Abstract
This study evaluated the impacts of a nitrification inhibitor (3,4-dimethylpyrazole phosphate, DMPP) and herbicides (atrazine and glyphosate) on nitrification, gross nitrite, and nitrate (NO2−-N + NO3−-N) consumption rate, nitrous oxide (N2O) emission, and abundances of microbial functional genes related to nitrogen (N) cycling in an Australian sugarcane soil. The experiment included four treatments: blank control (CK), DMPP application (NI), atrazine application (ATR), and glyphosate application (GLY). All treatments received (NH4)2SO4 at 50 mg N kg−1 dry soil and KNO3 at 50 mg N kg−1 dry soil and were incubated initially at 55% of water holding capacity (WHC) for 7 days and subsequently at 75% WHC for another 7 days (K15NO3 with 5 atom% 15N added at the beginning of each stage). Compared with the CK treatment, DMPP application significantly decreased N2O emissions throughout the incubation, while atrazine or glyphosate application significantly inhibited N2O emissions only during the 4–7-day period. DMPP application also decreased ammonium-oxidizing bacteria (AOB) amoA gene abundances, gross NO2−-N + NO3−-N consumption rates at 55 and 75% WHC, and nirS and nirK gene abundances of denitrifiers at 75% WHC. The atrazine and glyphosate applications decreased the gross nitrification and NO2−-N + NO3−-N consumption rates, abundances of both ammonium-oxidizing archaea (AOA) and AOB amoA genes at 55 and 75% WHC, and abundances of functional genes related to different reactions of the denitrification during the incubation. These results suggested that DMPP, atrazine, and glyphosate could decrease soil gross nitrification and denitrification rates perhaps by inhibiting microbial functional gene abundances and that application of DMPP could effectively reduce N2O emissions in the sugarcane cropping soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the largest sources of energy for human consumption and biofuel, sugarcane (Saccharum spp.) farming has significant economic and environmental implications (Thorburn et al. 2010). Sugarcane productivity largely relies on nitrogen (N) fertilizer input, with annual N application rates ranging from 100 to 300 kg N ha−1 (Nachimuthu et al. 2016; Wang et al. 2016b). Sugarcane is mainly cropped in sub-tropical and tropical areas. The local wet and warm climate can stimulate N loss from the cropping systems via leaching, runoff, and denitrification, which can have detrimental impacts on the environment through water pollution and nitrous oxide (N2O) emission (Kroon et al. 2016; Wang et al. 2016b).
Nitrification is a key process in N biogeochemistry and is mainly conducted by nitrifiers (Martens-Habbena et al. 2009; Sabba et al. 2015). During this process, ammonium (NH4+-N) is firstly oxidized to hydroxylamine (NH2OH), then to nitrite (NO2−-N) and eventually to nitrate (NO3−-N). The NO3−-N is also the substrate for denitrification in soils and can be easily washed into waterways. Excessive NO3−-N in surface water could lead to eutrophication, and high NO3−-N content in drinking water is harmful to human health (Soares et al. 2012). Moreover, nitrification and denitrification result in nitrous oxide (N2O) emissions from soils (Menéndez et al. 2012; Scheer et al. 2014). Nitrification inhibitors have been investigated extensively as potential means to reduce N2O emissions and enhance utilization efficiencies of applied N fertilizers (Decock 2014; Scheer et al. 2014). As one of the most popular nitrification inhibitors, 3,4-dimethylpyrazole phosphate (DMPP) has several distinct advantages: (1) DMPP is often more effective, relative to other products, and an application rate of 0.5–1.5 kg ha−1 is sufficient to obtain an optimal inhibition effect (Zerulla et al. 2001; Scheer et al. 2014); (2) DMPP application could reduce NO3−-N accumulations in crops and thus improve qualities of agricultural products (Zerulla et al. 2001; Chaves et al. 2006); (3) DMPP is less mobile in soils and may stay in the fertilized spot where NH4+-N is adsorbed (Yu et al. 2007; Kong et al. 2016); and (4) DMPP is mineralized slowly in soils, and its effect could last for a relatively long time (Weiske et al. 2001).
In sugarcane production, weeds are serious threats to yield (Kaur et al. 2016; Nachimuthu et al. 2016). Generally, weeds have well-developed root systems and higher efficiencies of nutrient transportation, and thus they have competitive advantages for nutrient uptake over the sugarcane crops (Anjum and Bajwa 2007; Fahad et al. 2014). Weeds also compete for space, water, and light with the sugarcane crops and release some allelochemicals into the rhizosphere (Fahad et al. 2014). Therefore, it is often essential to apply herbicides, such as atrazine and glyphosate, to control the weeds in sugarcane farming. However, once the herbicides enter into soils, they might generate non-target effects on soil microorganisms, including those involved in N reactions (Haney et al. 2002; Mahía et al. 2011; Nguyen et al. 2016).
Soil moisture is a key factor influencing the abundances of functional genes related to N cycling and the effects of applied agrochemicals (Jiang et al. 2015; Martins et al. 2015). Therefore, responses of nitrification, denitrification, and N2O emission to applied agrochemical could be affected by soil moisture (Martins et al. 2015; Bento et al. 2016; Cai et al. 2016). To the best of our knowledge, little information is available with respect to the effects of DMPP or herbicides on N cycling microorganisms in tropical or subtropical sugarcane cropping soils. In this study, we employed the isotope labeling method to quantify the effects of different agrochemicals (DMPP, atrazine, and glyphosate) on gross nitrification and NO2−-N + NO3−-N consumption rates at different moisture levels. In the meantime, soil N2O emissions and functional gene abundances were also determined. This study aimed to improve our understanding of the potential impacts of DMPP and herbicides on N transformations and N2O emissions and their relationships with biotic and abiotic factors in sugarcane cropping soils.
Materials and methods
Soil samples and chemicals
Soils were collected from a sugarcane field (18° 37′ S, 146° 07′ E) near the township of Ingham in Queensland, Australia. Mean annual temperature in this region is 24.0 °C, and mean annual rainfall is 2110 mm. The site had a fourth ratoon sugarcane crop (NQ239), initially planted in August 2012 on raised beds with a row spacing of 165 cm. The cropping field received about 150 kg N ha−1 in October 2016. Green cane trash (residue) blanketing had been carried out in this field since 1987, with the crop residues (approximately 10 t of dry matter-equivalent ha−1) being retained on the ground after harvest each year. Surface soil samples (0–10 cm) were randomly taken from the cropping beds, homogenized, and divided into two parts. One part of the soil sample was air-dried and sieved to < 2 mm for determinations of soil physical and chemical properties, and the other part was used for the incubation experiment. Main physical and chemical properties of the soil were clay content, 19%; sand content, 62%; pH (in water), 5.0; electrical conductivity, 3.4 ms m−1; total C content, 10.8 g kg−1 dry soil; and total N content, 0.84 g kg−1 dry soil (Wang 2016). The DMPP (ChemCruz™®, Santa Cruz Biotechnology, USA) was a water-soluble chemical agent grade powder containing 98% of the active ingredient. Atrazine (Gesaprim Granules®, Syngenta Crop Protection Pty Ltd., Australia) and glyphosate (Yates Zero®, Yates, Australia) were water-soluble commercial herbicides with active ingredient contents of 900 g kg−1 and 440 g L−1, respectively.
Experimental design
Four treatments were included in this study: (1) nitrification inhibitor DMPP application (NI) at 1% of the NH4+-N input, (2) atrazine application (ATR) at 2.25 mg active ingredient kg−1 soil (on dry mass basis, equivalent to 3.00 kg active ingredient ha−1), (3) glyphosate application (GLY) at 2.16 mg active ingredient kg−1 soil (equivalent to 2.88 kg active ingredient ha−1); and (4) the blank control without any agrochemical application (CK). Each treatment had three replicates. The DMPP, atrazine, and glyphosate application rates were based on the respective recommended rates (Bonfleur et al. 2015; Zhang et al. 2018). Soil samples (50 g dry weight) were placed into 500 mL glass jars (6.8 cm in external diameter × 14.5 cm in height). The agrochemicals were dissolved in double distilled H2O (ddH2O) and then added to the soils. Nine soil samples for each treatment were treated with (NH4)2SO4 at 50 mg N kg−1 soil, K15NO3 (5 atom% 15N) at 10 mg N kg−1 soil, and KNO3 (unlabeled) at 40 mg N kg−1 soil, and six soil samples for each treatment were initially treated with (NH4)2SO4 at 50 mg N kg−1 soil and KNO3 (unlabeled) at 50 mg N kg−1 soil. The soil samples were first adjusted to 55% water holding capacity (WHC). Three replicates of the nine soil samples treated with K15NO3 (5 atom% 15N) were destructively sampled on days 0, 3, and 7. To minimize possible impacts of 15NO3-N recycling between different N forms, K15NO3 (5 atom% 15N) solutions were added into the remaining six soil samples at 1.0 mg N kg−1 dry soil on day 8 so as to adjust the soil moistures to 75% WHC. Test soils were further incubated and sampled (three replicates) on days 10 and 14. During the incubation, the glass jars were covered with parafilm and incubated in the dark at 28 °C, and an amount of ddH2O equal to the weight loss was added to maintain soil moisture, when necessary. At each destructive sampling, the soils in each jar were homogenized and randomly taken to determine soil mineral N contents and microbial properties.
Determinations of mineral N contents and 15N abundances in NO2 −-N + NO3 −-N
Soil exchangeable NH4+-N and NO2−-N + NO3−-N were extracted by 2 M KCl (1:5 w/v). The extract was then filtered, and the filtrate (7–8 mL) was analyzed for mineral N (NH4+-N and NO2−-N + NO3−-N) content using a colorimetric method (Rayment and Lyons 2010). The same amount of KCl solution was also filtered and analyzed as the blank.
The 15N abundance in NO2−-N + NO3−-N was determined using the method described by Mary et al. (1998) with minor modification. Briefly, each filtrate (10 mL) was transferred into a 70-mL plastic jar. After adding 0.4 g of MgO into the filtrate, the jar was immediately sealed and placed on a rotary shaker for 7 days. The NH4+-N was transformed into NH3, and then the jar was opened to allow the NH3 to volatilize. A filter paper disk (0.5 cm in diameter) was treated with 5 μL of 2.5 M KHSO4, enclosed in Teflon tape and stuck to the lid of the jar. After addition of 0.2 g of reducing agent (Devarda’s alloy), the jar was immediately closed and placed in the rotary shaker at 25 °C for 7 days. The NO2−-N + NO3−-N was converted to the NH3 which was then absorbed by the KHSO4-impregnated filter paper disk. After drying in a desiccator with concentrated H2SO4, the filter paper disk was encapsulated into a tin cup, and the 15N atom% in NO2−-N + NO3−-N was determined with a mass spectrometer (Isoprime-EuroEA 3000, Milan, Italy). The (NH4)2SO4 was used as an elemental standard to calculate the total N content. Primary standards (IAEA-N1 and IAEA-N2) were employed to calibrate the instrument for the quantification of 15N abundance.
Gas sampling and determination
The incubation jar was flushed with compressed air and sealed for 24 h prior to gas sampling, with the exception of the first gas sampling (0–6-h period). At the end of the enclosure period, a gas sample was taken with a 25-mL syringe from the headspace of each jar and was injected into a pre-evacuated 12-mL vial. The gas sample was analyzed with a gas chromatograph (Varian CP-3800, Middelburgh, Netherlands) having an electron capture detector (ECD). High purity N2 was used as the carrier gas, and the column and ECD temperatures were 45 and 310 °C, respectively (Wang et al. 2011). A series of standard gas samples with different concentrations of N2O (0, 0.4, 5, 10, and 20 μL L−1) were used for calibrating the gas chromatograph. The cumulative N2O emissions were calculated assuming linear changes in N2O emission rates during the period between two consecutive gas sampling events.
Soil DNA extraction and real-time quantitative PCR (qPCR)
Soils (approximately 0.25 g) were extracted for the total genomic DNA by a DNeasy® PowerSoil® Kit (QIAGEN, Gmbh, Germany). After extraction, DNA quality and concentration were evaluated with a Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). The DNA samples were kept at − 20 °C prior to determinations of the microbial properties.
Abundances of N cycling functional genes, including ammonium-oxidizing archaea and bacteria (AOA and AOB) amoA genes and denitrifying NO3−-N reductase (narG), NO2−-N reductase (nirK and nirS), and N2O reductase (nosZ) genes, were quantified by the qPCR with a Bio-Rad Real-Time Detection System. The reaction system was 20.0 μL containing 0.5 μL of forward primer, 0.5 μL of reverse primer, 2.0 μL of DNA, 7.0 μL of sterile ddH2O, and 10.0 μL of SYBR® Premix Ex Taq™ (TaKaRa Biotech). The primers of functional genes with the related references and qPCR reaction conditions were shown in Table S1. The negative control with sterile ddH2O as amplification template was also used in each gene determination. The standard was obtained by performing serial dilutions of the plasmid with the target gene. After the determination of fluorescence, melting curve analysis was also conducted to confirm PCR product specificity (Zhang et al. 2018). In this study, the amplification efficiencies ranged from 95.1 to 118.9%, with R2 values > 98.5% for the calibration curves of functional genes.
Calculations and statistical analysis
Based on NO2−-N + NO3−-N contents and 15N atom% of NO2−-N + NO3−-N, net nitrification, gross nitrification, and gross NO2−-N + NO3−-N consumption (denitrification and biological immobilization) rates were calculated as follows (Mary et al. 1998; Gómez-Rey and González-Prieto 2015):
where N1 and N2 were the NO2−-N + NO3−-N contents at time T1 and T2, respectively. The EN1 and EN2 were the 15N atom % excess of NO2−-N + NO3−-N at time T1 and T2, respectively.
Two-way analysis of variance was conducted to determine significant differences among treatments, sampling time, and their interactions using SPSS v. 21.0, followed by Duncan’s multiple range test to determine significant differences (P < 0.05) among the different treatments. The MATLAB clustergram function was employed to generate a heat map for visual representation of gene abundances in different treatments and incubation times. The functional gene abundances, soil moistures, and mineral N contents were the candidate variables in the path analyses for gross nitrification and gross NO2−-N + NO3−-N consumption rates. Stepwise regression analyses were employed for fitting the best prediction models of daily N2O emissions with soil moistures, mineral N contents, and functional gene abundances. Moreover, the relationships among all functional gene abundances and daily N2O emissions under different soil moisture conditions were evaluated by the principal component analysis (PCA).
Results
Effects of DMPP and herbicides on gross nitrification and gross NO2 −-N + NO3 −-N consumption rates
Soil NO2−-N + NO3−-N contents in the CK treatment changed little during the first 7 days of incubation, but increased from 53.5 ± 1.2 mg N kg−1 soil on day 7 to 74.2 ± 1.7 mg N kg−1 soil on day 14 (Fig. S1A). The exchangeable NH4+-N content decreased from days 7 to 14 (Fig. S1B). The four treatments had approximately similar net nitrification rates at 55 or 75% WHC (the slopes of changes in NO2−-N + NO3−-N contents with time under each treatment in Fig. S1A). Compared with the CK treatment, DMPP, and atrazine applications significantly decreased gross nitrification rates from day 3 onwards (P < 0.05; Table 1). Glyphosate application decreased gross nitrification rates during the 0–3 and 10–14-day period to 34.0 and 45.2% of those in the CK treatment, respectively, but had negligible effect on gross nitrification rate during the 4–7-day period. Gross NO2−-N + NO3−-N consumption rates exhibited similar treatment effects to the gross nitrification rates (Table 1). The agrochemical applications generally decreased gross NO2−-N + NO3−-N consumption rates, except the ATR treatment during the 0–3-day period.
Effects of DMPP and herbicides on N2O emissions
As shown in Table 2, the NI treatment always had the lowest cumulative N2O emissions among the four treatments throughout the incubation. The N2O emissions in the ATR and GLY treatments were significantly lower than that in the CK treatment during the 4–7-day period (P < 0.05), but not during other incubation periods. In the whole incubation, compared with the CK treatment, DMPP application significantly decreased total N2O emissions (P < 0.05), and applications of atrazine and glyphosate also decreased total N2O emissions, with the differences between the CK and ATR or GLY treatments being not significant.
Effects of DMPP and herbicides on abundances of AOA amoA and AOB amoA genes
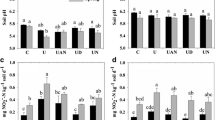
During the first 7 days at 55% WHC, the AOA amoA gene abundances increased with time in the CK treatment (Fig. 1a). The AOA amoA gene abundances in the NI treatment did not significantly differ with respect to those in the CK treatment during the 3–14-day period. However, DMPP application significantly decreased the AOB amoA gene abundances from day 3 onwards (P < 0.05). Atrazine or glyphosate application resulted in significantly lower abundances of both AOA and AOB amoA genes, relative to the CK treatment (Fig. 1). The atrazine and glyphosate had similarly negative impacts on the abundances of AOA and AOB amoA genes at 55% WHC, but the inhibitory effects of glyphosate diminished considerably at 75% WHC.
Effects of nitrification inhibitor and herbicides on abundances of soil a AOA amoA and b AOB amoA genes related to nitrification. CK blank control, NI nitrification inhibitor DMPP, ATR atrazine, and GLY glyphosate. Significant differences (P < 0.05) among different treatments for the same incubation time were shown with different lower case letters
Effects of DMPP and herbicides on abundances of functional genes related to denitrification
The DMPP and herbicide applications generated various negative effects on abundances of functional genes related to denitrification (Fig. 2). In the CK treatment, the narG gene abundance increased with time in the first 7 days of incubation and then changed slightly at 75% WHC (Fig. 2a). Compared with the CK treatment, the narG gene abundance was not significantly affected by the DMPP application. According to the results of Fig. 2b, atrazine application consistently reduced nirK gene abundances from day 3 onwards, and the nirK gene abundance in the ATR treatment was 29.6% of that in the CK treatment at the end of incubation. Compared with the CK treatment, DMPP application had negligible effects on nirK and nirS gene abundances at 55% WHC, but significantly decreased nirK and nirS gene abundances at 75% WHC (P < 0.05). Atrazine and glyphosate applications also decreased nirS gene abundances at 75% WHC (Fig. 2c). The nosZ gene abundances were decreased by the herbicides on day 3, but recovered to comparable levels to the CK treatment from day 7 onwards (Fig. 2d). At the end of incubation, there was no significant difference in nosZ gene abundances among the four treatments.
Effects of nitrification inhibitor and herbicides on abundances of soil a narG, b nirK, c nirS, and d nosZ genes related to denitrification. CK blank control, NI nitrification inhibitor DMPP, ATR atrazine, and GLY glyphosate. Significant differences (P < 0.05) among different treatments for the same incubation time were shown with different lower case letters
Relationships among functional gene abundances, mineral N contents, N2O emissions, and N transformation rates
The abundances of functional genes revealed treatment-induced effects from day 7 onwards (Fig. S2), and the samples of CK treatment on days 7, 10, and 14 were distinctly clustered, while earlier CK samples were mixed among the other treatments. The path analysis of factors potentially affecting gross nitrification rates (CMIN/df = 7.937, GFI = 0.891, and CFI = 0.911) indicated that soil moisture, exchangeable NH4+-N, NO2−-N + NO3−-N, AOA amoA, and AOB amoA explained 46.0% of the variance in gross nitrification rates (Fig. 3a). The increases in abundances of both AOA amoA and AOB amoA genes probably enhanced gross nitrification rates, and the effect of AOB amoA gene was stronger than that of AOA amoA gene. For gross NO2−-N + NO3−-N consumption rates, the path analysis also had an acceptable model (CMIN/df = 1.223, GFI = 0.975, and CFI = 0.997). Among the four functional genes, the nirS gene had the strongest impact on the gross NO2−-N + NO3−-N consumption rates (Fig. 3b).
Path analyses of the factors potentially affecting a gross nitrification and b gross NO2−-N + NO3−-N consumption rates during the incubation. Solid arrows showed positive effects, and dash arrows showed negative effects. Data on the arrows were standardized direct effects, and * and ** showed statistically significant at P < 0.05 and P < 0.01, respectively
The stepwise regression models for predicting daily N2O emissions differed for the different incubation periods (Table 3). Throughout the 14-day incubation, daily N2O emissions were positively correlated with AOB amoA abundances and soil moistures. At 55% WHC, daily N2O emissions were best described by the AOB amoA and nosZ gene abundances, with a positive and a negative relationship, respectively. However, when soil moisture was adjusted to 75% WHC, daily N2O emissions were best described by the nirS gene abundances. An overall PCA also demonstrated the relationships among functional gene abundances and daily N2O emissions at different soil moistures (Fig. 4). At 55% WHC, the two-dimensional PCA plot explained 80.85% of the total variance, with PCA1 accounting for a greater percentage (67.46%). The N2O emissions at 55% WHC were positively related to AOB amoA and AOA amoA gene abundances, but had negative relationship with nosZ gene abundances (Fig. 4a). At 75% WHC, the PCA1 and PCA2 explained 50.45 and 18.74% of the total variance, respectively, and the N2O emissions had close correlations with nirS, nosZ, and AOB amoA gene abundances (Fig. 4b).
Discussion
Ammonia oxidation is the rate-limiting step of soil nitrification, and previous studies have demonstrated that nitrification rates were positively correlated with AOA or AOB amoA gene abundances (Caffrey et al. 2007; He et al. 2007; Prosser and Nicol 2012). Relative to the CK treatment, DMPP application inhibited gross nitrification rates (Table 1), similar to what has already been reported (Florio et al. 2014; Liu et al. 2015). However, previous studies revealed different mechanisms of DMPP in controlling soil nitrification. Liu et al. (2015) suggested that DMPP application could inhibit soil nitrification by decreasing AOA amoA gene abundances rather than AOB amoA gene abundances in acid soils. Florio et al. (2014) also demonstrated that the transcription of AOA amoA gene was more sensitive to the DMPP than its bacterial counterpart. However, DMPP application was found to have negligible effects on AOA or AOB growth by Kong et al. (2016). Our study demonstrated that DMPP application significantly decreased AOB amoA gene abundances, whereas it had negligible effects on AOA amoA gene abundances (Fig. 1a). The decline in AOB amoA gene abundances confirmed the finding by Kleineidam et al. (2011) and coincided with the decline in gross nitrification rates. Therefore, DMPP application inhibited gross nitrification perhaps by decreasing the abundances of AOB amoA gene in this sugarcane cropping soil.
Atrazine or glyphosate application also decreased gross nitrification rates (Table 1). Previous studies demonstrated that atrazine application could generate non-target effects on soil microorganisms and decrease soil microbial biomass (Mahía et al. 2008, 2011). Indeed, both soil AOA and AOB amoA gene abundances decreased after atrazine application in the present study. The AOB amoA gene abundances also significantly decreased in response to glyphosate application (Fig. 1). The non-target suppressive effects of atrazine and glyphosate on the functional genes related to N cycling might be responsible for the inhibitions of gross nitrification and gross NO2−-N + NO3−-N consumption and thus the decrease in N2O emission. Jiang et al. (2015) also found that application of the herbicide butachlor reduced N2O emission by decreasing denitrifying bacteria abundance in a rice-wheat cropping system. Moreover, atrazine application could decrease soil urease activity and N mineralization rate, and glyphosate application could inhibit soil N fixation (Santos and Flores 1995; Mahía et al. 2011). These observations and our results suggest that most soil N transformations could be suppressed by atrazine and glyphosate applications. However, soil characteristics, such as pH value and moisture, could affect the longevity and impact of the applied herbicide (Bento et al. 2016; Nguyen et al. 2016). For example, in contrast to the above findings, Allegrini et al. (2017) found that in the Argentine soils, abundances of AOB amoA gene (copies ng−1 DNA) were not affected by three repeated glyphosate applications at 49.0 mg kg−1 soil per application. Mahía et al. (2011) showed that soil N availability was a key factor determining the degradation rate of atrazine. Future study should examine the effects of herbicides on other N cycling processes in relation to soil properties, and longer incubation periods are also needed to determine the longevities of the effects after herbicide applications.
The ratios of AOA to AOB amoA gene abundances in this study ranged from 5.79 to 47.49, confirming previous reports that the AOA amoA gene was more abundant than AOB amoA gene in some soils (Hayden et al. 2010; Martins et al. 2015). Generally, AOA could inhabit soils under a broad range of environmental conditions and are less susceptible to soil disturbances than AOB (Ouyang et al. 2016; Zhang et al. 2016), due to the differences in cellular compositions, energy metabolisms, and tolerances to soil xenobiotics (French et al. 2012; Hatzenpichler 2012). However, there are controversial views on the roles of AOA and AOB in soil nitrification. Prosser and Nicol (2012) found that AOA amoA gene abundance controlled soil nitrification, whereas we suggested that in this soil, AOB amoA gene was more important in determining gross nitrification rate than AOA amoA gene (Fig. 3), confirming the finding by Jia and Conrad (2009). Moreover, others showed that both AOA amoA and AOB amoA genes could influence soil nitrification (He et al. 2007; Wang et al. 2016a). Selective inhibitors, such as 1-octyne specifically inhibiting AOB ammonia monooxygenase, may be used to investigate the contributions of AOB and AOA to soil nitrification and N2O emission in future studies (Wang et al. 2016a).
As a key greenhouse gas, N2O can be emitted from both soil nitrification and denitrification. There has been no consensus on the relationships between soil N2O emissions and abundances of functional genes in soil N cycling (Liu et al. 2013; Wu et al. 2017). According to Liu et al. (2013), N2O emissions from forest soils were regulated by environmental factors rather than abundances of functional genes related to denitrification. In contrast, Wu et al. (2017) demonstrated that nirK and nirS gene abundances were explanatory variables in estimating N2O emissions from different agricultural soils. Considering that denitrification occurs under anaerobic conditions, nitrification should be the main process of N2O production at 55% WHC, since this moisture was not favorable for soil denitrification (MacGregor 1972; Lan et al. 2013). This hypothesis was supported by the positive correlations between N2O emissions and AOB amoA gene abundances (Table 3; Fig. 4a). However, at 75% WHC, denitrification activity could increase, and soil denitrification was probably the primary source of N2O emission under this wet condition (Duan et al. 2017). Consequently, the N2O emission was best described by the nirS gene abundance (Table 3). Similarly, Yang et al. (2017) revealed that there were significant correlations between soil N2O emissions and nirS or nirK gene abundances under the conditions favorable for denitrification. Duan et al. (2017) also observed a good correlation between nirS gene abundances and denitrification activities. Based on these observations and the lower gross nitrification and gross NO2−-N + NO3−-N consumption rates in the NI treatment (Table 1), we suggest that DMPP probably decreased N2O emissions by inhibiting AOB amoA gene abundances at 55% WHC and nirS gene abundances at 75% WHC. It should be mentioned that the primers used for the determinations of nirK and nirS gene abundances mainly detected the nirK- or nirS-denitrifiers of the phylum Proteobacteria (Wei et al. 2015). Recent studies showed that some denitrifiers belonging to Actinobacteria, Chloroflexi, or Spirochetes could not be quantified with the primers used in this study. Thus, the determined gene abundance might be lower than that of denitrifiers inhabiting the test soil (Wei et al. 2015; Bonilla-Rosso et al. 2016). Use of novel primers may provide a more comprehensive understanding of the impacts of agrochemicals on nirK- or nirS-denitrifiers and their roles in soil N2O emissions.
Conclusion
Compared with the CK treatment, DMPP application significantly inhibited gross nitrification rates and decreased AOB amoA gene abundances, but had no significant effects on AOA amoA gene abundances. Therefore, DMPP application inhibited gross nitrification in the sugarcane soil probably by reducing AOB amoA gene abundances. DMPP also decreased N2O emissions by inhibiting soil nitrification at 55% WHC and perhaps denitrification at 75% WHC. Both atrazine and glyphosate applications decreased gross nitrification rates perhaps by inhibiting abundances of both AOA and AOB amoA genes and decreased gross NO2−-N + NO3−-N consumption rates by inhibiting various denitrifying genes, resulting in lower cumulative N2O emissions. Further studies on herbicides should take into account other N transformation processes including gross N mineralization and N immobilization, and longer incubation periods are also needed to determine the longevities of the effects after herbicide applications in different soil types.
References
Allegrini M, Gomez EV, Zabaloy MC (2017) Repeated glyphosate exposure induces shifts in nitrifying communities and metabolism of phenylpropanoids. Soil Biol Biochem 105:206–215
Anjum T, Bajwa R (2007) Field appraisal of herbicide potential of sunflower leaf extract against Rumex dentatus. Field Crop Res 100:139–142
Bento CP, Yang X, Gort G, Xue S, van Dam R, Zomer P, Mol HG, Ritsema CJ, Geissen V (2016) Persistence of glyphosate and aminomethylphosphonic acid in loess soil under different combinations of temperature, soil moisture and light/darkness. Sci Total Environ 572:301–311
Bonfleur E, Tornisielo V, Regitano J, Lavorenti A (2015) The effects of glyphosate and atrazine mixture on soil microbial population and subsequent impacts on their fate in a tropical soil. Water Air Soil Pollut 226:21
Bonilla-Rosso G, Wittorf L, Jones CM, Hallin S (2016) Design and evaluation of primers targeting genes encoding NO-forming nitrite reductases: implications for ecological inference of denitrifying communities. Sci Rep 6:39208
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Cai Y, Chang SX, Ma B, Bork EW (2016) Watering increased DOC concentration but decreased N2O emission from a mixed grassland soil under different defoliation regimes. Biol Fertil Soils 52:987–996
Chaves B, Opoku A, De Neve S, Boeckx P, Van Cleemput O, Hofman G (2006) Influence of DCD and DMPP on soil N dynamics after incorporation of vegetable crop residues. Biol Fertil Soils 43:62–68
Decock C (2014) Mitigating nitrous oxide emissions from corn cropping systems in the midwestern US: potential and data gaps. Environ Sci Technol 48:4247–4256
Duan YF, Kong XW, Schramm A, Labouriau R, Eriksen J, Petersen SO (2017) Microbial N transformations and N2O emission after simulated grassland cultivation: effects of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP). Appl Environ Microbiol 83:e02019–e02016
Fahad S, Hussain S, Saud S, Hassan S, Muhammad H, Shan D, Chen C, Wu C, Xiong D, Khan S (2014) Consequences of narrow crop row spacing and delayed Echinochloa colona and Trianthema portulacastrum emergence for weed growth and crop yield loss in maize. Weed Res 54:475–483
Florio A, Clark IM, Hirsch PR, Jhurreea D, Benedetti A (2014) Effects of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) on abundance and activity of ammonia oxidizers in soil. Biol Fertil Soils 50:795–807
French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A (2012) Ecophysiological characterization of ammonia-oxidizing archaea and bacteria from freshwater. Appl Environ Microbiol 78:5773–5780
Gómez-Rey MX, González-Prieto SJ (2015) Soil gross N transformation rates after a wildfire and straw mulch application for burned soil emergency stabilisation. Biol Fertil Soils 51:493–505
Haney R, Senseman S, Krutz L, Hons F (2002) Soil carbon and nitrogen mineralization as affected by atrazine and glyphosate. Biol Fertil Soils 35:35–40
Hatzenpichler R (2012) Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl Environ Microbiol 78:7501–7510
Hayden HL, Drake J, Imhof M, Oxley AP, Norng S, Mele PM (2010) The abundance of nitrogen cycle genes amoA and nifH depends on land-uses and soil types in South-Eastern Australia. Soil Biol Biochem 42:1774–1783
He J, Shen J, Zhang L, Zhu Y, Zheng Y, Xu M, Di H (2007) Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ Microbiol 9:2364–2374
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671
Jiang J, Chen L, Sun Q, Sang M, Huang Y (2015) Application of herbicides is likely to reduce greenhouse gas (N2O and CH4) emissions from rice-wheat cropping systems. Atmos Environ 107:62–69
Kaur N, Bhullar M, Gill G (2016) Weed management in sugarcane-canola intercropping systems in northern India. Field Crop Res 188:1–9
Kleineidam K, Košmrlj K, Kublik S, Palmer I, Pfab H, Ruser R, Fiedler S, Schloter M (2011) Influence of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) on ammonia-oxidizing bacteria and archaea in rhizosphere and bulk soil. Chemosphere 84:182–186
Kong X, Duan Y, Schramm A, Eriksen J, Petersen SO (2016) 3, 4-Dimethylpyrazole phosphate (DMPP) reduces activity of ammonia oxidizers without adverse effects on non-target soil microorganisms and functions. Appl Soil Ecol 105:67–75
Kroon FJ, Thorburn P, Schaffelke B, Whitten S (2016) Towards protecting the great barrier reef from land-based pollution. Glob Chang Biol 22:1985–2002
Lan T, Han Y, Roelcke M, Nieder R, Cai Z (2013) Processes leading to N2O and NO emissions from two different Chinese soils under different soil moisture contents. Plant Soil 371:611–627
Liu X, Chen C, Wang W, Hughes J, Lewis HE, Shen J (2013) Soil environmental factors rather than denitrification gene abundance control N2O fluxes in a wet sclerophyll forest with different burning frequency. Soil Biol Biochem 57:292–300
Liu R, Hayden H, Suter H, He J, Chen D (2015) The effect of nitrification inhibitors in reducing nitrification and the ammonia oxidizer population in three contrasting soils. J Soils Sediments 15:1113–1118
MacGregor A (1972) Gaseous losses of nitrogen from freshly wetted desert soils. Soil Sci Soc Am J 36:594–596
Mahía J, Cabaneiro A, Carballas T, Díaz-Raviña M (2008) Microbial biomass and C mineralization in agricultural soils as affected by atrazine addition. Biol Fertil Soils 45:99–105
Mahía J, González-Prieto SJ, Martín A, Bååth E, Díaz-Raviña M (2011) Biochemical properties and microbial community structure of five different soils after atrazine addition. Biol Fertil Soils 47:577–589
Martens-Habbena W, Berube PM, Urakawa H, José R, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–979
Martins CS, Nazaries L, Macdonald CA, Anderson IC, Singh BK (2015) Water availability and abundance of microbial groups are key determinants of greenhouse gas fluxes in a dryland forest ecosystem. Soil Biol Biochem 86:5–16
Mary B, Recous S, Robin D (1998) A model for calculating nitrogen fluxes in soil using 15N tracing. Soil Biol Biochem 30:1963–1979
Menéndez S, Barrena I, Setien I, González-Murua C, Estavillo JM (2012) Efficiency of nitrification inhibitor DMPP to reduce nitrous oxide emissions under different temperature and moisture conditions. Soil Biol Biochem 53:82–89
Nachimuthu G, Halpin NV, Bell MJ (2016) Effect of sugarcane cropping systems on herbicide losses in surface runoff. Sci Total Environ 557:773–784
Nguyen DB, Rose MT, Rose TJ, Morris SG, Van Zwieten L (2016) Impact of glyphosate on soil microbial biomass and respiration: a meta-analysis. Soil Biol Biochem 92:50–57
Ouyang Y, Norton JM, Stark JM, Reeve JR, Habteselassie MY (2016) Ammonia-oxidizing bacteria are more responsive than archaea to nitrogen source in an agricultural soil. Soil Biol Biochem 96:4–15
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531
Rayment GE, Lyons DJ (2010) Soil chemical methods—Australasia. CSIRO Publishing, Melbourne, p 520
Sabba F, Picioreanu C, Pérez J, Nerenberg R (2015) Hydroxylamine diffusion can enhance N2O emissions in nitrifying biofilms: a modeling study. Environ Sci Technol 49:1486–1494
Santos A, Flores M (1995) Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria. Lett Appl Microbiol 20:349–352
Scheer C, Rowlings DW, Firrel M, Deuter P, Morris S, Grace PR (2014) Impact of nitrification inhibitor (DMPP) on soil nitrous oxide emissions from an intensive broccoli production system in sub-tropical Australia. Soil Biol Biochem 77:243–251
Soares JR, Cantarella H, de Campos Menegale ML (2012) Ammonia volatilization losses from surface-applied urea with urease and nitrification inhibitors. Soil Biol Biochem 52:82–89
Thorburn PJ, Biggs JS, Collins K, Probert ME (2010) Using the APSIM model to estimate nitrous oxide emissions from diverse Australian sugarcane production systems. Agric Ecosyst Environ 136:343–350
Wang W (2016) Effects of polymer-and nitrification inhibitor-coated urea on N2O emission, productivity and profitability in a wet tropical sugarcane crop in Australia. In ‘Proceedings of the International Nitrogen Initiative Conference, Melbourne, Australia’. http://www.ini2016.com/pdf-papers/INI2016_Wang_Weijin.pdf
Wang W, Dalal RC, Reeves SH, Bntterbach-Bahl K, Kiese R (2011) Greenhouse gas fluxes from an Australian subtropical cropland under long-term contrasting management regimes. Glob Chang Biol 17:3089–3101
Wang Q, Zhang LM, Shen JP, Du S, Han LL, He JZ (2016a) Nitrogen fertiliser-induced changes in N2O emissions are attributed more to ammonia-oxidising bacteria rather than archaea as revealed using 1-octyne and acetylene inhibitors in two arable soils. Biol Fertil Soils 52:1163–1171
Wang W, Reeves S, Salter B, Moody P, Dalal R (2016b) Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia. Agric Ecosyst Environ 216:137–146
Wei W, Isobe K, Nishizawa T, Zhu L, Shiratori Y, Ohte N, Koba K, Otsuka S, Senoo K (2015) Higher diversity and abundance of denitrifying microorganisms in environments than considered previously. ISME J 9:1954–1965
Weiske A, Benckiser G, Herbert T, Ottow J (2001) Influence of the nitrification inhibitor 3, 4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emissions, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments. Biol Fertil Soils 34:109–117
Wu X, Liu H, Fu B, Wang Q, Xu M, Wang H, Yang F, Liu G (2017) Effects of land-use change and fertilization on N2O and NO fluxes, the abundance of nitrifying and denitrifying microbial communities in a hilly red soil region of southern China. Appl Soil Ecol 120:111–120
Yang L, Zhang X, Ju X (2017) Linkage between N2O emission and functional gene abundance in an intensively managed calcareous fluvo-aquic soil. Sci Rep 7:43283
Yu Q, Chen Y, Ye X, Tian G, Zhang Z (2007) Influence of the DMPP (3, 4-dimethyl pyrazole phosphate) on nitrogen transformation and leaching in multi-layer soil columns. Chemosphere 69:825–831
Zerulla W, Barth T, Dressel J, Erhardt K, von Locquenghien KH, Pasda G, Rädle M, Wissemeier A (2001) 3, 4-Dimethylpyrazole phosphate (DMPP)—a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84
Zhang MY, Xu ZH, Teng Y, Christie P, Wang J, Ren WJ, Luo YM, Li ZG (2016) Non-target effects of repeated chlorothalonil application on soil nitrogen cycling: the key functional gene study. Sci Total Environ 543:636–643
Zhang MY, Wang WJ, Bai SH, Zhou X, Teng Y, Xu ZH (2018) Antagonistic effects of nitrification inhibitor 3, 4-dimethylpyrazole phosphate and fungicide iprodione on net nitrification in an agricultural soil. Soil Biol Biochem 116:167–170
Acknowledgments
Manyun Zhang was awarded a Griffith University Ph.D. scholarship for international students. The authors sincerely thank Geoffrey Lambert for his patient and detailed guidance in the determination of gross nitrification/denitrification rates, Linfeng Li for his support in the path analysis, and Dr. Xiuzhen Shi from the University of Melbourne for her help in the qPCR.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 317 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Wang, W., Tang, L. et al. Effects of nitrification inhibitor and herbicides on nitrification, nitrite and nitrate consumptions and nitrous oxide emission in an Australian sugarcane soil. Biol Fertil Soils 54, 697–706 (2018). https://doi.org/10.1007/s00374-018-1293-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-018-1293-6