surface of the endoplasmic reticulum and secreted to the extracellular space through vesicles (Winkel-Shirly, 2001) (Fig. 1).
Environmental factors in soils affecting the BNIs release from plant roots
The physical, chemical, and biological properties of soils are tremendously heterogeneous and dynamic, and they strongly affect the activities of plants and microorganisms. Therefore, the release of BNIs in soils should be influenced by various soil environmental factors. Soil fertility is one of the important factors that decide morphological and physiological adaptations of plant roots. For the BNI plants, NH4+ availability is a major factor to stimulate hydrophilic-BNIs release, although it is difficult to measure the released BNIs in situ. Our previous experiments using split roots indicated that sorghum roots incubated in the solution with NH4+ caused stronger release of BNIs as compared with that incubated with nitrate (Zhu et al. 2012). In addition, the release rate of BNIs from sorghum roots depended on NH4+ concentration (Zakir et al. 2008; Zeng et al. 2016). Further, the hydrophobic BNIs, such as sorgoleone, were also found positively correlated to exchangeable NH4+ content in soil (Sarr et al. 2020). Therefore, it may be important that the release of BNIs was triggered at the root zones, where NH4+ is available in soil. It is thus important for plants root to exudate BNIs to protect NH4+ from nitrifiers directly in situ in order to utilize the N source in soil with high efficiency.
The soil pH also plays an important role in the BNIs release by roots. In general, NH4+ uptake by roots causes strong acidification of rhizosphere (Marschner 2012; Zhu et al. 2009). Low pH increased the release of BNIs from roots of Brachiaria humidicola (Subbarao et al. 2007b), sorghum (Zakir et al. 2008; Zhu et al. 2012), and rice (Zhang et al. 2019), while pH above 7 depressed the release rate of BNIs by Brachiaria humidicola or sorghum (Subbarao et al. 2007b; Zhu et al. 2012). It was found that the nitrification inhibitory rate of Brachiaria humidicola root exudates was higher in an Andosol soil with pH 5.9 than in a Combisol soil with pH 6.9 (Gopalakrishnan et al. 2009). Further, it was found that light soils such as Alfisols of the semi-arid tropics India or sandy loams of West Africa are better suited to develop acidic rhizosphere (pH < 6.0) for the BNI function of sorghum, when compared to Vertisols soil (pH > 7.5) (Subbarao et al. 2013). In acid red soils (pH 4.26), the nitrification inhibition potential of the BNIs from rice root exudates was significantly stronger than that in neutral paddy soils (pH 6.25) or alkaline fluvo-aquic siols (pH 7.92) (Lu et al. 2018). These results indicated that the low soil pH enhanced the BNIs release and/or their activity. Since the suppression of nitrification in soil by BNIs can further improve NH4+ availability for plant roots, which in turn leads to the higher acidification of rhizosphere due to the uptake of NH4+, thus acting as a feedback loop for the efficient utilization of soil N.
The bulk density (BD) is an important soil property, which is an indicator of the amount of pore space in the soil (Dam et al. 2005). Bulk density can thus decide the difficulty of root penetration into the soil, and also the content of soil water, which can affect root growth and microbial activity. It is possible that bulk density is critical for the plant roots to release BNIs.
The soil moisture influences not only the plant growth and microbial activity but also the movement of various soluble substances in soil. Xeric moisture regimes are dry and may limit the movement of released BNIs from roots, which may cause the accumulation of BNIs in the rhizosphere and inhibit the root growth and subsequently the release capacity of BNIs. It has been documented that the sorgoleone content in soil is negatively correlated to the soil moisture (Sarr et al. 2020). The growth of terrestrial plants is inhibited under hydric regimes. Therefore, the mesic moisture regimes should be optimal for root growth of terrestrial plants and the release of BNIs in soil.
Besides the above factors, the stability and mobility of BNIs in soil should be considered. Since BNIs are mainly low molecular weight organic compounds, they could be absorbed by surface reactive soil particles, and this can reduce BNIs efficiency. BNIs could also be used as C sources by various soil microorganisms. It is still not clear whether the released BNIs could be degraded by some specific microorganisms. BNIs from root exudation of Brachiaria humidicola showed nitrification inhibitory ability after 60 days in Cambisol and Andosol soils (Gopalakrishnan et al. 2009). The nitrification inhibition in soils could last for 2.3 months by continuous release of sorgoleone from sorghum roots (Sarr et al. 2020). Thus, it seems that the most of BNIs in the soil are relatively stable and efficient. The duration of the nitrification inhibition of various BNIs in soils is worth further evaluation in the future.
Conclusions and perspectives
Controlling nitrification is critical to improve N retention in the soil. BNIs are active where plant root systems deliver powerful BNIs at nitrifier sites, and BNI is one of the best strategies to develop N-efficient production systems. Plants with high BNI-capacity root systems can be produced by using modern breeding tools and approaches. In addition, BNI-producing plants such as Brachiaria pasture grasses can be incorporated into soils (e.g., as green manures) (Subbarao et al. 2012, 2015), and BNI-producing plants can be inter-cropped or rotated with other crops to manage soil nitrifier activity to improve NUE of production systems (Karwat et al. 2017).
References
Afzal MR, Zhang M, Jin H, Wang G, Zhang M, Ding M, Raza S, Hu J, Zeng H, Gao X, Subbarao GV, Zhu Y (2020) Post-translational regulation of plasma membrane H+-ATPase is involved in the release of biological nitrification inhibitors from sorghum roots. Plant Soil 450:357–372. https://doi.org/10.1007/s11104-020-04511-6
Alsaadawi IS, Al-Uqaili JK, Alrubeaa AJ, Al-Hadithy SM (1986) Allelopathic suppression of weed and nitrification by selected cultivars of Sorghum bicolor (L.) moench. J Chem Ecol 12:209–219. https://doi.org/10.1007/BF01045604
Assmann SM, Haubrick LL (1996) Transport proteins of plant plasma membranes. Curr Opin Cell Biol 8:458–467. https://doi.org/10.1016/S0955-0674(96)80021-4
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Peña C, Jasinski M, Santelia D, Martinoia E, Sumner LW, Banta LM, Stermitz F, Vivanco JM (2008) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol 146:762–771. https://doi.org/10.5167/uzh-13651
Bais HP, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organ. In Vitro Cell Dev Biol-Plant 37:730–741. https://doi.org/10.1007/s11627-001-0122-y
Balzi E, Goffeau A (1994) Genetics and biochemistry of yeast multidrug resistance. Biochim Biophys Acta 1187:152–162. https://doi.org/10.1016/0005-2728(94)90102-3
Battey NH, Blackbourn HD (1993) The control of exocytosis in plant cells. New Phytol 125:307–338. https://doi.org/10.1111/j.1469-8137.1993.tb03883.x
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83. https://doi.org/10.1023/A:1026290508166
Buer CS, Muday GK, Djordjevic MA (2007) Flavonoids are differentially taken up and transported long distances in Arabidopsis. Plant Physiol 145:478–490. https://doi.org/10.4161/psb.3.6.5440
Coskun D, Britto DT, Shi W, Kronzucker HJ (2017) Nitrogen transformations in modern agriculture and the role of biological nitrification inhibition. Nature Plants 3:17074. https://doi.org/10.1038/nplants.2017.74
Czarnota MA, Paul RN, Weston LA, Duke SO (2003) Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int J Plant Sci 164:861–866. https://doi.org/10.1086/378661
Daims H, Lebedeva EV, Pjevac P, Han P, Herbold C, Albertsen M, Jehmlich N, Palatinszky M, Vierheilig J, Bulaev A, Kirkegaard RH, von Bergen M, Rattei T, Bendinger B, Nielsen PH, WagnerM, (2015) Complete nitrification by Nitrospira bacteria. Nature 528:504–509. https://doi.org/10.1038/nature16461
Dam RF, Mehdi BB, Burgess MSE, Madramootoo CA, Mehuys GR, Callum IR (2005) Soil bulk density and crop yield under eleven consecutive years of corn with different tillage and residue practices in a sandy loam soil in central Canada. Soil Tillage Res 84:41–53. https://doi.org/10.1016/j.still.2004.08.006
Dayan FE, Rimando AM, Pan Z, Baerson SR, Gimsing AL, Duke SO (2010) Sorgoleone Phytochem 71:1032–1039. https://doi.org/10.1016/j.phytochem.2010.03.011
Di T, Afzal MR, Yoshihashi T, Zhu DS, Y, Subbarao GV, (2018) Further insights into underlying mechanisms for the release of biological nitrification inhibitors from sorghum roots. Plant Soil 423:99–110. https://doi.org/10.1007/s11104-017-3505-5
Doshi R, McGrath AP, Piñeros M, Szewczyk P, Garza DM, Kochian LV, Chang G (2017) Functional characterization and discovery of modulators of SbMATE, the agronomically important aluminium tolerance transporter from Sorghum bicolor. Sci Rep 7:17996. https://doi.org/10.1038/s41598-017-18146-8
Egenolf K, Conrad J, Schöne J, Braunberger C, Beifuß U, Walker F, Nuñez J, Arango J, Karwat H, Cadisch G, Neumann G, Rasche F (2020) Brachialactone isomers and derivatives of Brachiaria humidicola reveal contrasting nitrification inhibiting activity. Plant Physiol Biochem 154:491–497. https://doi.org/10.1016/j.plaphy.2020.06.004
Fowler D, Coyle M, Skiba U, Sutton MA, Cape JN, Reis S, Sheppard LJ, Jenkins A, Grizzetti B, Galloway JN, Vitousek P, Leach A, Bouwman AF, Butterbach-Bahl K, Dentener F, Stevenson D, Amann M, Voss M (2013) The global nitrogen cycle in the twenty first century. Philos Trans Royal Soc B 368:20130164. https://doi.org/10.1098/rstb.2013.0164
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48:1081–1091. https://doi.org/10.1093/pcp/pcm091
Gooding MJ, Addisu M, Uppal RK, Snape JW, Jones HE (2012) Effect of wheat dwarfing genes on nitrogen-use efficiency. J Agricul Sci 150:3–22. https://doi.org/10.1017/S0021859611000414
Gopalakrishnan S, Watanabe T, Pearse SJ, Ito O, Hossain ZAKM, Subbarao GV (2009) Biological nitrification inhibition by Brachiarai humidicola roots varies with soil type and inhibits nitrifying bacteria, but not other major soil microorganisms. Soil Sci Plant Nutr 55:725–733. https://doi.org/10.1111/j.1747-0765.2009.00398.x
Guern J, Renaudin JP, Brown SC (1987) The compartmentation of secondary metabolites in plant cell cultures. In: Constabel F, Vasil IK (eds) Cell cultures and somatic cell genetics of plants. Academic Press, San Diego, pp 43–76
Higgins CF (1995) The ABC of channel regulation. Cell 82:693–696. https://doi.org/10.1016/0092-8674(95)90465-4
Karwat H, Moreta D, Arango J, Núńez J, Rao I, Rincón Á, Rasche F, Cadisch G (2017) Residual effect of BNI by Brachiarai humidicola pasture on nitrogen recovery and grain yield of subsequent maize. Plant Soil 420:389–406. https://doi.org/10.1007/s11104-017-3381-z
Kodama O, Miyakawa J, Akatsuka T, Kiyosawa S (1992) Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochem 31:3807–3809. https://doi.org/10.1016/S0031-9422(00)97532-0
Lam SK, Suter H, Mosier AR, Chen D (2017) Using nitrification inhibitors to mitigate agricultural N2O emission: a double-edged sword? Glob Change Biol 23:485–489. https://doi.org/10.1111/gcb.13338
Lewis OAM, James DM, Hewitt EJ (1982) Nitrogen assimilation in barley (Hordeum vulgare L. cv. Mazurka) in response to nitrate and ammonium nutrition. Ann Bot 49:39–49. https://doi.org/10.1093/oxfordjournals.aob.a086228
Liu Y, Wang R, Zhang P, Chen Q, Luo Q, Zhu Y, Xu J (2016) The nitrification inhibitor methyl 3-(4-hydroxyphenyl) propionate modulates root development by interfering with auxin signaling via the NO/ROS pathway. Plant Physiol 171:1686–1703. https://doi.org/10.1104/pp.16.00670
Lu YF, Zhang XN, Jiang JF, Kronzucker HJ, Shen WS, Shi WM (2018) Effects of the biological nitrification inhibitor 1,9-decanediol on nitrification and ammonia oxidizers in three agricultural soils. Soil Bio Biochem 129:48–59. https://doi.org/10.1016/j.soilbio.2018.11.008
Maathuis FJM, Filatov V, Herzyz P, Krijger GC, Axelsen KB, Chen S, Green BJ, Li Y, Madagan KL, Sánchez-Fernández R, Forde BG, Palmgren MG, Rea PA, Williams LE, Sanders D, Amtmann A (2003) Transcriptome analysis of root transporters reveals participation of multiple gene families in the response to cation stress. Plant J 35:675–692. https://doi.org/10.1046/j.1365-313x.2003.01839.x
Marschner P (2012) Marschner's miniral nutrition of higher plants, 3rd edn. Academic Press, London p 23. https://doi.org/10.1016/C2009-0-63043-9
Martinoia E, Klein M, Geisler M, Bovet L, Forestier C, Kolulisaoglu U, Muller-Rober B, Schluz B (2002) Multifunctionality of plant ABC transporters: more than just detoxifiers. Planta 214:345–355. https://doi.org/10.1007/s004250100661
Meinshausen M, Meinshausen N, Hare W, Raper SCB, Frieler K, Knutti R, Frame DJ, Allen MR (2009) Greenhouse-gas emission targets for limiting global warming to 2 ℃. Nature 458:1158–1162. https://doi.org/10.1038/nature08017
Nardi P, Akutsu M, Pariasca-Tanaka J, Wissuwa M (2013) Effect of methyl 3–4-hydroxyphenyl propionate, a Sorghum root exudate, on N dynamic, potential nitrification activity and abundance of ammonia-oxidizing bacteria and archaea. Plant Soil 367:627–637. https://doi.org/10.1007/s11104-012-1494-y
Nardi P, Laanbroek HJ, Nicol GW, Renella G, Cardinale M, Pietramellara G, Weckwerth W, Trinchera A, Ghatak A, Nannipieri P (2020) Biological nitrification inhibition in the rhizosphere: determining interactions and impact on microbially mediated processes and potential applications. FEMS Microbiol Rev 44:874–908. https://doi.org/10.1093/femsre/fuaa037
O’Sullivan CA, Fillery IRP, Poper MM, Richards RA (2016) Identification of several wheat land races with biological nitrification inhibition capacity. Plant Soil 404:61–74. https://doi.org/10.1007/s11104-016-2822-4
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52:817–845. https://doi.org/10.1146/annurev.arplant.52.1.817
Pariasca-Tanaka J, Nardi P, Wissuwa M (2010) Nitrification inhibition activity, a novel trait in root exudates of rice. AoB Plants 2010:plq014. https://doi.org/10.1093/aobpla/plq014
Rea PA (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58:347–375. https://doi.org/10.1146/annurev.arplant.57.032905.105406
Sarr PS, Ando Y, Nakamura S, Deshpande S, Subbarao GV (2020) Sorgoleone release from sorghum roots shapes the composition of nitrifying populations, total bacteria, and archaea and determines the level of nitrification. Biol Fertil Soil 56:145–166. https://doi.org/10.1007/s00374-019-01405-3
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Zeitschr Pflanzenernähr Bodenk 160:275–281. https://doi.org/10.1002/jpln.19971600222
Shen H, He LF, Sasaki T, Yamamoto Y, Zheng SJ, Ligaba A, Yan XL, Ahn SJ, Yamaguchi M, Sasakawa H, Matsumoto H (2005) Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Up-regulation of transcription, translation, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiol 138:287–96. https://doi.org/10.1104/pp.104.058065
Sivaguru M, Liu J, Kochian LV (2013) Targeted expression of SbMATE in the root distal transitionzone is responsible for sorghum aluminum resistance. Plant J 76:297–307. https://doi.org/10.1111/tpj.12290
Slangen J, Kerkhoff P (1984) Nitrification inhibitors in agriculture and horticulture: a literature review. Fertil Res 5:1–76. https://doi.org/10.1007/bf01049492
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006a) Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Crit Rev Plant Sci 25:303–335. https://doi.org/10.1080/07352680600794232
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006b) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiariahumidicola. Plant Soil 288:101–112. https://doi.org/10.1007/s11104-006-9094-3
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007a) Biological nitrification inhibition (BNI) – is it a widespread phenomenon? Plant Soil 294:5–18. https://doi.org/10.1270/jsbbs.59.529
Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL (2007b) NH4+ triggers the synthesis and release of biological nitrification inhibition compounds in Brachiarahumidicola roots. Plant Soil 290:245–257. https://doi.org/10.1007/s11104-006-9156-6
Subbarao GV, Ban T, Kishi M, Ito O, Samejima H, Wang HY, Pearse SJ, Gopalakrishnan S, Nakahara K, Zakir AKMZ, Tsujimoto H, Berry WL (2007c) Can biological nitrification inhibition (BNI) genes from perennial Leymusracemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 299:55–64. https://doi.org/10.1007/s11104-007-9360-z
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Nat Acad Sci (PNAS) (USA) 106:17302–17307. https://doi.org/10.1073/pnas.0903694106
Subbarao GV, Sahrawat KL, Nakahara K, Ishikawa T, Kishii M, Rao IM, Hash CT, George TS, Srinivasarao P, Nardi P, Bonnett D, Berry W, Suenaga K, Lata JC (2012) Biological nitrification inhibition (BNI) – a novel strategy to regulate nitrification in agricultural systems. Adv Agron 114:249–302. https://doi.org/10.1016/B978-0-12-394275-3.00001-8
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, ZhuY ZHAKM, Deshpande SP, Hash CT, Sahrawat KL (2013) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259. https://doi.org/10.1007/s11104-012-1419-9
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata JC, Kishii M, Hans-Joachim B (2015) Suppression of soil nitrification by plants. Plant Sci 233:155–164. https://doi.org/10.1016/j.plantsci.2015.01.012
Subbarao GV, Arango J, Masahiro K, Hooper AM, Yoshihashi T, Ando Y, Nakahara K, Deshpande S, Ortiz-Monasterio I, Ishitani M, Peters M, Chirinda N, Wollenberg L, Lata JC, Gerard B, Tobita S, Rao IM, Braun HJ, Kommerell V, Tohme J, Iwanaga M (2017) Genetic mitigation strategies to tackle agricultural GHG emissions: the case for biological nitrification inhibition technology. Plant Sci 262:165–168. https://doi.org/10.1016/j.plantsci.2017.05.004
Sun L, Lu YF, Yu FW, Kronzucker HJ, Shi WM (2016) Biological nitrification inhibition by rice root exudates and its relationship with nitrogen use efficiency. New Phytol 212:646–656. https://doi.org/10.1111/nph.14057
Tyerman SD (1992) Anion channels in plants. Annu Rev Plant Physiol Plant Mol Biol 43:351–357. https://doi.org/10.1146/annurev.pp.43.060192.002031
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003) Root exudation and rhizosphere biology. Plant Physiol 132:44–51. https://doi.org/10.1104/pp.102.019661
Winkel-Shirly B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493. https://doi.org/10.1104/pp.126.2.485
Yan F, Zhu Y, Müller C, Zörb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63. https://doi.org/10.1104/pp.010869
Zakir AKMZ, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol 180:442–451. https://doi.org/10.1111/j.1469-8137.2008.02576.x
Zeng H, Di T, Zhu Y, Subbarao GV (2016) Transcriptional response of plasma membrane H+-ATPase genes to ammonium nutrition and its functional link to the release of biological nitrification inhibitors from sorghum roots. Plant Soil 398:301–312. https://doi.org/10.1007/s11104-015-2675-2
Zerulla W, Barth T, Dressel J, Erhardt K, Von-Locquenghien KH, Pasda G, Radle M, Wissemeier H (2001) 3,4- dimethylpyrazole phosphate (DMPP)-a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soil 34:79–84. https://doi.org/10.1007/s003740100380
Zhang WH, Ryan PR, Tyerman SD (2004) Citrate-permeable channels in the plasma membrane of cluster roots of white lupin. Plant Physiol 136:3771–3783. https://doi.org/10.1104/pp.104.046201
Zhang X, Lu Y, Yang T, Kronzuck HJ, Shi W (2019) Fractors influening the release of the biological nitrification inhibitor 1,9-decanediol from rice (Oryza sativa L.) roots. Plant Soil 436:253–265. https://doi.org/10.1007/s11104-019-03933-1
Zhang M, Wang Y, Chen X, Xu F, Ding M, Ye W, Kawai Y, Toda Y, Hayashi Y, Zeng H, Xiao L, Xiao X, Xu J, Guo S, Yan F, Shen Q, Xu G, Kinoshita T, Zhu Y (2021) Overexpression of plasma membrane H+-ATPase OSA1 increases rice grain yield via simultaneous enhancement of nutrient uptake and photosynthesis. Nat Commun 12(1):735. https://doi.org/10.1038/s41467-021-20964-4
Zhao J, Dixon RA (2009) MATE transporters facilitate vacuolar uptake of epicatechin 3-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21:2323–2340. https://doi.org/10.1105/tpc.109.067819
Zhu Y, Yan F, Zörb C, Schubert S (2005) A link between citrate and proton release by proteoid roots of white lupin (Lupinusalbus L.) grown under phosphorus-deficient conditions? Plant Cell Physiol 46:892–901. https://doi.org/10.1093/pcp/pci094
Zhu Y, Di T, Hu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environm 32:1428–1440. https://doi.org/10.1111/j.1365-3040.2009.02009.x
Zhu Y, Zeng H, Shen Q, Ishikawa T, Subbarao GV (2012) Interplay among NH4+ uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant Soil 358:131–141. https://doi.org/10.1007/s11104-012-1151-5
Egenolf K, Verma S, Schöne J, Klaiber I, Arango J, Cadisch G, Neumann G, Rasche F (2021) Rhizosphere pH and cation-anion balance determinc the exudation of nitrification inhibitor 3-epi-brachialactone suggesting release via secondary transport. Physiol Plantar 1-8. https://doi.org/10.1111/ppl.13300
Funding
The research presented here is funded by Natural Science Foundation of China (NSFC 31172035) and grant-in-Aid for scientific research from Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) to JIRCAS under BNI project.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, M., Zeng, H., Afzal, M.R. et al. BNI-release mechanisms in plant root systems: current status of understanding . Biol Fertil Soils 58, 225–233 (2022). https://doi.org/10.1007/s00374-021-01568-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-021-01568-y