Abstract
Purpose
2,4-D (2,4-dichlorophenoxy acetic acid) is an herbicide used in weed control worldwide; however, there is no information regarding its effects on the nitrification process in volcanic soils fertilized with urea and how its bioavailability can be modified during this process.
Methods
This study was carried out under microcosm incubation of an Andisol amended with an equivalent field dose of 200 kg N ha−1 and 0.672 kg 2,4-D ha−1 for 30 days. In this study were evaluated the changes produced on enzymatic activity, nitrogen kinetic mineralization, ammonia-oxidizing bacteria (AOB) abundance and herbicide persistence. Additionally, a 2,4-D adsorption study was carried out in batch with urea application.
Results
The main results on fertilized soils show that 2,4-D stimulated temporarily urease and dehydrogenase activity. Herbicide application increased N-NH4+ concentration by approximately 23%, and N-NO3− significantly decreased by 18%, compared to fertilized soil. The abundance of AOB amoA gene decreased at all times. On the other hand, 2,4-D adsorption decreased by urea application, but its persistence was not affected, with a low half-life of approximately 4 days.
Conclusions
This study indicated that 2,4-D influences the urea mineralization process by increasing ammonium and decreasing nitrate levels as a consequence of increased urease activity and the inhibition of AOB populations. On the other hand, the bioavailability of 2,4-D decreased due to the pH modifications produced by the application of urea. In general, we conclude that the combined application of fertilizers and some herbicides should be studied to estimate the efficacy and environmental effect of both agrochemicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
At present, there is great concern for intensifying crop production and its impact on the environment, focusing on the excessive use of nitrogen fertilizers and pesticides. Both nitrogen fertilizers and herbicides could mutually influence each other in their action (Ding et al. 2017; Muñoz-Leoz et al. 2012; Palma et al. 2016; Tan et al. 2013). In soil, the urea mineralization process initially involves an increase in soil pH with an increase in dissolved organic carbon content. Subsequently, soil acidification occurs, where the soil pH decreases with time (Cartes et al. 2009; Palma et al. 2016). In this process, nitrification is a key process in which microorganism ammonium oxidizers, including bacteria (AOB) and archaea (AOA), transform ammonium to nitrate. The relative abundance of AOB and AOA is influenced by diverse environmental factors, with AOB abundance being higher in acidified soils under nitrogen fertilization (Jia and Conrad 2009; Prosser et al. 2012; Sun et al. 2019). 2,4-D is a systemic herbicide absorbed by foliage and roots and widely used in postemergence to control broadleaf weeds in cereals, pastures and fruits (Peterson et al. 2016). It is an acidic compound with a low pKa value of 3.4 (PPDB 2013) and is mainly found in anionic form in soils. It is weakly adsorbed by soil and therefore has the potential to be leachable and become a water contaminant (Abigail et al. 2017; USEPA 2005). It is adsorbed onto soil particles through hydrophobic interactions and hydrogen bonding, and its adsorption process is strongly influenced by the organic matter (OM) content and pH (Kah and Brown 2006; Ololade et al. 2015; Picton and Farenhorst 2004). In this context, the application of urea can substantially modify the bioavailability of 2,4-D. In this sense, diverse responses of other herbicides to fertilizer application have been reported, such as MCPA, an acid herbicide studied in a Andisol (Palma et al. 2016), terbuthylazine (Pinna et al. 2014), and metribuzin (Singh 2006), both classified as basic herbicides applied in soils with low OM content and different pH values.
Pesticide effects on microorganisms have been extensively studied, and it was concluded that pesticides exert a short-term toxic effect, although this effect could last longer considering that the repeated application of pesticides can result in pesticide residue accumulation in soils and induce pesticide-tolerant communities (Cycón et al. 2011; Katsoula et al. 2020; Zabaloy et al. 2010). 2,4-D is classified as moderately persistent with a low half-life in the range of 4–28 days (Cycón et al. 2011; PPDB 2013).
New approaches have emerged in recent years, particularly in relation to the pesticide effect on nontarget soil microorganisms involved in the N cycle, although this effect has not been extensively addressed. Studies have considered some herbicides, fungicides and insecticides to evaluate the response of some microbial communities of the N cycle, mainly fixation or nitrification, and very diverse responses were observed (Cycoń and Piotrowska-Seget 2015; Meena et al. 2020; Muñoz-Leoz et al. 2012; Rahman et al. 2021; Sim et al. 2022; Yamaguchi et al. 2021; Zabaloy et al. 2017). In particular, Sim et al. (2022) included 20 pesticides in their study and found that the fungicides azoxystrobin and flutriafol, the herbicide chlorsulfuron and the insecticide fipronil had some negative effects on enzymatic activities, potential nitrification, and the abundance of functional genes involved in N cycling. This study was performed in acid and basic soils with OM content in the range of 5–11%, approximately. Specific studies carried out by Kucharski et al. (2009) on the effects of dimethalin, isoproturon, chlopyralid and dicamba on the urea ammonification process showed that only dicamba, an acidic herbicide, substantially decreased ammonification in a slightly acid soil with a relatively high OM content of 10%, approximately.
There are few reports on 2,4-D in the context of this study. In this regard, studies carried out by Martens and Bremmer (1993), in acid and basic soils, with a high OM content. on the effect of 28 herbicides on urea transformations in soil showed minor effects for most of the herbicides, depending on the dose of herbicide used. Specifically, 2,4-D was reported to retard urea nitrification. Studies by Rose et al. (2018) involving six herbicides, including 2,4-D applied at the recommended rate, showed a transient effect or no effect on soil nitrogen mineralization in acid and basic soils, with a low OM content. For doses five times the recommended dose, 2,4-D increased N-NH4+ and reduced N-NO3− concentrations. Ding et al. (2017) evaluated the effect of 2,4-D butyl ester on diverse microorganisms involved in the soil nitrogen cycle, observing diverse responses depending on the dose and soil type. In field studies conducted by Narain JP (1992) using two types of commercial formulations of 2,4-D, he found that the nitrogen mineralization process and soil mineralization potential were reduced only by the butyl ester formulation of 2,4-D and not the amine formulation.
A better understanding is required regarding how both agrochemicals influence each other and how their behavior is modified when they are both in the soil environment. This study was carried out in a volcanic soil characterized by high organic matter content and acid pH. The aim of this study was to examine how the application of urea fertilizer could modify 2,4-D bioavailability and at the same time the 2,4-D influence on urea mineralization process. This study includes adsorption and degradation studies on 2,4-D in fertilized soils, enzyme studies, and urea mineralization and qPCR analyses on nitrifying AOB bacteria.
2 Material and Methods
2.1 Soil
Soil samples (Andisol, Freire Series) were collected at 0–20 cm depth at the Maquehue Experimental Station of La Frontera University (38° 50’ S, 72° 41’ W) in southern Chile, passed through a 2-mm sieve, and stored at 4 °C until its use for the experiments. Soil physico-chemical analyses were performed according to the methodology described in Marileo et al. (2016).
2.2 Herbicide
The analytical standard of 2,4-D (2,4-dichlorophenoxy acetic acid), 99.9% purity, used in.the experiments were provided by Chem Service (West Chester, PA, USA). All reagents used were analytical or HPLC grade.
2.3 Microcosm Set up and Sampling
Before setting up the experiment, the soil was incubated at 20 °C and 60% of the water holding capacity for two weeks in darkness. For the microcosm set up, approximately 500 g of soil (dry weight basis) for each treatment in three replicates, were placed into plastic vessels. Urea was applied at an equivalent dose of 200 kg N ha−1 (573 mg N kg−1), and after 24 h, 2,4-D was applied at an equivalent dose of 0.672 kg ha−1 (1.792 mg kg−1 of the active ingredient (a.i.), assuming a distribution in the first 5 cm of depth and a bulk density for a silty loam soil of 0.75 g mL−1. Both doses corresponded to the recommended dose in field conditions for cereals. The treatments were as follows: control soil, soil without urea and 2,4-D (0N0H), soil with 2,4-D (0N1H), soil with urea without 2,4-D (2N0H) and soil with urea and 2,4-D (2N1H). Aqueous solutions of urea and 2,4 D were applied to the soil with a small spray bottle and thoroughly mixed and shaken manually and moisture content was adjusted by weight. Immediately after the microcosms were prepared, the soils were distributed in smaller plastic vessels, considering a destructive sampling for each time (OECD 2002) .
Approximately 50 g of these subsamples were added into each plastic vessels, maintained semi-open and incubated in the dark at 20 °C for 30 days. and sampled after 1, 5, 10, 20 and 30 days. The soil moisture content was controlled by weighing the tubes weekly and adding a necessary amount of distilled water. The subsamples were used for the determination of residual herbicide, dehydrogenase activity, urease activity, ammonia and nitrate contents, pH record and DNA extraction for copy numbers of AOB amoA gene estimation. The samples were kept at -20 °C for further analysis.
2.4 pH Record in Soil-Urea Microcosms
The soil pH was measured in 1:2.5 soil–water suspension in deionized water using a glass electrode. Samples for each treatment were taken over 30 days.
2.5 Determination of N-NH4 + and N-NO3 −
Mineralization of urea fertilizer was measured by extractable N-NH4+ and N-NO3− from microcosms at all sampling dates. They were extracted from 2.5 g of each soil sample using 12.5 ml of 1 mol L−1 KCl shaken for 30 min, centrifuged at 3000 rpm for 15 min and filtered. The ammonium concentration in the extract was measured by the colorimetric Kandeler and Gerber (1988) method. Briefly, 1 mL of filtrate was diluted to 10 mL with deionized water, and 2 ml of 0.1% sodium dichloroisocyanurate and 5 ml sodium salicylate solutions were added. The sodium salicylate solution was prepared by mixing 100 mL of 0.12% sodium nitroprusside, 100 mL of 17% sodium salicylate and 100 mL of water. Ammonium was determined after 30 min of incubation at room temperature at 620 nm.
Nitrate in soil was determined by the method based on the use of Vanadium (III) and Griess reagents and quantified by spectrophotometry at 540 nm (Doane and Horwath 2003; Keeney and Nelson 1982). Briefly, 200 mg sulfanilamide and 10 mg of N-(1-naphthyl) ethylenediamine dihydrochloride were added to 400 mL of distilled water, shaken and added to a vanadium solution of 400 mg of vanadium (III) chloride, prepared in 50 mL of 1 mol L−1 HCl. Then, 0.4 mL of the previously obtained filtrate was mixed with 2 mL of the reagent and incubated in at 60 °C for 2 h. The samples were kept at room temperature for 20 min before nitrate measurement.
2.6 Soil Microbial Activities
Dehydrogenase activity was determined by method based on the reduction of 2,3,5-triphenyltetrazoliumchloride (TTC) to triphenyl formazan (TPF), according to Serra-Witting et al. 1995. Briefly, 3 g of soil samples were mixed with TTC solution (3%) and de deionized water (1:1) and incubated at 37 °C for 24 h in darkness. After incubation, TPF produced was extracted with methanol, filtrated and determined by spectrophotometry at 485 nm.
Urease (UR) activity was determined according to the method described by Kandeler and Gerber (1988). Briefly, 1 g of soil was incubated with 1.5 mL of urea solution 0.08 mol L−1 at 37 °C for 2 h, then 13.5 mL of 2 mol L−1 KCl solution was added and shaken for 30 min. The resulting suspension was filtered and the filtrates analysed for ammonia by the colorimetric method described previously for ammonia determination.
2.7 Quantitative Real-Time PCR (qPCR)
Total genomic DNA was extracted from 0.5 g of soil samples using the PowerSoil DNA isolation Kit (Mo-Bio, Carlsbad, USA) according to manufacturer’s instructions. The extracted DNA was stored at -80 °C for qPCR assays. Copy numbers of the AOB amoA gene were estimated by quantitative real-time PCR (qPCR) using the specific primer set and qPCR conditions. The primer set amoA-1F (5′-GGGGTTTCTACTGGTGGT-3′) and amoA-2R (5′-CCCCTCKGSAAAGCCTTCTTC-3′) were used (Rotthauwe et al. 1997; Tan et al. 2013; Marileo et al. 2016). All qPCRs were performed in triplicate with 25 ng μL−1 of total DNA in a 7300 Real Time PCR System (Applied Biosystems) using BrilliantIII Ultra-Fast SYBR Green qPCR Master Mix (Strategene) following the manufacturer’s instructions. The PCR conditions included an enzyme activation step at 95 °C for 5 min followed by 35 cycles of 15 s at 95 °C and 1 min of annealing plus extension at 60 °C. The specificity of the amplification products was confirmed by a melting-curve analysis (60 °C to 95 °C at 0.2 °C intervals of 15 s each). The expected size of the amplified fragments was confirmed with a 1.5% (w/v) agarose gel. A dilution series (10–107 amoA copies) consisting of a known amount of a synthetic gene by Invitrogen GeneArt Gene Synthesis (Nitrosospira multiformis ATCC 25196 genomic DNA) was used as a standard. The amplification efficiency was 94%, the R2 value was > 0.994.
2.8 Determination of Residual 2,4-D in soil
The extraction of 2,4-D from soil microcosm samples involved three successive extractions with methanol:water (60:40 v/v) acidified with 0.1% H3PO4 extractive solution. Briefly, 5.0 g of fresh soil and 25 mL of solvent extractive solution were placed into a centrifuge tube, vortexed for 1 min, sonicated for 10 min, and centrifuged at 3000 rpm for 10 min. The supernatants were filtered and analyzed separately (Marileo et al. 2016).
The herbicide was analyzed using a Shimadzu Prominence HPLC chromatograph LC-20AT with a diode array detector (SPD-M20A) and a prontoSil column RP-C18 (250 × 4. 6 mm). The mobile phase used was a 50:50 (v/v) mixture of acetonitrile and water acidified to pH 2 with phosphoric acid. The detection wavelength was 228 nm. The injection volume was 20 μL, the flow rate was 1.0 mL min-1, and the oven temperature was 40 °C. The detection limit and quantification limits were 0.010 and 0.034 mg L−1, respectively. The precision (relative standard deviation) was < 5%, and the chromatographic response for the calibration curve was linear up to 25 mg L-1 (R2 = 0.999). Blank soil sample without herbicide was used to evaluate soil matrix effect (Spuler et al. 2019). No significant interference was recorded. Additionally, recovery experiment in a soil sample was performed in triplicate using two concentrations of herbicide (2.0, 10 mg kg−1) with recovery ranging from 92- 95%.
The residual amount and half-life of 2,4-D were calculated by the equations Ct = C0e−kt and t1/2 = ln2/k, respectively, where Ct represents the concentration of the pesticide residue at time t, C0 represents the initial concentration after application, k is a dissipation coefficient and t1/2 is the time required for the pesticide residue level to degrade to half of the initial application level.
2.9 Adsorption Experiments
Sorption experiments were carried out according to the method of Palma et al. (2016) development for acidic herbicides. For the 2,4-D isotherm, duplicate 2.0 g soil samples were mixed with approximately 10 mL of a 0.01 M CaCl2 aqueous solution in 50 mL centrifuge tubes (polypropylene copolymer). Herbicide stock solution prepared in 0.01 M CaCl2 was added to yield final concentrations of 0.05, 0.25, 0.5, 1.0, 2.0, 5.0, 10.0, and 20.0 mg L−1 with final volumes of 20 mL. The tubes were shaken at 20 ± 1 °C in a rotary shaker for 24 h. The tubes were then centrifuged at 3000 rpm for 15 min. The supernatants were filtered using 0.22 µm membranes (Durapore, PVDF from Millipore), and 5 mL aliquots were transferred to HPLC vials for analysis. The initial and final pH values were recorded in each tube.
The urea-herbicide isotherms were conducted using the same herbicide concentrations as mentioned above. A 1000 mg L−1 stock urea solution was used to obtain a urea concentration of 56.7 mg L−1 in each tube, which is equivalent to 200 kg N ha-1 (0.572 g urea per kg of soil). The sorption experiments were performed in triplicate. Sorption was described by the Freundlich model Cs = Kf Ce 1/n, and the constants were determined by linear fitting.where Cs (mg kg−1) is the herbicide adsorbed by the soil, and Ce (mg L−1) is the equilibrium concentration in the solution. Kf and 1/n are empirical constants.
2.10 Statistical Analysis
All of the experiments were performed using three independent replicates. The results were expressed as means value with their corresponding standard deviations. The data were subjected to statistical analyses of variance (ANOVA), and all mean separations were determined using the Tukey test (p ˂ 0.05).
3 Results
3.1 Soil Properties
The soil used in this study has been previously used by the authors of this work and has been previously characterized according to the methodology described in Marileo et al. (2016). Some properties were 5.20 pH, 19 mg P kg−1, 32 mg N kg−1, 152 mg K kg−1, 15% organic matter, 8.2 cmol ( +) kg−1 cation exchange capacity, 1.46% Al saturation, and a silty loam texture with 39.7% sand, 42.9% silt, and 17.3% clay.
3.2 Effects of 2,4-D on Soil Enzyme Activities
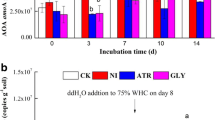
In the present study, the effect of 2,4-D application on soil enzyme activities, included urease activity and dehydrogenase activity measured for all treatments over 30 days. Figure 1a and b show the behavior of soil enzymatic activity during the incubation period, respectively. Compared to soil (N0H0), urease activity due to the application of herbicide (0NH1), urea (2N0H) and the combined application of urea and herbicide (2N1H) progressively increased up to day 10 by approximately 20, 24 and 49%, respectively. Urease activity increased by approximately 20% due to 2,4-D over the activity shown by urea application.
a) Urease activity and b) dehydrogenase activity in: control soil, without urea and 2,4-D (0N0H), soil without urea and with 2,4-D (0N1H), soil with urea and without 2,4-D (2N0H), soil with urea and 2,4-D (2N1H). All data show the means ± SD of three replicates. Different letters indicated significant differences between treatments according Tukey test (P < 0.05)
Regarding dehydrogenase activity, the highest activity was observed at 10 days. Compared to soil (N0H0), dehydrogenase activity due to the application of herbicide (0NH1), urea (2N0H) and the combined application of urea and herbicide (2N1H) increased by approximately 23, 49 and 53%, respectively.
3.3 Effects of 2,4-D on Urea Mineralization
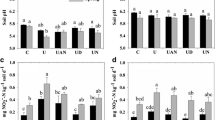
Figure 2a and b show the N-NH4+ and N-NO3− production from the urea mineralization process during the incubation period, respectively. The highest ammonium production occurred in the first 10 days of the experiment for the treatments with urea application (2N0H) and the combined urea-herbicide treatment (2N1H). At 1, 5 and 10 days, herbicide application increased ammonium concentration by approximately 12, 5 and 23%, respectively, compared to the ammonium concentration of 2N0H.
a) N-NH4+and b) N-NO3− in: control soil, without urea and 2,4-D (0N0H), soil without urea and with 2,4-D (0N1H), soil with urea and without 2,4-D (2N0H) and soil with urea and 2,4-D (2N1H). All data show the means ± SD of three replicates. Different letters indicated significant differences between treatments according Tukey test (P < 0.05)
N-NO3− production in the fertilized soil (2N0H) increased progressively between 10–30 days, but the combined urea application and herbicide (2N1H) significantly decreased the N-N03− production by 18, 15 and 6%, respectively, compared to N-NO3− production in fertilized soil (2N0H).
3.4 AOB AmoA Gene Abundance
The results of the treatments on the abundance of the ammonia-oxidizing bacteria AOB amoA genes were obtained using quantitative real-time PCR at four sampling days and are shown in Fig. 3. The abundance of amoA genes in the fertilized soil (2N0H) was the highest and increased progressively from 3.02 × 10 12 to 15.7 × 1012 copies per gram of dry soil during the first 20 days of the experiment. For the combined application of urea and herbicide (2N1H), abundance values decreased significantly to 1.9 × 1012 to 7.75 × 1012 copies/g of soil at the same period of time compared to the abundance values of 2N0H. 2,4-D application (0N1H) sharply decreased the AOB abundance at 10 days from 2.7 × 1012 to 1.3 × 1012 AOB amoA gene copies numbers g−1 soil dw compared to that of soil (0N0H), although after this time, a significant increase in the number of copies was observed, similar to that of the 2N1H treatment at 20 days, increasing to 10.4 × 10 12 copies.
3.5 2,4-D Bioavailability
The pH results of the microcosms (Fig. 4) show the changes in pH that occurred during the 35 days of the experiments. 2,4-D (0N1H) did not produce significant changes in pH during the experiment, although a small degree of acidification was recorded. The main pH changes were produced by the application of urea during the mineralization process. The urea treatment (2N0H) increased the pH value from 5.4 to 6.1 at 24 h after application, and acidification subsequently occurred, shifting the pH to 4.4 by the end of the experiments.
The persistence results of 2,4-D (Table 1) fit well to the first-order kinetic model (R2 ≥ 0.98), showing rapid degradation and no significant differences in the rate constant and half-life obtained from the model. However, it is possible to observe some differences in the concentration of 2,4-D in the soil between the 3–5 days. For example, the concentration of 2,4-D was approximately 28% lower in soils treated with urea (2N1H) than in soils with herbicide and without urea application (0N1H). After 20 days, herbicide residues were not detected in either treatment. The 2,4-D detection and quantification limits were determined to be 0.010 and 0.034 mg L−1, respectively.
The adsorption isotherm data (Table 2) showed a good fit to the Freundlich model (R2 ≥ 0.99). The Kf value of 16.3 with urea was considerably lower than the Kf value without urea (21.2). The Koc values were 189.6 and 246.1, respectively. In the range of concentrations studied, the adsorption percentage for the treatment without urea application (0N) was approximately 37–75%; for the combined urea-herbicide treatment (2N), it was 30–60%.
4 Discussion
The objective of this study was to establish the behavior of the herbicide in a Andisol fertilized with urea, in the doses applied in the field and how both agrochemicals can mutually modify their behavior and therefore their effectiveness in both weed control and crop nutrition and eventually affect the environment. The nitrogen and pesticides application in the long term could be expected to affect both soil quality and crop productivity. In this regard, the availability of soil nutrients, potentially toxic metals and soil acidification are intimately related and could be affected as a whole (Takousting et al. 2016; Triantafyllidis et al. 2020).
According to the obtained results, the herbicide 2,4-D applied in soils fertilized with urea had diverse effects on the enzymatic activities, urea mineralization process and AOB populations. Two enzyme activities were considered in this study. Urease involve in urea mineralization and dehydrogenase enzyme used as a general indicator of soil microbial activity. Urease was stimulated due to the application of 2,4-D and urea, and this effect was even greater when they were both applied only at 10 days, in the other dates levels differences were not significant. On the other hand, dehydrogenase activity was also stimulated by the application of 2,4-D and urea, until day 10, without showing major changes in the combined application of both agrochemicals except at day 1. Several studies have shown that urease and dehydrogenase activity may be stimulated by fertilization, but pesticide application may decrease, increase or have no effect (Du et al. 2018; Nivelle et al. 2017; Riah et al. 2014; Zabaloy et al. 2017). The effect of 2,4-D on these enzymes have been little documented; although there are some works that reported the inhibition of urease activity or no significant changes (Bécaert et al. 2006; Narain 1992). Studies conducted by Zabaloy et al. (2008) on 2,4-D at higher doses reported an increase of dehydrogenase activity by approximately 20%. The different responses of both enzymes can be understood by considering the toxic effect of some pesticides on microorganisms or microorganisms adaptation due to repeated pesticide application. The soil used in this study has a history of pesticide application associated with cereal crops and grasslands, although in the last two years, it has not been subjected to pesticide application.
In relation to the urea mineralization process, it is known that urea first undergoes hydrolysis to ammonium and then oxidation to nitrate, with pH changes during this process. For the soil under study, it was reported that the hydrolysis process of urea occurred within the first 5–10 days after application and decreased progressively. At the same time, the nitrate concentration began to increase until it reached its maximum concentration between 10–20 days, depending on the temperature and urea dosage (Cartes et al. 2009). In fact, the highest ammonium production occurred in the first 10 days and its subsequent oxidation to nitrate occurred within 10–30 days. During this period, the herbicide produced a significant increase in ammonium concentration and a decrease in nitrate concentration of approximately 20%. This increase in urea hydrolysis can be explained by the stimulation of urease activity mentioned above and due to the acidic character of 2,4-D (pKa 3.4), which catalyzes the urea hydrolysis process. Similar results were obtained with MCPA (pKa 3.7), a phenoxyacetic acid herbicide of the same 2,4-D chemical family (Palma et al. 2016). Our results are in agreement with those of studies conducted by Rose et al. (2018), conducted in acid soils and low OM content for 2,4-D. They reported an increase in NH4+ concentration and a decrease in nitrate concentration in some soils depending on the herbicide rate in a short-term experiment. Similarly, studies conducted by Martens and Bremmer (1993) showed that at doses much higher than the recommended doses, 2,4-D temporarily inhibited nitrification. These results are consistent with the fact that 2,4-D decreased nitrifying bacteria abundance in soil and in fertilized soils. Although no specific reports were found for the effect of 2,4-D on the abundance of nitrifying bacteria, AOB amoA genes, similar results were reported for other herbicides, such as mesotrione, mancozeb, dazomet and chlorimuron-ethyl, which inhibit the nitrification process (Du et al. 2018; Feld et al. 2015; Tan et al. 2013).
On the other hand, the effect of soil pH changes during the urea mineralization process on the bioavailability of 2,4-D showed that although there was an important effect on the adsorption process, it did not impact the degradation of the herbicide. 2,4-D had a low persistence in this soil. The adsorption and mineralization of 2,4-D in soils with diverse physicochemical characteristics has been extensively addressed. In general, these studies show a weak adsorption of 2,4-D, with Freundlich distribution coefficient values below 2.89 and a relatively short half-life of less than 20 days, for acid and neutral soils with an OM content lower than 8% (Paszko et al. 2016; Picton and Farenhorst 2004).
The decrease in the adsorption of 2,4-D due to the application of urea (Kf value from 21.2 to 16.3) can be explained first by taking into consideration the pH variations produced by urea, which determine the chemical forms of the herbicide in the soil solution and its adsorption mechanisms. The pH records showed that urea increased the soil pH from 5.4 to 6.1 at 24 h and then decreased it progressively, reaching a value of 4.40 after 35 days (Fig. 4). In a similar pH range (pH 4–6), the ratio of the neutral to the anionic form of 2,4-D varied from 20–0.3% (Schwarzenbach et al. 2003) The adsorption mechanisms of 2,4-D involve 2,4-D mainly in its molecular form interacting with organic matter through H-bridging of carboxylic groups and hydrophobic interactions, and anionic adsorption is less likely. Additionally, in previous studies, we reported an increase in DOC at higher pH, which could explain the lower adsorption of the herbicide due to competition with the adsorption sites (Palma et al. 2016). Similar arguments have been used to explain the adsorption decrease of terbuthylazine and metribuzin (Pinna et al. 2014; Singh 2006) in soils with the addition of nitrogen fertilizers. These results are in agreement with those of studies carried out by our group on the adsorption kinetics of 2,4-D (Spuler et al. 2019) and MCPA herbicide, herbicide chemically similar to 2,4-D (Palma et al. 2016). The results obtained in this research are an important contribution considering that Andisols are soils widely distributed throughout the world, and supporting a high agricultural production. Some Andisol characteristics are a high organic matter content, low pH and high allophone mineral fraction (Escudey et al. 2004). However, the herbicide behaviour in soil is strongly determined its chemical structure (Kah and Brown 2006; Sarmah et al. 2004). In this regard, it is necessary to advance in further studies including other pesticides widely used in agricultural production.
5 Conclusions
The main conclusions of this short-term study show that 2,4-D (2,4-dichlorophenoxy acetic acid) in fertilized soils produces an increase in urease activity and ammonium concentration, possibly due to the acidic character of the herbicide which contributes to increase urea hydrolysis. The effect of 2,4-D on the enzymatic activities is recovered during the evaluation period, which could be directly related to the low persistence of 2,4-D. On the other hand, the decrease in the nitrification process in accordance with the decrease in the abundance of ammonia-oxidizing bacteria AOB amoA genes can be explained by the toxic effect of the herbicide on these microbial populations. However, a decrease in the oxidation process could mean a lower environmental impact due to nitrate.
On the other hand, urea reduced the adsorption of the herbicide, which can be attributed to the increase in soluble organic matter that competes for adsorption sites, as previously established, and to the higher percentage of the anionic form of the herbicide in solution.
These results, obtained under controlled conditions contribute to understand the behavior of fertilizers and pesticides in soil, being necessary to advance in studies under field conditions to establish the impact of both agrochemicals on the environment and crop production.
Data Availability
The online version contains all necessary data related to the manuscript available at https://doi.org/10.1007/s42729-023-01350-z.
References
Abigail EA, Samuel M, Needhidasan S, Ramalingam Ch (2017) Stratagems employed for 2,4-dichlorophenoxyacetic acid removal from polluted water sources. Clean Technol Environ Policy 19:1607–1620. https://doi.org/10.1007/s10098-017-1371-8
Bécaert V, Samson R, Deschênes L (2006) Effect of 2,4-D contamination on soil functional stability evaluated using the relative soil stability index (RSSI). Chemosphere 64:1713–1721. https://doi.org/10.1016/j.chemosphere.2006.01.008
Cartes P, Jara A, Demanet R, Mora ML (2009) Urease activity and nitrogen mineralization kinetics as affected by temperature and urea input rate in southern Chilean Andisols. J Soil Sci Plant Nutr 9:69–82
Cycoń M, Piotrowska-Seget Z (2015) Community structure of ammonia-oxidizing archaea and ammonia-oxidizing bacteria in soil treated with the insecticide imidacloprid. Biomed Res Int 582938:1–12. https://doi.org/10.1155/2015/582938
Cycoń M, Zmijowska A, Piotrowska-Seget Z (2011) Biodegradation kinetics of 2,4-D by bacterial strains isolated from soil. Cent Eur J Biol 6:188–198. https://doi.org/10.2478/s11535-011-0005-0
Ding H, Zhang J, Fang Y, Zheng X, Zhang Y, Chen D (2017) Impact of herbicide 24-dichlorophenoxyacetic acid butyl ester on soil nitrogen-transforming bacterial populations in two soils. Int J Agric Biol 19:812–816. https://doi.org/10.17957/IJAB/15.0367
Doane TA, Horwáth WR (2003) Spectrophotometric determination of nitrate with a single reagent. Anal Lett 36:2713–2722. https://doi.org/10.1081/AL-120024647
Du Z, Zhu Y, Zhu L, Zhang J, Li B, Wang J, Wang J, Zhang Ch, Cheng Ch (2018) Effects of the herbicide mesotrione on soil enzyme activity and microbial. Commun Ecotox Environ Safe 164:571–578. https://doi.org/10.1016/j.ecoenv.2018.08.075
Escudey M, Forster J, Galindo G (2004) Relevance of organic matter in some chemical and physical characteristics of volcanic ash-derived soils. Commun Soil Sci Plant Anal 35:781–797. https://doi.org/10.1081/CSS-120030358
Feld L, Hjelmsø MH, Nielsen MS, Jacobsen AD, Rønn R, Ekelund F, Krogh PH, Strobel BW, Jacobsen CS (2015) Pesticide side effects in an agricultural soil ecosystem as measured by amoA expression quantification and bacterial diversity changes. PLoS One 10:e0126080. https://doi.org/10.1371/journal.pone.0126080
Jia Z, Conrad R (2009) Bacteria rather than archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671. https://doi.org/10.1111/j.1462-2920.2009.01891.x
Kah M, Brown CD (2006) Adsorption of ionisable pesticides in soils. Rev Environ Contam Toxicol 188:149–217. https://doi.org/10.1007/978-0-387-32964-2_5
Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fertil Soils 6:68–77. https://doi.org/10.1007/BF00257924
Katsoula A, Vasileiadis S, Sapountzi M, Karpouzas DG (2020) The response of soil and phyllosphere microbial communities to repeated application of the fungicide iprodione: accelerated biodegradation or toxicity? FEMS Microbiol Ecol 96:fiaa056. https://doi.org/10.1093/femsec/fiaa056
Keeney DR, Nelson DW (1982) Nitrogen-inorganic forms. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis part 2: Chemical and microbiological properties, 2nd edn. American Society of Agronomy Madison, WI, pp 643–698
Kucharski J, Bacmaga M, Wyszkowska J (2009) Effect of herbicides on the course of ammonification in soil. J Elementol 14:477–487
Marileo L, Jorquera M, Hernández M, Briceño G, Mora ML, Demanet R, Palma G (2016) Changes in bacterial communities by postemergent herbicides in an Andisol fertilized with urea as revealed by DGGE. App Soil Ecol 101:141–151. https://doi.org/10.1016/j.apsoil.2016.02.003
Martens DA, Bremner JM (1993) Influence of herbicides on transformations of urea nitrogen in soil. J Environ Sci Health Part B 28:377–395. https://doi.org/10.1080/03601239309372831
Meena RS, Kumar S, Datta R, Lal R, Vijayakumar V, Brtnicky M, Sharma M, Yadav G, Jhariya M, Jangir Ch, Pathan S, Dokulilova T, Pecina V, Marfo T (2020) Impact of agrochemicals on soil microbiota and management: a review. Land 9:34. https://doi.org/10.3390/land9020034
Muñoz-Leoz B, Garbisu C, Antiguedad I, Ruiz-Romera E (2012) Fertilization can modify the non-target effects of pesticides on soil microbial communities. Soil Biol Biochem 48:125–134. https://doi.org/10.1016/j.soilbio.2012.01.021
Narain JP (1992) Effect of long-term 2,4-D application on microbial populations and biochemical processes in cultivated soil. Biol Fertil Soils 13:187–191. https://doi.org/10.1007/BF00336278
Nivelle E, Verzeaux J, Chabot A, Roger D, Spicher F, Lacoux J, Nava-Saucedo JE, Catterou M, Tétu T (2017) Does nitrogen fertilization history affects short-term microbial responses and chemical properties of soils submitted to different glyphosate concentrations? PLoS One 12:e0178342. https://doi.org/10.1371/journal.pone.0178342
OECD (2002) Test No. 307 Aerobic and anaerobic transformation in soil. OECD guidelines for the testing of chemicals. https://doi.org/10.1787/9789264070509-en
Ololade IA, Alomaja F, Oladoja NA, Ololade O, Oloye F (2015) Kinetics and isotherm analysis of 2,4-dichlorophenoxyl acetic acid adsorption onto soil components under oxic and anoxic conditions. J Environ Sci Health Part B-Pestic Contam Agric Wastes 50:492–503. https://doi.org/10.1080/03601234.2015.1018762
Palma G, Jorquera M, Demanet R, Elgueta S, Briceño G, Mora ML (2016) Urea fertilizer and pH influence on sorption process of flumetsulam and MCPA acidic herbicides in a volcanic soil. J Environ Qual 45:323–330. https://doi.org/10.2134/jeq2015.07.0358
Paszko T, Muszynski P, Materska M, Bojanowska M, Kostecka M, Jackowska I (2016) Adsorption and degradation of phenoxyalkanoic acid herbicides in soils: a review. Environ Toxicol Chem 35:271–286. https://doi.org/10.1002/etc.3212
Pesticide Properties DataBase (PPDB) (2013) Agriculture and environment research unit. University of Hertfordshire, UK. http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm
Peterson MA, McMaster SA, Riechers DE, Skelton J, Stahlman PW (2016) 2,4-D Past, present, and future: a review. Weed Technol 30:303–345. https://doi.org/10.1614/WT-D-15-00131.1
Picton P, Farenhorst A (2004) Factors influencing 2,4-D sorption and mineralization in soil. J Environ Sci Health Part B-Pestic Contam Agric Wastes 39:367–379. https://doi.org/10.1081/PFC-120035923
Pinna MV, Roggero P, Seddaiu G, Pusino A (2014) Soil sorption and leaching of active ingredients of lumax® under mineral or organic fertilization. Chemosphere 111:372–378. https://doi.org/10.1016/j.chemosphere.2014.03.124
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia- oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531. https://doi.org/10.1016/j.tim.2012.08.001
Rahman MM, Khanom A, Biswas S (2021) Effect of pesticides and chemical fertilizers on the nitrogen cycle and functional microbial communities in paddy soils: Bangladesh perspective. Bull Environ Contam Toxicol 106:243–249. https://doi.org/10.1007/s00128-020-03092-5
Riah W, Laval K, Laroche-Ajzenberg E, Mougin C, Latour X, Trinsoutrot-Gattin I (2014) Effects of pesticides on soil enzymes: a review. Environ Chem Lett 12:257–273. https://doi.org/10.1007/s10311-014-0458-2
Rose MT, Ng EL, Weng Z, Wood R, Rose TJ, Zwieten VL (2018) Minor effects of herbicides on microbial activity in agricultural soils are detected by N-transformation but not enzyme activity assays. Eur J Soil Biol 87:72–79. https://doi.org/10.1016/j.ejsobi.2018.04.003
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712. https://doi.org/10.1128/2Faem.63.12.4704-4712.1997
Sarmah AK, Müller K, Ahmad R (2004) Fate and behaviour of pesticides in the agroecosystem - A review with a New Zealand perspective. Aust J Soil Res 42:125–154. https://doi.org/10.1071/SR03100
Schwarzenbach RP, Gschwend PM, Imboden M (2003) Environmental organic chemistry, 2nd edn. John Wiley & Sons, Hoboken, NJ
Serra-Wittling C, Houots S, Barriuso E (1995) Soil enzymatic response to addition of municipal solid-waste compost. Biol Fertil Soils 20:226–236. https://doi.org/10.1007/BF00336082
Sim J, Doolette C, Vasileiadis S, Drigo B, Wyrsch E, Djordjevic S, Karpouzas C, Lombi E (2022) Pesticide effects on nitrogen cycle related microbial functions and community composition. Sci Total Environ 807:150734. https://doi.org/10.1016/j.scitotenv.2021.150734
Singh N (2006) Metribuzin mobility in soil columns as affected by urea fertilizer. Pest Manage Sci 62:402–406. https://doi.org/10.1002/ps.1173
Spuler MJ, Briceño G, Duprat F, Jorquera M, Céspedes C, Palma G (2019) Sorption kinetics of 2,4-D and diuron herbicides in a urea-fertilized Andisol. J Soil Sci Plant Nutr 19:313–320. https://doi.org/10.1007/s42729-019-00031-0
Sun R, Myrold D, Wang D, Guo X, Chu H (2019) AOA and AOB communities respond differently to changes of soil pH under long-term fertilization. Soil Ecol Lett 1:126–135. https://doi.org/10.1007/s42832-019-0016-8
Takoutsing B, Weber J, Aynekulu E, Rodríguez JA, Shepherd K, Sila A, Tchoundjeu Z, Diby L (2016) Assessment of soil health indicators for sustainable production of maize in smallholder farming systems in the highlands of Cameroon. Geoderma 276:64–73. https://doi.org/10.1016/j.geoderma.2016.04.027
Tan H, Xu M, Li X, Zhang H, Zhang C (2013) Effects of chlorimuron-ethyl application with or without urea fertilization on soil ammonia-oxidizing bacteria and archaea. J Hazard Mater 260:368–374. https://doi.org/10.1016/j.jhazmat.2013.05.043
Triantafyllidis V, Zotos A, Kosma C, Kokkotos E (2020) Effect of land-use types on edaphic properties and plant species diversity in mediterranean agroecosystem. Saudi J Biol Sci 27:3676–3690. https://doi.org/10.1016/j.sjbs.2020.08.012
United States Environmental Protection Agency (USEPA) (2005) Reregistration eligibility decision for 2,4-D. EPA 738-R-05-002, June 2005. https://archive.epa.gov/pesticides/reregistration/web/pdf/24d_red.pdf
Yamaguchi T, Mahmood A, Ito T, Kataoka R (2021) Non-target impact of dinotefuran and azoxystrobin on soil bacterial community and nitrification. Bull Environ Contam Toxicol 106:996–1002. https://doi.org/10.1007/s00128-021-03163-1
Zabaloy MC, Garland JL, Gómez MA (2008) An integrated approach to evaluate the impacts of the herbicides glyphosate, 2, 4-D and metsulfuron-methyl on soil microbial communities in the Pampas region, Argentina. Appl Soil Ecol 40:1–12. https://doi.org/10.1016/j.apsoil.2008.02.004
Zabaloy MC, Garland J, Gómez M (2010) Assessment of the impact of 2,4-idichlorophenoxyacetic acid (2,4-D) on indigenous herbicide-degrading bacteria and microbial community function in an agricultural soil. Appl Soil Ecol 46:240–246. https://doi.org/10.1016/j.apsoil.2010.08.006
Zabaloy MC, Allegrini M, Tebbe D, Schuster K, Gómez E (2017) Nitrifying bacteria and archaea withstanding glyphosate in fertilized soil microcosms. Appl Soil Ecol 117–118:88–95. https://doi.org/10.1016/j.apsoil.2017.04.012
Funding
This work was financed by Universidad de La Frontera (Project DI20-0051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palma, G., Spuler, M.J., Jorquera, M. et al. Effects of the Combined Application of Nitrogen Fertilizer and 2,4-D on Nitrification Ammonia Oxidizers and Herbicide Bioavailability in a Volcanic Soil. A Microcosm Study. J Soil Sci Plant Nutr 23, 4309–4317 (2023). https://doi.org/10.1007/s42729-023-01350-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-023-01350-z