Abstract
Freeze-tolerant animals survive sub-zero temperatures and long-term starvation associated with the winter by lowering their metabolic rate using a variety of transcriptional, translational, and post-translational regulatory methods. Histone methylation is one mechanism that is known to regulate gene expression at the transcriptional level. Here, we measured relative protein levels of seven histone methyltransferases (SMYD2, SETD7, ASH2L, RBBP5, SUV39H1, EHMT2, and SET8), four methylated histone H3 residues (H3K4me1, H3K9me3, H3K27me1, and H3K36me2), the methyltransferase activity on H3K4, and methylation of p53 (p53K370me2 and p53K372me1) in the skeletal muscle and liver of the freeze-tolerant wood frog (Rana sylvatica) during the freeze–thaw cycle. Overall, the results reveal a tissue-specific expression of histone methyltransferases and the methylation sites on histone H3 during freezing and thaw. In liver, H3K4me1 significantly decreased during freezing, H3K9me3 remained constant across conditions, H3K27me1 increased only during thaw, and H3K36me2 increased during freezing and then decreased during thaw (p < 0.05, n = 4). In skeletal muscle, H3K4me1 and H3K27me1 both decreased during freezing, whereas H3K9me3 and H3K36me2 were maintained across freezing and thaw (p < 0.05, n = 4). Methylation of p53 was also tissue-specific, where no changes were seen in liver tissue; however, p53 in skeletal muscle was differentially methylated. Overall, these results provide an evidence for the potential role methylation of histones and non-histone proteins play in freezing survival and entrance into a hypometabolic state.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most well-studied naturally freeze-tolerant vertebrates is the wood frog, Rana sylvatica (Storey and Storey 1984, 1988; Layne and Lee 1987; Lee et al. 1992). This animal enacts multiple adaptations to survive full-body freezing, including control of ice crystal formation using ice nucleating proteins, accumulation of massive amounts of intracellular glucose to prevent intracellular ice and maintain cell volume, and, perhaps, most crucially, a drastic reduction in metabolic rate to survive long periods while frozen (Storey 1984; Storey and Storey 1985; Wolanczyk et al. 1990; Costanzo et al. 1993). These mechanisms allow the wood frog to recover from days to weeks at sub-zero temperatures with up to ~65% of total body water frozen (Lee et al. 1992).
At sub-zero temperatures, ice crystal formation causes the onset of ischemia in the wood frog due to the cessation of breathing and blood flow. This produces a switch to anaerobic glycolysis in the form of lactic acid fermentation, which the wood frog must rely on for ATP generation for days to weeks until thawing occurs. As a result, all cellular energetic requirements depend on internal glucose stores accumulated before ice nucleation begins. Since the animal is limited to a finite supply of energy, reprioritization of ATP expenditure to only essential processes occurs until thawing takes place, resulting in global metabolic rate depression for the duration of the freezing period.
Some of the most energetically expensive processes are gene expression and protein synthesis. These two cell processes use 1–10% of the total energy production of the cell (Rolfe and Brown 1997) and, thus, are key targets for stringent regulation during periods of environmental stress (Land et al. 1993; Frerichs et al. 1998; Fuery et al. 1998; Storey and Storey 2004). Given that the expression of some genes is necessary for survival, the reduction of transcription and translation is done in a selective manner. Genes that are vital for surviving freezing temperatures, such as fibrinogen, antioxidants, novel freeze-responsive genes, and anti-apoptotic genes (Joanisse and Storey 1996; Cai and Storey 1997a, b; McNally et al. 2002, 2003; Gerber et al. 2016) are upregulated, while those unnecessary due to energy expenditure limitations, such as those involved in cell cycle progression, and glycolysis, are downregulated (Storey 1987a; Zhang and Storey 2012). Given the importance of controlling transcription at the global level in these animals, we have begun to explore the role that epigenetics play in the wood frog, and other species that undergo metabolic rate depression.
Epigenetics refers to heritable, molecular mechanisms that are capable of altering gene expression without changing the DNA sequence (Wolffe and Matzke 1999). There are two main categories of epigenetic mechanisms: DNA methylation, and histone modification. DNA methylation occurs mainly on the 5th position of cytosine residues (5mC), and is generally associated with silencing gene expression (Bogdanović and Veenstra 2009). Similar to DNA, histones can be modified by the addition of methyl groups, as well as a host of other chemical modifications, resulting in a multitude of transcriptional consequences (Peterson and Laniel 2004). Recent evidence has shown that epigenetic mechanisms are vital during times of cellular stress and environmental perturbation (Storey 2015). In the context of metabolic rate depression, DNA methylation and histone acetylation have shown to be differentially regulated between control and hypometabolic states in a variety of animals such as hibernating squirrels (Biggar and Storey 2014) and anoxia tolerant turtles (Krivoruchko and Storey 2010; Wijenayake and Storey 2016). The results from these animals lead to the idea that other epigenetic mechanisms, such as histone methylation, may also be involved in the coordinated regulation of metabolic rate depression.
Histone methylation is one such mechanism that has been strongly linked to transcriptional regulation (Zhang and Reinberg 2001; Kouzarides 2002; Martin and Zhang 2005; Liu et al. 2010; Greer and Shi 2012). The methylation of histones is carried out by histone methyltransferases (HMTs) specific to each residue (Fig. 1). The addition of uncharged methyl groups to certain amino acid residues creates effector protein binding sites (Lachner et al. 2001; Kim et al. 2006; Kouzarides 2007; Adams-Cioaba and Min 2009). The creation of new binding sites, therefore, allows histone methylation to act as either a transcriptional activator or repressor, depending on which proteins are recruited. Up to three methyl groups can be added to each histone lysine residue, resulting in mono-, di-, or tri-methylation (me1, me2, and me3, respectively). Each lysine residue, and the degree to which it is methylated, can have functionally distinct outcomes within the nucleus. For instance, active gene transcription and euchromatin has been strongly associated with mono-methylation of lysine 4 and mono-methylation of lysine 27 of histone H3 (H3K4me1 and H3K27me1, respectively) (Schneider et al. 2004; Martin and Zhang 2005), whereas gene silencing and heterochromatic regions are linked to tri-methylation of lysine 9 and di-methylation of lysine 36 (H3K9me3 and H3K36me2, respectively) (Peters et al. 2002; Keogh et al. 2005; Youdell et al. 2008; Li et al. 2009; Hsia et al. 2010). The role of histone methylation in metabolic rate depression and freeze tolerance has yet to be elucidated, and like other epigenetic processes mentioned previously, may regulate the hypometabolic state.
Histone lysine residues methylated by histone methyltransferases. Histone methyltransferases (HMTs) such as SETD7, RBBP5, ASH2L, SMYD2, SUV39H1, EHMT2, and SET8 transfer methyl groups from S-adenosyl methionine (SAM) to recipient histone lysine residues. Each residue can accept up to three methyl groups (me1, me2, and me3, respectively)
Lysine methylation is not limited to histone proteins, but rather has been shown to regulate non-histone targets, and influence a wide variety of signaling pathways and cellular processes (reviewed in Biggar and Li 2015). One of the many transcription factors that are regulated by lysine methylation is p53 (Chuikov et al. 2004; Huang et al. 2006; Shi et al. 2007). Lysine 370 and 372 both act to increase activity and stability of p53 when di- and mono-methylated, respectively (p53K370me2 and p53K372me1). Given that p53 is involved in cell cycle arrest, DNA damage repair, and apoptosis signaling, all of which have implications for freeze tolerance and metabolic rate depression, the methylation of p53 is of particular interest in the context of this study.
This study presents the first exploration of site-specific histone methylation in the context of freeze tolerance in R. sylvatica. The protein expression levels of seven histone methyltransferases (SETD7, RBBP5, ASH2L, SMYD2, SUV39H1, EHMT2, and SET8), global levels of corresponding methylated histone residues (H3K4me1, H3K9me3, H3K27me1, and H3K36me2), non-histone targets (p53K370me2 and p53K372me1), and total methyltransferase enzymatic activity on H3K4 were measured in the liver and skeletal muscle of R. sylvatica in response to 24 h freezing and 8 h thaw. Overall, the results show tissue-specific changes in HMTs and HMT target expression levels, as well as changes in HMT activity under freezing conditions.
Materials and methods
Animal care and treatment
Male wood frogs (R. sylvatica) were caught during the spring from breeding ponds in Ottawa, Ontario, kept in coolers with crushed ice from the edges of the ponds, and transported to Carleton University. All frogs were washed in tetracycline and then placed in plastic containers with sphagnum moss for 1 week in an incubator at 5 °C. Control frogs were sampled from this condition after the 1 week period.
For the frozen condition, frogs were placed in a plastic container with damp paper towel and put into an incubator at −4.0 °C for 45 min to lower the frog’s body temperature to below zero. The temperature was then raised to −2.5 °C for 24 h and frogs were randomly selected for sampling. The remaining frogs were placed back into an incubator at 5 °C for 8 h to allow thawing before sampling.
Control, 24 h frozen, and 8 h thaw frogs were euthanized by pithing, and then, skeletal muscle and liver tissues were excised and flash frozen in liquid N2 for a total of n = 4 of each tissue per condition. All tissues were then stored at −80 °C for future use.
All protocols were conducted with permission of the Carleton University Animal Care Committee and within the guidelines of the Canadian Council on Animal Care.
Total protein extraction
Muscle and liver tissues from control, 24 h frozen, and 8 h thaw conditions were weighed, then crushed with a mortar and pestle in liquid N2 and homogenized with a P10 homogenizer, 1:2 w/v in chilled homogenization buffer (20 mM HEPES, 200 mM NaCl, 0.1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, and 10 mM β-glycerophosphate, pH 7.4) with a few crystals of PMSF and 1 μL/mL of protease inhibitor (Catalogue # PIC002; BioShop Canada Inc., Burlington, ON, Canada). The homogenates were then centrifuged at 10,000×g for 15 min at 4 °C and the supernatant containing the soluble proteins was collected as samples. Total protein concentration for each sample was determined using the BioRad protein assay (Catalogue # 5000002; BioRad Laboratories, Hercules, CA, USA) at 595 nm on an MR5000 microplate reader (Dynatech Laboratories, Chantilly, VA, USA). Concentrations were then adjusted to 10 μg/μL using the same homogenization buffer as before. Aliquots of samples were taken at this stage to be used for HMT activity assays. The remaining sample volumes were used for western blotting. These samples were mixed 1:1 v/v with SDS buffer (100 mM Tris-base, 4% w/v SDS, 20% v/v glycerol, 0.2% w/v bromophenol blue, and 10% v/v 2-mercaptoethanol), ending with a final concentration of 5 μg/μL. All samples were then boiled in a water bath for 10 min to fully denature and linearize the proteins. Samples were stored at −40 °C until use.
Western blotting
Equal amounts of control, 24 h frozen, and 8 h thaw (15–40 μg, depending on tissue, and protein to be probed) were loaded into 6–15% discontinuous SDS–polyacrylamide gels (percentage of acrylamide in resolving gel dependent on size protein to be probed) with 4 μL of PiNK Plus pre-stained protein ladder (10.5–175 kDa; Catalogue # PM005-0500K; FroggaBio, Toronto, ON, Canada). The upper stacking gel (pH 6.8) was comprised of 5% acrylamide v/v in pH 6.8 Tris buffer (1 M Tris) with 0.1% SDS (Sodium dodecyl sulfate), 0.1% APS (ammonium persulphate), and 0.1% TEMED (N,N,N′,N′-Tetramethylethane-1,2-diamine), whereas resolving gels were 6–15% acrylamide v/v in pH 8.8 Tris buffer (1.5 M Tris), with 0.1% SDS, 0.1% APS, and 0.1% TEMED.
Loaded gels were run in the BioRad Mini Protean III system (BioRad Laboratories, Hercules, CA, USA) for 30–150 min at 180 V in running buffer (25 mM Tris-base, 190 mM glycine, 0.1% w/v SDS, pH 7.6). Proteins from the gels were then transferred onto 0.45 μm pore PVDF membranes by electroblotting at room temperature for 60–180 min at 160 mA in 1X transfer buffer (25 mM Tris-base, 192 mM glycine 10% v/v methanol, pH 8.5).
To prevent non-specific binding of primary and secondary antibodies, the membranes were incubated with skim milk (1–10%, on a rocker at room temperature for 30 min) in 1X TBST (20 mM Tris-base, 140 mM NaCl, 0.05% Tween-20) or M w 30,000–70,000 PVA (1 mg/mL, on a rocker at room temperature for 30–90 s) in 1X TBST. Membranes were then probed with primary antibodies (1:1000 v/v dilution in 1X TBST) at 4 °C for 18 h. Blots were washed 3 × 5 min with 1X TBST and probed with HRP-conjugated goat anti-rabbit secondary antibodies (1:8000 v/v dilution in TBST; Catalogue # APA002P, BioShop Canada Inc., Burlington, ON, Canada) at room temperature for 30 min. Membranes were washed 3 × 5 min again in 1X TBST and were visualized by chemiluminescence (1:1 v/v H2O2, Luminol) using the ChemiGenius Bio Imaging System (Syngene, Frederick, MD, USA). Membranes were then stained with Coomassie blue (0.25% w/v Coomassie brilliant blue, 7.5% v/v acetic acid, 50% methanol) to visualize all protein bands for loading standardization. Histone methyltransferase antibodies were purchased from Cell Signal (Lysine Methyltransferase Antibody Sampler Kit-#8694). Antibodies for H3K4me1 and H3K9me3 were purchased from Abcam (H3K4me1-ab8895; H3K9me3-ab8898), and antibodies for H3K27me1 and H3K36me2 were purchased from ActiveMotif (H3K27me1-39890; H3K36me2-39256). The primary antibody for p53 was purchased from Abcam (Catalogue # ab28), p53K370me2 was purchased from Ameritech Biomedicines (Catalogue # ATB-H0007), and the antibody for p53K372me1 was from Genetex (Catalogue # GTX117515).
HMT activity assays
Total methyltransferase activity on H3K4 was measured using the EpiQuik Histone Methyltransferase Activity/Inhibition Assay Kits (Epigentek, Catalogue # P-3002-96) following the manufacturer’s instructions. Activity on H3K4 was chosen for three reasons: (1) the HMTs that methylate H3K4 were shown to be consistently downregulated in both tissues, (2) levels of H3K4me1 were significantly decreased in both tissues, and (3) the connection between H3K4me1 and transcription activation has been concretely determined. Briefly, a dilution curve ranging from 20 to 100 µg of protein was tested using a pooled sample containing 10 µL of total protein extracts from each sample point to determine the linear range of protein needed for the assay. Based on the dilution curve, 30 and 60 µg of total protein extracts from liver and skeletal muscle, respectively, of control, 24 h frozen, and 8 h thaw wood frog tissue were incubated with the supplied adomet (Epigentek), and biotinylated substrate (Epigentek) in the provided 96-well microplate for 60 min at 37 °C. Along with the protein samples, in each run, a blank well was prepared by replacing total protein extracts with the provided assay buffer, and a positive control was prepared by replacing total protein extracts with the provided control enzyme (300 µg/mL; Epigentek). After incubation, each well was washed three times with 150 µL of 1X wash buffer. 50 µL of capture antibody provided with the kit (100 µg/mL; Epigentek) diluted 1:100 in 1X wash buffer was then added to each well and incubated for 60 min at room temperature on an orbital shaker. The wells were washed again three times with 150 µL of 1X wash buffer before adding 50 µL of supplied detection antibody (100 µg/mL; Epigentek) diluted 1:1000 in 1X wash buffer, and the wells were incubated for 30 min at room temperature. After a final five washes with 150 µL of 1X wash buffer, 100 µL of developing solution supplied with the kit was added to each well and incubated for 10 min at room temperature until the color of the positive control wells had reached a dark blue (indicating the presence of methylated histone H3K4). At this stage, 50 µL of the supplied stop solution was added to each well, and the sample wells were read using a microplate reader (Multiscan Spectrum, Thermo Labsystems) at 450 nm.
A standard curve was created by replacing total protein extracts with supplied HMT standard at different concentrations (0.1–5.0 ng/µL) as per the manufacturer’s instructions. The protein concentrations that were used fit on the linear portion of the standard curve, and the slope of this curve was calculated and used to calculate HMT activity using the following equation:
Quantification and statistics
Chemiluminescent protein bands on the immunoblots were quantified by densitometry using the ChemiGenius Bio Imaging System GeneTools Software (Syngene, Frederick, MD, USA). Band densities were standardized against the combined intensity of Coomassie blue stained bands in the same lane that did not show differential expression between conditions. Data for each experimental condition are expressed as mean ± SEM with n = 4 samples from different animals. Statistical analysis was performed by one-way ANOVAs and Tukey’s post-hoc test (p < 0.05) using SigmaPlot 12 statistical package software (Systat Software Inc., San Jose, CA, USA).
Results
HMT protein expression in response to 24 h freezing exposure and 8 h thaw
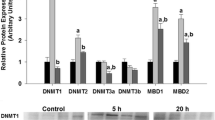
Relative protein expression levels of seven HMTs (SETD7, RBBP5, ASH2L, SMYD2, SUV39H1, EHMT2, and SET8) were measured in the liver (Fig. 2) and skeletal muscle (Fig. 3) of R. sylvatica. Control frogs are compared with frogs exposed to 24 h freezing temperatures and 8 h thaw. In liver (Fig. 2), SMYD2 and ASH2L were significantly downregulated to 30 ± 8 and 20 ± 2% of control, respectively, during 24 h freezing compared to the control (p < 0.05), and while ASH2L returned to above control levels (140 ± 13% of control levels), SMYD2 remained downregulated after 8 h thaw (10 ± 2% of control). RBBP5 did not change significantly across the three conditions, whereas SETD7 increased to 160 ± 8% of control values during 24 h freezing, and remained elevated at 180 ± 16% of control values during 8 h thaw compared to the control (p < 0.05). The HMTs that methylate H3K9 (SUV39H1, EHMT2) and SET8 remained unchanged.
Effects of 24 h frozen and 8 h thaw on relative proteins levels of SETD7, RBBP5, ASH2L, SMYD2, SUV39H1, EHMT2, and SET8 methyltransferases in R. sylvatica liver as determined by Western immunoblotting. Data are mean ± SEM, n = 4 independent trials on samples from different animals. a Not significantly different from corresponding control. b Significantly different from corresponding control (p < 0.05). c Significantly different from corresponding control and 24 h frozen (p < 0.05)
Effects of 24 h frozen and 8 h thaw on relative proteins levels of SETD7, RBBP5, ASH2L, SMYD2, SUV39H1, EHMT2, and SET8 methyltransferases in R. sylvatica skeletal muscle. All other information as in Fig. 2
In skeletal muscle (Fig. 3), ASH2L protein levels decreased to 50 ± 5% of control values during 24 h freezing (p < 0.05) and remained at that level during 8 h thaw (50 ± 13% of control). SETD7, RBBP5, and SMYD2 showed no significant change across experimental conditions. As for the HMTs that methylate H3K9, SUV39H1 increased to 140 ± 13% of control values during 8 h thaw (p < 0.05), while EHMT2 had no significant change. SET8 decreased to 40 ± 6% of control values during 24 h freezing (p < 0.05) and remained at that level for 8 h thaw.
Analysis of methylated histone residues levels
Relative protein levels of H3K4me1, H3K9me3, H3K27me1, and H3K36me2 in liver (Fig. 4) and skeletal muscle (Fig. 5) were measured during control, 24 h freezing, and 8 h thaw conditions. In liver tissue, expression levels of H3K4me1 were observed to significantly decrease to 24 ± 5% of control values during 24 h freezing and remained decreased at 30 ± 3% during 8 h thaw (p < 0.05) compared to the control, whereas H3K9me3 did not change between the three conditions. Total levels of H3K27me1 did not change between control and 24 h freezing; however, it increased to 280 ± 62% of control values after 8 h thaw (p < 0.05). H3K36me2 increased significantly to 130 ± 5% of control values during 24 h freezing (p < 0.05) and then decreased to 60 ± 9% of the control value during 8 h thaw (p < 0.05).
Effects of 24 h frozen and 8 h thaw on relative protein levels of H3K4me1, H3K9me3, H3K27me1, and H3K36me2 in R. sylvatica liver. All other information as in Fig. 2
Effects of 24 h frozen and 8 h thaw on relative protein levels of H3K4me1, H3K9me3, H3K27me1, and H3K36me2 in R. sylvatica skeletal muscle. All other information as in Fig. 2
H3K4me1 levels in skeletal muscle significantly decreased to 2 ± 1 and 4 ± 1% of control values during both 24 h freezing and 8 h thaw, respectively (p < 0.05) compared to the control. H3K9me3 levels remained constant across the three conditions. H3K27me1 decreased significantly to 20 ± 4% of control values during 24 h freezing and stayed at that level during 8 h thaw (p < 0.05), while H3K36me2 levels did not change between all experimental conditions.
Analysis of HMT activity
Total HMT activity levels on H3K4 were measured in total soluble protein extracts of liver and skeletal muscle (Fig. 6) comparing 24 h freezing and 8 h thaw to the control. In liver, total mono-methylating activity on H3K4 decreased significantly to 20 ± 7% of control values in 24 h freezing (p < 0.05) and 30 ± 4% of control values after 8 h thaw. The activity on H3K4 in skeletal muscle was also observed to decrease significantly to 50 ± 10% of control after 24 h freezing, and continued to decrease to 26 ± 10% of control values in response to an 8 h thaw (p < 0.05).
Relative HMT activity on H3K4 in the liver and skeletal muscle of R. sylvatica comparing the effects of 24 h frozen and 8 h thaw. Enzyme activities are expressed in relative enzymatic activity levels. Data are mean ± SEM, n = 4 independent trials on samples from different animals. a Not significantly different from corresponding control. b Significantly different from corresponding control (p < 0.05). a, b Not significantly different from corresponding control and 24 h frozen (p < 0.05)
Relative levels of methylated p53 in response to freezing temperatures
The relative protein levels of total p53, p53K370me2, and p53K372me1 were measured in liver and skeletal muscle of R. sylvatica in control, 24 h frozen, and 8 h thaw frogs. In the liver (Fig. 7), total p53, p53K370me2, and p53K372me1 were not affected by freezing temperatures and subsequent thawing. In skeletal muscle (Fig. 8), total p53 did not change; however, relative levels of p53K370me2 increased to 200 ± 13% relative to the control (p < 0.05) during 24 h freezing, and increased further to 230 ± 34% during 8 h thaw. Levels of p53K372me1 decreased to 70 ± 5% of control values during 24 h freezing and continued to decrease to 40 ± 1% relative to the control in response to 8 h thaw.
Effects of 24 h frozen and 8 h thaw on relative protein levels of total p53, p53K370me2, and p53K372me1 in R. sylvatica liver. All other information as in Fig. 2
Effects of 24 h frozen and 8 h thaw on relative protein levels of total p53, p53K370me2, and p53K372me1 in R. sylvatica skeletal muscle. All other information as in Fig. 2
Discussion
The wood frog, R. sylvatica, belongs to a small group of vertebrates that can survive full-body freezing over long periods of time. Although several biochemical adaptations for freezing survival have been elucidated in the wood frog (Storey 1987b; Greenway and Storey 2000; Cowan and Storey 2001; Rider et al. 2006; Woods and Storey 2006; Dieni and Storey 2008, 2011; Zhang and Storey 2013; Abboud and Storey 2013), the role of histone methylation has yet to be explored. Histone lysine methylation plays a major role in the regulation of protein expression and the overall function of these modifications depends on the residue and the degree to which it is methylated. This gives histone modifications both a transcriptional activator and repressor role (Kouzarides 2002, 2007), making it of particular interest in the wood frog as many genes are differentially regulated in response to freeze temperatures.
The HMTs that methylate H3K4 (SETD7, RBBP5, ASH2L, and SMYD2) showed either a significant downregulation or no change in protein levels during 24 h freezing in both tissues when compared to control, except for SETD7 in the liver (Figs. 2, 3). The potential net decrease in these HMTs that methylate H3K4 is consistent with the fact that they are transcription activators, and thus, their downregulation may be to decrease transcription, while the wood frog is frozen. To further support this finding, levels of H3K4me1 decreased significantly in muscle and liver during freezing (Figs. 4, 5) and total methyltransferase activity on H3K4 in both tissues was reduced a comparable amount (Fig. 6). If reducing the amount of methylated H3K4 is a contributing factor to metabolic rate depression and subsequent freezing survival, we would expect to see the overall decrease in HMT protein levels and/or methyltransferase activity, and ultimately the reduced amount of H3K4me1 as seen in these results.
Once ice nucleation begins in the wood frog, glycogen stores in the liver decrease substantially, fueling the synthesis of extremely high levels of glucose in the liver (Storey and Storey 1984, 1985). The glucose is then shuttled into the blood for distribution to all other tissues, where it acts as a cryoprotectant. Glycogenolysis in the liver continues even after extracellular freezing has reached its full extent (Storey and Storey 1984) showing a need for the regulation of some liver function, while the wood frog is in a frozen state. Aside from histones, SETD7 also methylates the farnesoid X receptor (FXR), regulating the expression of FXR target genes specific to the liver (Balasubramaniyan et al. 2012). FXR expression has been shown to be upregulated in response to glucose (Duran-Sandoval et al. 2004), and results in reducing glucose production, and increasing insulin sensitivity (Ma et al. 2006). SETD7 protein levels increase during 24 h freezing in the liver and remain elevated during 8 h thaw, which suggests that it may be expressed in anticipation of rising temperatures when the wood frog thaws and must return glucose to basal levels. Not only would the elevated levels of glucose be toxic when the animal returns to euthermic temperatures, the replenishment of glycogen levels must also occur for the animal to be prepared for the next freezing event.
Unlike the HMTs that methylate H3K4, the ones that act on H3K9 (SUV39H1, EHMT2) did not change significantly (Figs. 2, 3), nor did levels of H3K9me3 (Figs. 4, 5) in either tissue. On the other hand, H3K36me2, which recruits the histone deacetylase complex Rpd3S to act on Histone H4 (Li et al. 2009), did change. We found a significant increase in the levels of H3K36me2 in liver tissue during freezing exposure (Fig. 4); however, no change was seen in skeletal muscle during the same conditions (Fig. 5). One of the downstream actions of H3K36me2, recruitment of histone deacetylases, has previously been shown as a contributing factor in animals that undergo metabolic rate depression (Krivoruchko and Storey 2010), alluding to a potential role for histone deacetylation and H3K36me2 in the wood frog as it enters a metabolically depressed state.
H3K27me1 is a histone modification associated with active gene transcription (Barski et al. 2007; Ferrari et al. 2014), and of the other histone modification examined in this study, it is most functionally similar to H3K4me1. It is of interest to note that H3K4me1 significantly decreased during 24 h freezing and remained at that level during the 8 h thaw, whereas H3K27me1 did not change during 24 h freezing, but increased during the 8 h thaw (Fig. 4). In an animal that reduces its metabolic rate to a dramatically low level, the difference seen between these two functionally similar modifications may be due to the role the liver plays in freezing survival. As mentioned previously, the liver has a unique and vital role during freezing and remains metabolically active to produce and release high levels of glucose that is needed for survival, while most other tissues such as skeletal muscle are dormant (Storey and Storey 1986). This reduced but still present metabolic activity in the liver requires a transcriptionally permissive histone modification during freezing to continue the production of enzymes involved in processes such as glycogenolysis. Once the wood frog begins to thaw, the liver facilitates multiple methods to decrease glucose levels, which otherwise would be toxic (Storey and Storey 1986; Layne et al. 1996; Costanzo et al. 1997). These immediate steps taken by the liver to return to euglycemic levels require coordinated regulation from the transcriptional level down to modulating the activity of metabolic enzymes. Thus, a transcriptionally permissive histone modification could be involved, leaving open the possibility that the increase in H3K27me1 during 8 h thaw is to regulate the return to metabolic baseline. In contrast to liver tissue, skeletal muscle in the wood frog does not have as high of a level of function during freezing. Myofibril contraction is blocked by ice formed around the muscle, and although cryoprotective glucose increases in the muscle during the freezing period, it is not derived from muscle glycogen stores, but rather is transported from the liver where it is produced (Storey 1984). It, therefore, comes as no surprise that the changes seen in H3K27me1, a dramatic decrease during freezing and thaw, match those seen in H3K4me1 in skeletal muscle (Fig. 5) given the fact that there are low metabolic requirements in the tissue compared to the liver.
In recent years, the number of non-histone proteins discovered to be controlled by methylation has grown substantially and is expected to continue rising (Biggar and Li 2015). The transcription factor p53 is heavily regulated by many post-translational modifications (Tibbetts et al. 1999; Barlev et al. 2001; Tang et al. 2008; Lee and Gu 2009), including methylation, making it particularly interesting in the context of this study. Exerting control over many processes such as the cell cycle (Agarwal et al. 1995), apoptosis (Lowe et al. 1993), and energy metabolism (Puzio-Kuter 2011), p53 could play a major role in the wood frog’s survival during periods of freezing. Here, we look at two positive regulating methyl-lysine residues on p53 (p53K370me2 and p53K372me1). Although there were no changes in the liver (Fig. 7), there was a significant differential regulation of methylated lysine residues in skeletal muscle (Fig. 8). The most prominent change, an increase to 200% of control values in p53K370me2 during freezing and thawing, suggests an active role for p53 during and after periods of whole body freezing in the skeletal muscle. One possibility for the change in methylation status of p53 in skeletal muscle tissue, but not liver, is the difference in metabolic output and function during freezing stress. Whereas some liver function remains, while the frog is in a frozen state, skeletal muscle is less metabolically active, relative to euthermic conditions, given that all muscle movement is halted due to ice formation. The previous studies have shown both an increase in anti-apoptotic proteins, and cell cycle suppression proteins in skeletal muscle when the wood frog enters a hypometabolic state (Zhang and Storey 2012; Gerber et al. 2016), thus potentially leading to a resistance to changes in muscle mass. Given that methylation and subsequent activation of p53 may be a mechanism for inducing cell cycle arrest, it may be the case that the increases in p53 methylation during freezing and thaw may be a mechanism to halt the cell cycle for the wood frog and thus halt the expenditure of scarce energy resources. Active p53 has been shown to increase in other hypometabolic animals (Zhang et al. 2013; Biggar et al. 2015). For example, p53 in the anoxia tolerant red-eared slider turtle translocates to the nucleus and is phosphorylated at multiple sites, leading to the increased transcription of p53 downstream genes (Zhang et al. 2013). Since p53 activation may be seen in multiple species that undergo metabolic rate depression, it could be possible that p53 methylation is a contributing mechanism for increasing p53 activity. Further investigation into whether there is a direct causal link between the methylation of p53 and suppression of the cell cycle in the wood frog will be needed to expand our understanding of cellular regulation by protein methylation.
Conclusion
In summary, the present study demonstrates tissue-specific changes in HMTs, their activity, and the histone and non-histone proteins that they methylate in response to freezing and thaw. Crucial to freezing survival is the reprioritization of finite energy reserves, the wood frog accumulates prior to temperatures dropping below zero. This is made possible through top level controls (i.e. transcriptional regulation) to enter a state of hypometabolism. Differentially regulated histone methylation may provide the base for downregulating pathways that are not vital, while simultaneously maintaining the select few that are. These results are not without their limitations; global changes in histone modification can only signify general trends across the genome. Ultimately histone modifications act on a gene-to-gene level, and thus, how exactly these global changes map to individual genes is the direction that this research must take. Directly connecting changes in histone modifications at individual genes will help broaden our understanding of the role histone modifications play, and how they enable survival of whole body freezing by the wood frog.
References
Abboud J, Storey KB (2013) Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ 1:e12
Adams-Cioaba MA, Min J (2009) Structure and function of histone methylation binding proteins. Biochem Cell Biol 87:93–105
Agarwal ML, Agarwal A, Taylor WR, Stark GR (1995) p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci USA 92:8493–8497
Balasubramaniyan N, Ananthanarayanan M, Suchy FJ (2012) Direct methylation of FXR by Set7/9, a lysine methyltransferase, regulates the expression of FXR target genes. Am J Physiol Liver Physiol 302:G937–G947
Barlev NA, Liu L, Chehab NH et al (2001) Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol Cell 8:1243–1254
Barski A, Cuddapah S, Cui K et al (2007) High-resolution profiling of histone methylations in the human genome. Cell 129:823–837
Biggar KK, Li SS-C (2015) Non-histone protein methylation as a regulator of cellular signalling and function. Nat Rev Mol Cell Biol 16:5–17
Biggar Y, Storey KB (2014) Global DNA modifications suppress transcription in brown adipose tissue during hibernation. Cryobiology 69:333–338
Biggar KK, Wu C-W, Tessier SN et al (2015) Primate torpor: regulation of stress-activated protein kinases during daily torpor in the gray mouse lemur, Microcebus murinus. Genom Proteom Bioinform 13:81–90
Bogdanović O, Veenstra GJC (2009) DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma 118:549–565
Cai Q, Storey KB (1997a) Freezing-induced genes in wood frog (Rana sylvatica): fibrinogen upregulation by freezing and dehydration. Am J Physiol 272:R1480–R1492
Cai Q, Storey KB (1997b) Upregulation of a novel gene by freezing exposure in the freeze-tolerant wood frog (Rana sylvatica). Gene 198:305–312
Chuikov S, Kurash JK, Wilson JR et al (2004) Regulation of p53 activity through lysine methylation. Nature 432:353–360
Costanzo JP, Lee RE, Lortz PH (1993) Glucose concentration regulates freeze tolerance in the wood frog Rana sylvatica. J Exp Biol 181:245–255
Costanzo JP, Callahan PA, Lee RE Jr, Wright MF (1997) Frogs reabsorb glucose from urinary bladder. Nature 389:343–344
Cowan KJ, Storey KB (2001) Freeze–thaw effects on metabolic enzymes in wood frog organs. Cryobiology 43:32–45
Dieni CA, Storey KB (2008) Regulation of 5′-adenosine monophosphate deaminase in the freeze tolerant wood frog, Rana sylvatica. BMC Biochem 9:12
Dieni CA, Storey KB (2011) Regulation of hexokinase by reversible phosphorylation in skeletal muscle of a freeze-tolerant frog. Comp Biochem Physiol Part B Biochem Mol Biol 159:236–243
Duran-Sandoval D, Mautino G, Martin G et al (2004) Glucose regulates the expression of the farnesoid X receptor in liver. Diabetes 53:890–898
Ferrari KJ, Scelfo A, Jammula S et al (2014) Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol Cell 53:49–62
Frerichs KU, Smith CB, Brenner M et al (1998) Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci USA 95:14511–14516
Fuery CJ, Withers PC, Hobbs AA, Guppy M (1998) The role of protein synthesis during metabolic depression in the Australian desert frog Neobatrachus centralis. Comp Biochem Physiol Part A Mol Integr Physiol 119:469–476
Gerber VEM, Wijenayake S, Storey KB (2016) Anti-apoptotic response during anoxia and recovery in a freeze-tolerant wood frog (Rana sylvatica). PeerJ 4:e1834
Greenway SC, Storey KB (2000) Activation of mitogen-activated protein kinases during natural freezing and thawing in the wood frog. Mol Cell Biochem 209:29–37
Greer EL, Shi Y (2012) Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet 13:343–357
Hsia DA, Tepper CG, Pochampalli MR et al (2010) KDM8, a H3K36me2 histone demethylase that acts in the cyclin A1 coding region to regulate cancer cell proliferation. Proc Natl Acad Sci USA 107:9671–9676
Huang J, Perez-Burgos L, Placek BJ et al (2006) Repression of p53 activity by Smyd2-mediated methylation. Nature 444:629–632
Joanisse DR, Storey KB (1996) Oxidative damage and antioxidants in Rana sylvatica, the freeze-tolerant wood frog. Am J Physiol 271:R545–R553
Keogh M-C, Kurdistani SK, Morris SA et al (2005) Cotranscriptional Set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 123:593–605
Kim J, Daniel J, Espejo AB et al (2006) Tudor, MBT and chromo domains gauge the degree of lysine methylation. EMBO Rep 7:89–91
Kouzarides T (2002) Histone methylation in transcriptional control. Curr Opin Genet Dev 12:198–209
Kouzarides T (2007) Chromatin modifications and their function. Cell 128:693–705
Krivoruchko A, Storey KB (2010) Epigenetics in anoxia tolerance: a role for histone deacetylases. Mol Cell Biochem 342:151–161
Lachner M, O’Carroll D, Rea S et al (2001) Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410:116–120
Land SC, Buck LT, Hochachka PW (1993) Response of protein synthesis to anoxia and recovery in anoxia-tolerant hepatocytes. Am J Physiol 265:R41–R48
Layne JR, Lee RE (1987) Freeze tolerance and the dynamics of ice formation in wood frogs (Rana sylvatica) from southern Ohio. Can J Zool 65:2062–2065
Layne JR, Lee RE, Cutwa MM (1996) Post-hibernation excretion of glucose in urine of the freeze tolerant frog Rana sylvatica. J Herpetol 30:85
Lee JT, Gu W (2009) The multiple levels of regulation by p53 ubiquitination. Cell Death Differ 17:86–92
Lee RE, Costanzo JP, Davidson EC, Layne JR (1992) Dynamics of body water during freezing and thawing in a freeze-tolerant frog (Rana sylvatica). J Therm Biol 17:263–266
Li B, Jackson J, Simon MD et al (2009) Histone H3 lysine 36 dimethylation (H3K36me2) is sufficient to recruit the Rpd3s histone deacetylase complex and to repress spurious transcription. J Biol Chem 284:7970–7976
Liu C, Lu F, Cui X, Cao X (2010) Histone methylation in higher plants. Annu Rev Plant Biol 61:395–420
Lowe SW, Schmitt EM, Smith SW et al (1993) p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362:847–849
Ma K, Saha PK, Chan L, Moore DD (2006) Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest 116:1102
Martin C, Zhang Y (2005) The diverse functions of histone lysine methylation. Nat Rev Mol Cell Biol 6:838–849
McNally JD, Wu S-B, Sturgeon CM, Storey KB (2002) Identification and characterization of a novel freezing-inducible gene, li16, in the wood frog Rana sylvatica. FASEB J 16:902–904
McNally JD, Sturgeon CM, Storey KB (2003) Freeze-induced expression of a novel gene, fr47, in the liver of the freeze-tolerant wood frog, Rana sylvatica. Biochim Biophys Acta BBA Gene Struct Expr 1625:183–191
Peters AHFM, Mermoud JE, O’Carroll D et al (2002) Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet 30:77–80
Peterson CL, Laniel M-A (2004) Histones and histone modifications. Curr Biol 14:R546–R551
Puzio-Kuter AM (2011) The role of p53 in metabolic regulation. Genes Cancer 2:385–391
Rider MH, Hussain N, Horman S et al (2006) Stress-induced activation of the AMP-activated protein kinase in the freeze-tolerant frog Rana sylvatica. Cryobiology 53:297–309
Rolfe DFS, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758
Schneider R, Bannister AJ, Myers FA et al (2004) Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol 6:73–77
Shi X, Kachirskaia I, Yamaguchi H et al (2007) Modulation of p53 function by SET8-mediated methylation at lysine 382. Mol Cell 27:636–646
Storey KB (1984) Freeze tolerance in the frog, Rana sylvatica. Experientia 40:1261–1262
Storey KB (1987a) Glycolysis and the regulation of cryoprotectant synthesis in liver of the freeze tolerant wood frog. J Comp Physiol B 157:373–380
Storey KB (1987b) Organ-specific metabolism during freezing and thawing in a freeze-tolerant frog. Am J Physiol Integr Comp Physiol 253:R292–R297
Storey KB (2015) Regulation of hypometabolism: insights into epigenetic controls. J Exp Biol 218:150–159
Storey KB, Storey JM (1984) Biochemical adaption for freezing tolerance in the wood frog, Rana sylvatica. J Comp Physiol B 155:29–36
Storey JM, Storey KB (1985) Triggering of cryoprotectant synthesis by the initiation of ice nucleation in the freeze tolerant frog, Rana sylvatica. J Comp Physiol B 156:191–195
Storey KB, Storey JM (1986) Freeze tolerant frogs—cryoprotectants and tissue metabolism during freeze thaw cycles. Can J Zool 64:49–56
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev 68:27–84
Storey KB, Storey JM (2004) Metabolic rate depression in animals: transcriptional and translational controls. Biol Rev 79:207–233
Tang Y, Zhao W, Chen Y et al (2008) Acetylation is indispensable for p53 activation. Cell 133:612–626
Tibbetts RS, Brumbaugh KM, Williams JM et al (1999) A role for ATR in the DNA damage-induced phosphorylation of p53. Genes Dev 13:152–157
Wijenayake S, Storey KB (2016) The role of DNA methylation during anoxia tolerance in a freshwater turtle (Trachemys scripta elegans). J Comp Physiol B 186:333–342
Wolanczyk JP, Storey KB, Baust JG (1990) Ice nucleating activity in the blood of the freeze-tolerant frog, Rana sylvatica. Cryobiology 27:328–335
Wolffe AP, Matzke MA (1999) Epigenetics: regulation through repression. Science 286:481–486
Woods AK, Storey KB (2006) Vertebrate freezing survival: regulation of the multicatalytic proteinase complex and controls on protein degradation. Biochim Biophys Acta BBA Gen Subj 1760:395–403
Youdell ML, Kizer KO, Kisseleva-Romanova E et al (2008) Roles for Ctk1 and Spt6 in regulating the different methylation states of histone H3 lysine 36. Mol Cell Biol 28:4915–4926
Zhang Y, Reinberg D (2001) Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev 15:2343–2360
Zhang J, Storey KB (2012) Cell cycle regulation in the freeze-tolerant wood frog, Rana sylvatica. Cell Cycle 11:1727–1742
Zhang J, Storey KB (2013) Akt signaling and freezing survival in the wood frog, Rana sylvatica. Biochim Biophys Acta BBA Gen Subj 1830:4828–4837
Zhang J, Biggar KK, Storey KB (2013) Regulation of p53 by reversible post-transcriptional and post-translational mechanisms in liver and skeletal muscle of an anoxia tolerant turtle, Trachemys scripta. Gene 513:147–155
Acknowledgements
The authors thank J.M. Storey for assistance in the editing of the manuscript. This work was supported by a Discovery Grant (Grant # 6793) from the Natural Sciences and Engineering Research Council (NSERC) of Canada. KBS holds the Canada Research Chair in Molecular Physiology. LJH holds a Queen Elizabeth II Graduate Scholarship in Science and Technology award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Hawkins, L.J., Storey, K.B. Histone methylation in the freeze-tolerant wood frog (Rana sylvatica). J Comp Physiol B 188, 113–125 (2018). https://doi.org/10.1007/s00360-017-1112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1112-7