Abstract
Aims and background
The ability to suppress soil nitrification through the release of nitrification inhibitors from plant roots is termed ‘biological nitrification inhibition’ (BNI). Earlier, we reported that sorghum roots release higher BNI-activity when grown with NH +4 , but not with NO -3 as N source. Also for BNI release, rhizosphere pH of <5.0 is needed; beyond this, a negative effect on BNI release was observed with nearly 80% loss of BNI activity at pH >7.0. This study is aimed at understanding the inter-functional relationships associated with NH +4 uptake, rhizosphere-pH and plasma membrane H+-ATPase (PM H+-ATPase) activity in regulating the release of BNIs (biological nitrification inhibitors) from sorghum roots.
Methods
Sorghum was grown hydroponically and root exudates were collected from intact plants using a pH-stat system to separate the secondary acidification effects by NH +4 uptake on BNIs release. A recombinant luminescent Nitrosomonas europaea bioassay was used to determine BNI-activity. Root plasma membrane was isolated using a two-phase partitioning system. Hydrolytic H+-ATPase activity was determined. Split-root system setup was deployed to understand the localized responses to NH +4 , H+-ATPase-stimulator (fusicoccin) or H+-ATPase-inhibitor (vanadates) on BNI release by sorghum.
Results
Presence of NH +4 in the rhizosphere stimulated the expression of H+-ATPase activity and enhanced the release of BNIs from sorghum roots. Fusicoccin, which stimulates H+-ATPase activity, also stimulated BNIs release in the absence of NH +4 ; vanadate, which suppresses H+-ATPase activity, also suppressed the release of BNIs. NH +4 levels (in rhizosphere) positively influenced BNIs release and root H+-ATPase activity in the concentration range of 0-1.0 mM, indicating a close relationship between BNI release and root H+-ATPase activity with a possible involvement of carrier-mediated transport for the release of BNIs in sorghum.
Conclusion
Our results suggest that NH +4 uptake, PM H+-ATPase activity, and rhizosphere acidification are functionally inter-connected with BNI release in sorghum. Such knowledge is critical to gain insights into why BNI function is more effective in light-textured, mildly acidic soils compared to other soil types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrification is an important soil biological process in nitrogen (N) cycling, whereby ammonium is oxidized to nitrite, and subsequently to nitrate by nitrifying bacteria (Nitrosomonas and Nitrobacter, respectively). Nitrogen use efficiency (NUE) for global cereal production is about 33%. The unaccounted 67% represents a $US16.7 billion annual loss, assuming fertilizer–soil equilibrium (Raun and Johnson 1999). Nitrification is one of the main causes of N loss and associated environmental pollution as a result of NO -3 leaching and denitrification. Nitrification and the subsequent denitrification, are the primary drivers of N2O production from agricultural systems (Subbarao et al. 2009, 2012a). N2O is the third most important greenhouse gas contributing to global warming (Parker 1972; Meinshausen et al. 2009). If the nitrification process is depressed, N recovery by crop uptake can be improved, and NO -3 leaching and N2O emission can be reduced (Slangen and Kerkhoff 1984; Subbarao et al. 2006a; 2012a).
Nitrification can be reduced by the release of inhibitors from plant roots or by the application of synthetic nitrification inhibitors (Smart and Bloom 2001; Weiske et al. 2001). Application of synthetic nitrification inhibitors is currently the only method used to reduce soil nitrification in agricultural systems (Slangen and Kerkhoff 1984; Amberger 1989; Zerulla et al. 2001). Plant-derived nitrification inhibitors have been known for several decades (Moore and Waid 1971; Lata et al. 1999, 2004). In situ production of nitrification inhibitors, either by plant roots or microorganisms within the rhizosphere of growing crops or pastures, is an appealing low-cost alternative to synthetic nitrification inhibitors (Fillery 2007; Subbarao et al. 2009). Recently, a bioassay system was developed that enabled quantification of the inhibitory effects from roots. The phenomenon is termed biological nitrification inhibition (BNI) (Iizumi and Nakamura 1997; Iizumi et al. 1998; Subbarao et al. 2006b).
Cultivated sorghum was found to have significant BNI capacity (Hossain et al. 2008; Subbarao et al. 2012b), while other important cereals such as wheat (Triticum aestivum), barley (Hordeum vulgare), rice (Oryza sativa) and maize (Zea mays) did not show BNI capacity (Subbarao et al. 2007a). Our earlier results indicated that the release of BNIs is not dependent on the BNI concentration gradient across the plant cell membrane, but is rather regulated by an unknown transport process (Subbarao et al. 2007a). BNIs release is stimulated by NH +4 in the rhizosphere (Hossain et al. 2008; Subbarao et al. 2007b, c).
NH +4 uptake is known to depolarize the electrical membrane potential, and thereby increases net proton release (Wang et al. 1993; Schubert and Yan 1997). In contrast, the uptake of NO -3 results in H+ uptake by a plasmalemma H+ co-transport system (Mistrik and Ullrich 1996), leading to alkalization of the rhizosphere (Marschner and Römheld 1983; Moorby et al. 1985). In addition, the assimilation of NH +4 is a proton-generating process, whereas NO -3 assimilation is a proton-consuming process (Mengel et al. 1983; Pearson and Stewart 1993).
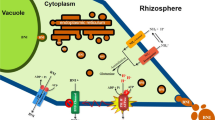
The PM H+-ATPase is a universal electrogenic H+ pump, which hydrolyzes ATP to pump H+ outside the plant cells. The key function of this enzyme is to generate an H+ electrochemical gradient, thereby providing the driving force for the active transport, i.e. influx and efflux of ions and metabolites across the plasma membrane (Palmgren and Harper 1999). In addition, PM H+-ATPase may significantly contribute to pH homeostasis of plant cells (Serrano 1989; Palmgren and Harper 1999). Accordingly, it was concluded that the enhanced activity of PM H+-ATPase could be responsible for the pumping of H+ against a higher H+ electrochemical gradient at low root medium pH in association with NH +4 uptake (Zhu et al. 2009). It is possible that such a mechanism in relation to NH +4 uptake may contribute to the release of BNIs from sorghum roots (Fig. 1). The present study is an attempt to understand the inter-functional relationships associated with NH +4 uptake, rhizosphere-pH and plasma membrane H+-ATPase activity in regulating the release of BNIs from sorghum roots.
Materials and methods
Cultivation of sorghum plants
Seeds of sorghum (Sorghum bicolor L. Moench var. hybrid sorgo) were germinated in trays containing vermiculite. Plants were grown in a growth chamber with a day : night temperature regime of 30 : 28°C, a photosynthetic photon flux, averaging at 300 μmol m−2 s−1 and a 14 : 10 h light : dark photoperiod. One-week-old seedlings were transferred to continuously aerated nutrient solution in 70 l tanks on styrofoam blocks with 45 holes and 4 plants per hole, supported with sponge. The composition of the nutrient solution (mg l−1) was as follows: KH2PO4, 38.31; K2SO4, 31.02; CaCl2·2H2O, 10.5; MgSO4·7H2O, 36.93; Fe-EDTA, 15.1; H3BO3, 0.57; CuSO4·5H2O, 0.078; MnSO4·6H2O, 2.35; Na2MoO4·2H2O, 0.126; ZnSO4·7H2O, 0.220. Nitrogen at 1 mM was added as (NH4)2SO4 or KNO3 to the nutrient solution; nutrient solutions were replaced at 3 d interval. The pH of the nutrient solution was adjusted to 5.0 with 1.0 M NaOH or 0.5 M H2SO4.
Root exudate collection
Root exudate is collected from intact plants at 14 DAT (days after transplantation). Intact plant roots (12 plants for each sample and replicated three times) were removed from the nutrient solution, rinsed with distilled water, then immersed for 4 h (from 10:00 am to 14:00 pm) in one L aerated collection solution (with 0.5 mM KCl, 1 mM CaCl2 and 1 mm NH4Cl or 1 mM KNO3). The solution pH was kept at 5 by pH-stat (NPH-660 NDE, Nissin, Japan) (Fig. 2). A pH-stat system (NPH-660 NDE, Nissin, Japan) was used in selected experiments where the pH of the root exudate collection solution is maintained at a constant pH of either 3.0 or 7.0 as per the treatment (Fig. 2). Long-term effects (i.e. 2 weeks) of N forms (NH +4 vs NO -3 ) in nutrient solutions on the BNI release, were investigated at a constant nutrient solution pH of either 3 or 7 (using pH-stat systems) to eliminate the secondary effects of pH changes associated with the uptake of NH +4 or NO -3 . Also, short-term effects (i.e. 4 h collection period) of N forms (NH +4 vs NO -3 ) in root exudate collection solutions on BNI release was investigated using sorghum plants raised with NH +4 or NO -3 as N source at a constant pH of 3 or 7. For the investigation of pharmacological agents, fusicoccin (stimulator of PM-ATPase) or vanadate (inhibitor of PM-ATPase) was added for root-treatment solutions. For fusicoccin root-treatment, fusicoccin was dissolved in ethanol and diluted to a concentration of 0.5 μM and 1.0 μM in the treatment solution; for vanadate root-treatment, sodium vanadate was added to the solution to a concentration of 0.1 mM and 0.5 mM, while the standard 1 mM NH4Cl solution was used as control solution. After keeping the roots for 4 h in these treatment solutions, root exudates are collected using standard root exudate collection solution (i.e. 1 mM NH4Cl) for a 4 h period to determine BNI release. After root exudate collection, roots were separated and weighted. The fresh roots are used directly for plasma membrane isolation. For the split-root experiment, sorghum plants were raised in nutrient solution with both NH +4 and NO -3 (i.e. NH4NO3 as N source) and then the roots were separated into two root compartments as shown in the Fig. 3. The fusicoccin root-treatment and vanadate-root treatment for the split-root system was similar to that described above for the whole plant system experiments.
Nitrification inhibition determination
For extraction of BNI compounds, root exudates were evaporated to dryness using a rotary evaporator (Buchi, V-850, Flawil, Switzerland) under vacuum at 45°C, followed by extraction with 20 ml of methanol. The methanol extract was further evaporated to dryness using a rotary evaporator at 40°C and the residue was extracted with 50 μl of dimethyl sulphoxide (DMSO). The DMSO extract was then used to determine the BNI activity using bioassay (Subbarao et al. 2006b).
The nitrification inhibition (NI) activity of the samples was determined using a modified bioassay that employs recombinant luminescent N. europaea (Iizumi et al. 1998; Subbarao et al. 2006b). The BNI activity of the samples is expressed in units defined in terms of the action of a standard inhibitor, allylthiourea (AT); the inhibitory effect of 0.22 μm AT in an assay containing 18.9 mm of NH +4 is defined as one ATU (AT unit) of activity (Subbarao et al. 2006b).
Plasma membrane isolation and determination of H±-ATPase activity
Root plasma membrane from sorghum was isolated according to Yan et al. (2002). Roots were cut and washed with de-ionized water and ground in ice-cold homogenization buffer. The homogenization buffer contained 250 mM sucrose, 250 mM KI, 2 mM EGTA, 10% (v/v) glycerol, 0.5% (w/v) bovine serum albumin, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 5 mM 2-mercaptoethanol, 50 mM 1,3-bis (tris [hydroxymethyl] methylamino) propane (BTP), and was adjusted to pH 7.8 with MES. The homogenate (adjusted to a grinding medium/tissue ratio of 4 mL g-1 fresh weight) was filtered through two layers of Miracloth (Calbiochem-Novabiochem, San Diego, USA) and centrifuged in a fixed rotor at 11,700 g (RP42A rotor, 94 mL*6, HITACHI Koki; Himac CP100WX Hitachi Ultra Centrifuge) and 0°C for 10 min. The supernatant was centrifuged at 106,000 g for 35 min. The microsomal pellets were re-suspended in BTP buffer, which contained 250 mM sucrose, 3 mM KCl and 5 mM BTP (pH 7.8).
The microsomal membrane preparation was fractionated by two-phase partitioning in aqueous dextran T-500 (Sigma) and polyethylene glycol (Sigma) according to the method of Larsson (1985). Phase separations were carried out in a series of 12-g phase systems that contained: 6.1% (w/w) dextran T-500, 6.1% (w/w) PEG 3350, 250 mM sucrose, 3 mM KCl, 5 mM BTP (pH 7.8). Stock solutions of polymers were prepared with concentrations of 20% and 40% (w/w) for dextran and polyethylene glycol, respectively. The concentration of dextran stock solution was determined by optical rotation (Larsson 1985). The phase stock was weighed and diluted to 6.1% (w/w, each polymer) with phase buffer to a final weight of 10 g. Polymers in "start tubes" were diluted to 26 g. Six g. microsomal re-suspension (in phase buffer) was added to the upper phase of each start tube. The tubes were sealed with Parafilm (American National Can, Greenwich, CT) and mixed by inversion (30 times). Phase separation was achieved at 4°C by centrifugation at 720 g (RPS40T swing rotor, 13 ml*6) for 23 min followed by two washing steps in identical phases. Centrifugation times for the second through fourth phases were 15, 10, and 5 min, respectively. The upper phases obtained after four separations were diluted with BTP buffer (see above) and centrifuged at 188,000 g (RP42A rotor, 94 mL*6) for 40 min. The pellets were re-suspended with BTP buffer, divided into aliquots and immediately stored in liquid nitrogen.
ATPase activity was determined by measuring the Pi amount after a 30 min hydrolysis reaction. The 0.5 mL reaction mixtures were composed of 30 mM BTP/MES, 5 mM MgSO4, 50 mM KCl, 50 mM KNO3, 1 mM Na2MoO4, 1 mM NaN3, 0.02% (w/v) Brij 58, and 5 mM disodium-ATP. Each reaction was initiated by the addition of 1 to 2 μg membrane protein, then allowed to proceed for 30 min at 30°C, before stopping the reaction with 1 mL of stopping reagent [2% (v/v) concentrated H2SO4, 5% (w/v) SDS, and 0.7% (w/v) (NH4)2MoO4)] followed immediately by 50 μL of 10% (w/v) ascorbic acid. After 10 min, 1.45 mL of arsenite-citrate reagent (2% [w/v] sodium citrate, 2% [w/v] sodium m-arsenite, and 2% [w/v] glacial acetic acid] was added to prevent the measurement of phosphate liberated because of ATPase activity from ATP hydrolysis under acidic conditions (Baginski et al. 1967). Color development was completed after 30 min and A720 was measured by means of a spectrophotometer. ATPase activity was calculated as the phosphate liberated in excess of a boiled-membrane control.
Statistical analysis of data
Variation is indicated by ± standard error (SE) (if bars exceed symbols in figures in figures). All experiments were repeated three times. Data from experiments were pooled for calculations of means and standard deviation (SD) and analyzed by one-way ANOVA followed by the LSD test at P < 0.05 to determine the statistical significance of the difference between individual treatments. All statistical evaluations were made with the SPSS (version 13.0) statistical software (SPSS Inc., Chicago, IL).
Results
Long-term effect of NH +4 vs. NO -3 on the release of BNIs and root PM H+-ATPase activity
Long-term effect of N-forms (i.e. 2 week growing period) significantly affected the pH of the nutrient solution. The NH +4 -fed sorghum plants strongly acidified the root medium close to pH 3.0, whereas the NO -3 -fed sorghum plants alkalized the medium to nearly pH 7.0 (data not shown). Based on these preliminary observations, the pH of the nutrient solutions for NH +4 treatment was set at 7.0 and that for the NO -3 treatment, at 3.0 using pH-stat systems during the plant growing period; this was done to separate the pH effects from N form influence on BNI release in sorghum roots.
After 2 weeks of plant growth in treatment solutions, root exudate was collected in collection solution pH of either 3.0 or 7.0. NH +4 cultivated plants released more BNIs than NO -3 cultivated plants at identical pH of the exudate collection solutions. In addition, both NH +4 and NO -3 cultivated sorghum plants released more BNI activity at pH 3 than at pH 7 (Fig. 4a). N-forms and pH of the root exudate collection solutions also affected PM H+-ATPase activity in a similar way (Fig. 4b). The simultaneous change in the BNIs release and PM H+-ATPase activity under NH +4 vs NO -3 at different medium pH, indicated that BNIs release is likely to be functionally linked to PM H+-ATPase.
Influence of N-forms (i.e. 1 mM N as NH +4 vs. NO -3 ) and root exudate collection solution pH (solution pH 3.0 vs. 7.0) on BNI release and root PM H+-ATPase in sorghum grown hydroponically for 14 d with NH +4 or NO -3 as N source. Error bars indicate ± SE (standard error) of 3 replications. Means with the same letter are not significantly different at P ≤ 0.05, according to one-way ANOVA followed by the LSD test
Short-term treatment of NH +4 vs NO -3 on BNIs release and root PM H+-ATPase activity
Sorghum plants were cultivated with NH +4 or NO -3 at a nutrient solution pH 5 for 2 weeks. These plants were used for root exudate collection using treatment solutions (1 mM NH +4 or NO -3 ) for 4 h. Addition of NH +4 in the root exudate solutions enhanced the release of BNIs from NO -3 -raised plants, which is nearly two-times higher than the addition of NO -3 (Fig. 5a) However, the BNI activity in the root exudate of NH +4 raised plants did not show a significant difference due the presence of NH +4 or NO -3 in the collection solution in this study (Fig. 5a). The PM H+-ATPase activity of NO -3 cultivated plant roots has increased after 4 h incubation with NH +4 , as compared that in roots fed with NO -3 . In addition, PM H+-ATPase activity of NH +4 cultivated plant roots did not change significantly after incubation with NO -3 (Fig. 5b). It indicated that NH +4 , rather than NO -3 , had a strong effect on the BNI release.
Short-term treatment of different concentration of NH +4 on the release of BNIs and PM H+-ATPase
For this experiment, sorghum plants were raised for 13 d with 1 mM N as NH +4 in the nutrient solution, and plants were shifted to N-free solutions for 24 h (i.e. 14th d) before using them for collecting root exudates in 0.0, 0.1, 0.5, and 1.0 mM NH4Cl solutions for a 4 h period, followed by extraction of the root tissue for PM H+-ATPase activity. The BNI release from sorghum roots increased with NH +4 concentration in the root exudate collection solution (Fig. 6a). Similarly, PM H+-ATPase activity increased with NH +4 concentration (0.0 to 1.0 mM) in the collection solution (Fig. 6b). The relationship between BNI release by roots and root PM H+ ATPase showed a linear relationship (Fig. 7).
BNIs release in the roots of sorghum (intact plants) and PM H+-ATPase activity in plants grown with NH +4 as the sole N source. Root exudates were collected with NH4Cl solutions ranging from 0.1, 1.0 and 5.0 mM. Error bars indicate ± SE of 3 replications. Means with the same letter are not significantly different at P ≤ 0.05, according to one-way ANOVA followed by the LSD test
Modification of PM H+-ATPase on BNIs release
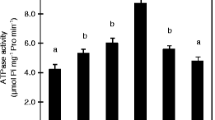
To understand further the role of PM H+-ATPase on BNIs release, we modified the root PM H+-ATPase activity by keeping the intact plant roots in solutions of fusicoccin and vanadate before the collection of root exudates. Fusicoccin, a phytotoxin produced by Fusicoccum amygdali, is a known stimulator of PM H+-ATPase activity in plant cells (Reid et al. 1985). Vanadate is an inhibitor of P-type ATPases, an analog of phosphate, which binds to the enzyme and thus blocks the reaction cycle (Palmgren and Harper 1999). In the earlier studies, we used NH +4 cultivated plants to test the effect of PM H+-ATPase on the release of BNIs. Fusicoccin at 1 μM significantly increased the BNIs exudation (Fig. 8). In contrast, BNI release was significantly suppressed by 0.5 mM vanadate (Fig. 8). Our results indicated that modification of PM H+-ATPase had a direct impact on the release of BNIs. Based on the above result, we selected 1 μM fusicoccin and 0.5 mM vanadate as treatments for the following split root system experiment.
Exposure of intact plant roots to treatment solutions of Fusicoccin and Vanadate on BNIs release in sorghum. Plants were grown with NH +4 as the sole N source before used for the collection of root exudates. Error bars indicate ± SE of 3 replications. Means with the same letter are not significantly different at P ≤ 0.05, according to one-way ANOVA followed by the LSD test
For the split root experiment, we cultivated sorghum with both NH +4 and NO -3 to eliminate the effect of N-forms on plant metabolism during plant growing period. Root exudate was collected using the following treatment solutions; 1 mM NH +4 , 1 mM NO -3 , 1 μM fussicoccin or 0.5 mM vanadate. Release of the BNIs was significantly higher in the part of roots supplied with 1 mM NH +4 compared with 1 mM NO -3 (Fig. 9a). In addition, without NH +4 , fusicoccin alone stimulated the BNI release, while vanadate completely inhibited the BNI release (Fig. 9b). These results further established a functional link between PM H+-ATPase activity and BNI release in sorghum. .
Influence of N-forms (1 mM N as NH +4 vs. NO -3 ) and H+-ATPase stimulator, fusicoccin (1 μM) or H+-ATPase inhibitor, vanadate (0.5 mM) on BNI release in sorghum in a split-root system setup. Error bars indicate ± SE of 3 replications. Means with the same letter are not significantly different at P ≤ 0.05, according to one-way ANOVA followed by the LSD test
Discussion
Earlier reports indicated an inhibitory effect on nitrification by sorghum root exudates (Alsaadawi et al. 1986; 19888). Our previous work also confirmed a direct inhibitory effect of sorghum root exudates on Nitrosomonas bacteria (Subbarao et al. 2007a, 2008, 2012a; b). In addition, our results demonstrated that the release of inhibitory compounds from sorghum roots is stimulated by the presence of NH +4 in root zone at low pH. However, the mechanism of the phenomenon is still not clear. In this study, we provided substantial evidence indicating a functional link between PM H+-ATPase activity and BNI release in sorghum.
NH4 + stimulated the PM H+-ATPase activity and BNI release in sorghum
Our results also indicate the role of NH +4 in stimulating PM H+-ATPase activity and release of BNI in both NH +4 and NO -3 fed sorghum. Unlike earlier studies (Subbarao et al. 2007b, c; 2009), we have separated the primary effects of NH +4 uptake and assimilation from the secondary effects of rhizosphere acidification on BNI release using pH-stat systems. Our results show that NH +4 as compared to NO -3 , stimulated the release of BNIs at all pH levels. Even NO -3 cultivated sorghum released BNIs in the presence of NH +4 (in exudate collection solutions), suggesting the critical role of NH +4 in the release of BNIs.
Uptake of NH +4 and NO -3 results in the depolarization of plasma membrane (Ullrich and Novacky 1990; Wang et al. 1994), which needs to be regenerated by PM H+-ATPase to maintain the uptake of nutrients for plant growth (Yamashita et al. 1995; Santi et al. 2003). Uptake of NH +4 by root cells does not consume H+, resulting in net H+ release from roots, hence the rhizosphere acidification. In contrast, NO -3 co-transport with H+ across the PM and its assimilation in root cells consume part of the H+ extruded by the PM+-ATPase, resulting in rhizosphere alkalization. In addition, NH +4 causes a stronger depolarization of PM than done by NO -3 ; thus, a higher membrane depolarization from NH +4 nutrition may further benefit PM H+-ATPase activity (Wang et al. 1993; Schubert and Yan 1997). From the results presented in this study, it is evident that PM H+-ATPase levels were substantially higher under NH +4 than in NO -3 . Also, the release of BNIs was stimulated by NH +4 (compared to NO -3 ) in the exudate collection solutions, irrespective of the rhizosphere pH. These results demonstrated the role of NH +4 in stimulating PM H+-ATPase activity and BNI release in sorghum. The simultaneous changes observed in PM H+-ATPase activity and BNI release from a series of experiments indicate the probability of a functional link.
Modification of PM H+-ATPase had a direct influence on BNIs release
It is generally agreed that in plant cells H+ is pumped out by the PM H+-ATPase activity. According to the hypothesis of ‘tissue-specific expression model’ (Palmgren and Harper 1999), the higher demands on H+ pumping activity in plant cells grown with NH +4 would cause a high regulation of the PM H+-ATPase activity. This implies that root cells pump more net H+ under NH +4 than under NO -3 nutrition. In our previous study, the BNIs were confirmed as anionic substances (Subbarao et al. 2006b). In plant cells, various anion channels were identified in plasma membrane, which are in general membrane potential dependent (Santi et al. 2003). ATP is a power house for most of the transport processes (Palmgren 2001). If the BNIs are transported through some voltage dependent anion channels, their release will be closely related to the regulation of PM H+-ATPase. This way, our approach of deploying pharmacological agents (i.e. fusicossin or vanadite) to alter H+-ATPase activity to understand its role in BNI release provided the direct evidence for our hypothesis (Fig. 1).
Fusicoccin, a fungal toxin, can bind to the phosphorylation site of plasma membrane H+-ATPase and release the auto-inhibitor domain in the C terminal. The phosphorylated PM H+-ATPase has a higher activity. In this study, the addition of 1 μM fusicoccin significantly increased the BNI release, either in the presence- or absence of NH +4 . Vanadate is a P-type ATPase inhibitor and competes with phosphate of ATP at the phosphorylation site, which blocks the reaction cycle of PM H+-ATPase. At 0.5 mM concentration, vanadate strongly inhibited the BNI release irrespective of NH +4 status in the rhizosphere, providing the first direct evidence for the functional significance of H+-ATPase in BNI release.
Conclusions and possible implications
The functional link between PM H+-ATPase and BNI release is further evident from studies where PM H+-ATPase and BNI release linearly responded to NH +4 concentration (0 to 1.0 mM range) in exudate collection solutions, providing a strong linear relationship with a probable involvement of carrier-mediated transport in BNI release. Our results suggest that NH +4 uptake, PM H+-ATPase activity and rhizosphere acidification are functionally inter-connected with BNI release in sorghum. Such knowledge is critical to gain insights on as to why BNI function can be more effective in light-textured slightly acidic soils compared to other soil types (Gopalakrishnan et al. 2009; Subbarao et al. 2012a, b).
References
Alsaadawi IS (1988) Biological suppression of nitrification by selected cultivars of Helianthus annuus L. J Chem Ecol 14:733–741
Alsaadawi IS, Al-Uquili JK, Alrubeaa AJ, Al-Hadithy SM (1986) Allelopathic suppression of weed and nitrification by selected cultivars of Sorghum bicolor (L.) Moench. JChem Ecol 12:209–219
Amberger A (1989) Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plant Anal 20:1933–1955
Baginski ES, Foa PP, Zak B (1967) Determination of phosphate: study of labile organic phosphate interference. Clinica Chimica Acta 15:155–158
Fillery IRP (2007) Plant-based manipulation of nitrification in soils: a new approach to managing N loss? Plant Soil 294:1–4
Gopalakrishnan S, Watanabe T, Pearse SJ, Ito O, Hossain AZKM, Subbarao GV (2009) Biological nitrification inhibition by Brachiaria humidicola roots varies with soil type and inhibits nitrifying bacteria, but not other major soil microorganisms. Soil Sci Plant Nutr 55:725–733
Hossain AKMZ, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol 180:442–451
Iizumi T, Nakamura K (1997) Cloning, nucleotide sequence, and regulatory analysis of the Nitrosomonas europaea dnaK gene. Appl Environ Microbiol 63:1777–1784
Iizumi T, Mizumoto M, Nakamura KA (1998) Bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. ApplEnviron Microbiol 64:3656–3662
Larsson C (1985) Plasma membrane. In Modern Methods of Plant Analysis (eds. HF Linskens & JF Jackson), pp. 85–104, Springer-Verlag, Berlin, Germany
Lata JC, Durand J, Lensi R, Abbadie L (1999) Stable coexistence of contrasted nitrification statuses in a wet tropical savanna system. Funct Ecol 13:762–763
Lata JC, Degrange V, Raynaud X, Maron PA, Lensi R, Abbadie L (2004) Grass populations control nitrification in savanna soils. Funct Ecol 18:605–611
Marschner H, Römheld V (1983) In vivo measurement of root induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Zeitschrift für Panzenphysiologie und Bodenkunde 111:241–251
Meinshausen M, Meinshausen N, Hare W, Raper SCB, Frieler K, Knutti R, Frame DJ, Allen MR (2009) Greenhouse-gas emission targets for limiting global warming to 2 C. Nature 458:1158–1162
Mengel K, Robin P, Salsac L (1983) Nitrate reductase activity in shoots and roots of maize seedlings as affected by the form of nitrogen nutrition and the pH of the nutrient solution. Plant Physiol 71:618–622
Mistrik I, Ulrich C (1996) Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO3- and H+/H2PO4- stoichiometrics. Plant Physiol Biochem 34:629–636
Moorby H, Nye P, White R (1985) The influence of nitrate nutrition on H + efflux by young rape plants (Brassica napus cv. emerald). Plant Soil 84:403–415
Moore DRE, Waid JS (1971) The influence of washing of living roots on nitrification. Soil Biol Biochem 3:69–83
Palmgren MG (2001) Plant plasma membrane H + −ATPase: powerhouses for nutrient uptake. Anu Rev Plant Mol Biol 52:817–845
Palmgren M, Harper J (1999) Pumping with plant P-type ATPases. J Exptl Bot 50:883–893
Parker JH (1972) How fertilizer moves and reacts in soil. Crops Soils 72:7–11
Pearson J, Stewart GR (1993) The deposition of atmospheric ammonia and its effects on plants. New Phytol 125:283–305
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363
Reid RJ, Field LD, Pitman MG (1985) Effects of external pH, fusicoccin and butyrate on the cytoplasmic pH in barley root tips measured by 31P-nuclear magnetic resonance spectroscopy. Planta 166:341–347
Santi S, Locci G, Monte R, Pinton R, Varanini Z (2003) Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. J Exptl Bot 54:1851–1864
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Zeitschrift für Panzenphysiologie und Bodenkunde 160:275–281
Serrano R (1989) Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol 40:61–94
Slangen J, Kerkhoff P (1984) Nitrification inhibitors in agriculture and horticulture: a literature review. Fertil Res 5:1–76
Smart DR, Bloom AJ (2001) Wheat leaves emit nitrous oxide during nitrate assimilation. Proc Nat Acad Sci (USA) 98:7875–787
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006a) Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Crit Rev Plant Sci 25:303–335
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006b) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007a) Biological nitrification inhibition (BNI) - is it a widespread phenomenon? Plant Soil 294:5–18
Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL (2007b) NH +4 triggers the synthesis and release of biological nitrification inhibition compounds in Brachiara humidicola roots. Plant Soil 290:245–257
Subbarao GV, Ban T, Kishi M, Ito O, Samejima H, Wang HY, Pearse SJ, Gopalakrishnan S, Nakahara K, Hossain AKMZ, Tsujimoto H, Berry WL (2007c) Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 299:55–64
Subbarao GV, Nakahara K, Ishikawa T, Yoshihashi T, Ito O, Ono H, Ohnishi-Kameyama M, Yoshida M, Kawano N, Berry WL (2008) Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 313:89–99
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Nat Acad Sci (PNAS) (USA) 106:17302–17307
Subbarao GV, Sahrawat KL, Nakahara K, Ishikawa T, Kishii M, Rao IM, Hash CT, George TS, Srinivasa rao P, Nardi P, Bonnett D, Berry W, Suenaga K, Lata JC (2012a) Biological nitrification inhibition (BNI) – A novel Strategy to regulate nitrification in agricultural systems. Adv Agron 114:249–302
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Yiyong Zhu, Zakir HAKM, Deshpande SP, Hash CT, Sahrawat KL (2012b) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil (accepted).
Ullrich CI, Novacky A (1990) Extra- and intracellular pH and membrane potential changes induced by K+, Cl-, H2PO -4 and NO -3 uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol 94:1561–1567
Wang MY, Siddiqi MY, Ruth TJ, Glass A (1993) Ammonium uptake by rice roots. II. Kinetics of 13NH +4 influx across the plasmalemma. Plant Physiol 103:1259–1267
Wang MY, Glass ADM, Shaff JE, Kochian LV (1994) Ammonium uptake by rice roots. Plant Physiol 104:899–906
Weiske A, Benckiser G, Ottow JCG (2001) Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr Cycl Agroecosys 60:57–64
Yamashita K, Kasai M, Ezaki B, Shibasaka M, Yamamoto Y, Matsumoto H, Sasakawa H (1995) Stimulation of H+ extrusion and plasma membrane H+-ATPase activity of barley roots by ammonium-treatment. Soil Sci Plant Nutr 41:133–140
Yan F, Zhu Y, Muller C, Zorb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Zerulla W, Barth T, Dressel J, Erhardt K, Von-Locquenghien KH, Pasda G, Radle M, Wissemeier H (2001) 3, 4-Dimethylpyrazole phosphate (DMPP)-a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Acknowledgements
This work was supported by a Grant-in-Aid for scientific research from the Ministry of Agriculture, Forestry and Fisheries of Japan and Natural Science Foundation of China (NSFC 31172035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Zhu, Y., Zeng, H., Shen, Q. et al. Interplay among NH +4 uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant Soil 358, 131–141 (2012). https://doi.org/10.1007/s11104-012-1151-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1151-5