Abstract
The frog Nanorana parkeri (Dicroglossidae) is endemic to the Tibetan Plateau, and overwinters shallow pond within damp caves for up to 6 months. Herein, we investigate the freeze tolerance of this species and profile changes in liver and skeletal muscle metabolite levels using an untargeted LC–MS-based metabolomic approach to investigate molecular mechanisms that may contribute to freezing survival. We found that three of seven specimens of N. parkeri could survive after being frozen for 12 h at − 2.0 °C with 39.91% ± 5.4% (n = 7) of total body water converted to ice. Freezing exposure induced partial dehydration of the muscle, which contributed to decreasing the amount of freezable water within the muscle and could be protective for the myocytes themselves. A comparative metabolomic analysis showed that freezing elicited significant responses, and a total of 33 and 36 differentially expressed metabolites were identified in the liver and muscle, respectively. These metabolites mainly participate in alanine, aspartic acid and glutamic acid metabolism, arginine and proline metabolism, and D-glutamine and D-glutamate metabolism. After freezing exposure, the contents of ornithine, melezitose, and maltotriose rose significantly; these may act as cryoprotectants. Additionally, the content of 8-hydroxy-2-deoxyguanine, 7-Ketocholesterol and hypoxanthine showed a marked increase, suggesting that freezing induced oxidative stress in the frogs. In summary, N. parkeri can tolerate a brief and partial freezing of their body, which was accompanied by substantial changes in metabolomic profiles after freezing exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ectothermic animals have developed different physiological strategies to cope with the extreme cold of winter including freeze avoidance and freeze tolerance in addition to overwintering deep in the ground or in water bodies (Costanzo 2019; Storey and Storey 2017). Freeze-avoiding species (including many insects and other invertebrates, as well as some marine fish) primarily depend on supercooling, and can seasonally produce antifreeze proteins and low molecular weight cryoprotectants (usually glycerol) (Duman 2015). Freeze-tolerant species have adapted to endure ice formation in extracellular spaces, controlling the sites and growth rates of ice and protecting the intracellular space with high levels of cryoprotectants (Storey and Storey 1988, 2017; Costanzo and Lee 2013). Physiological and biochemical responses known to occur in freeze-tolerant ectotherms include (1) the triggering of extracellular ice formation by ice nucleators (e.g., specific ice-nucleating proteins in body fluids or non-specific nucleation by microbes in skin or gut); (2) the accumulation of high concentrations of low molecular weight cryoprotectants inside cells to restrict intracellular dehydration and stabilize macromolecular structure; and (3) the partial dehydration of tissues (Schiller et al. 2008; Costanzo and Lee 2008; Costanzo et al. 2006). Freeze tolerance, as a survival strategy of amphibians in cold environments, has been extensively investigated in species living in Europe and North America (Storey and Storey 2017; Costanzo 2019; Voituron et al. 2009; Geiss et al. 2019). However, the issues and mechanisms of cold hardiness among the frog species that are indigenous to the Tibetan plateau, the highest altitude on Earth where frogs are found, are still not well understood.
Advances in some emerging “omics” technologies, such as transcriptome, proteome, and metabolome analysis, have created new opportunities to explore the adaptive mechanisms of freeze tolerance and prolonged hibernation in animals, and greatly facilitate comparative physiological studies, especially in non-model animals (Storey and Storey 2013; Storey 2015; D’Alessandro et al. 2016; Vesala et al. 2012). Metabolomics provides a comprehensive understanding of fluctuations in metabolite levels in response to environmental stressors, identifying and quantifying the low molecular weight endogenous compounds that respond to stress (Dunn and Ellis 2005; Rochfort 2005). The most commonly used analytical technologies for metabolomic analysis are gas chromatography-mass spectrometry (GC–MS), liquid chromatography-mass spectrometry (LC–MS), and nuclear magnetic resonance (NMR). These have been widely adopted to explore the relationships between organisms and the natural environment, and the response to heat shock, freezing, drying, hypoxia and cold acclimation from a molecular perspective (Viant 2008; Michaud et al. 2008; Lardon et al. 2013; Angelcheva et al. 2014; Koštál et al. 2011). For instance, to provide new insight into the metabolic regulation of hibernation, non-targeted LC–MS metabolomics was adopted to detect the changes of liver and plasma metabolites in 13-lined ground squirrels in different activity states (Nelson et al. 2009, 2010). Among freeze-tolerant organisms, GC–MS analysis showed that freezing of Antarctic midges induced a remarkable increase in the content of alanine, aspartate, and urea that function as cryoprotectants, while a significant reduction was observed in three polyols (glycerol, mannitol, erythritol) and serine (Michaud et al. 2008). Therefore, a comparison of metabolic profiling between unfrozen and frozen states, using metabolomics technology, could conceivably reveal changes in the low molecular weight metabolites and identify both novel cryoprotectant(s) and metabolic reorganization strategies that are needed for cell/tissue survival in the frozen state. However, previous studies on the molecular physiology of freeze tolerance focused mainly on the changes in several common low molecular weight cryoprotectants (glucose, urea, and glycerol) (Storey and Storey 2017; Costanzo et al. 2015), lacking a comprehensive understanding of the changes of all low molecular weight metabolites in organs, and even the regulation of metabolic pathways responding to freezing exposure.
The frog Nanorana parkeri is widely distributed on the southern Tibet Plateau, and figures among the highest altitude amphibians in the world (Lu et al. 2016). It is an ideal model for exploring the adaptive mechanisms to high elevations, and many investigations have been conducted over the past decade (Sun et al. 2015; Wang et al. 2018; Niu et al. 2018a). Moreover, the Tibetan Plateau is characterized by seasonal and permanent low temperatures, and the average temperature of the coldest month was as low as − 7.8 °C (data from https://data.cma.cn/) in the native habitats of N. parkeri during the hibernation season (October–April). Thus, this species is an excellent model for studying how amphibians respond to cold stress because, as organisms with relatively limited mobility options to elude low temperatures, they rely primarily on physiological adaptations to endure prolonged exposures to severe environments.
The objective of the present study was to explore the capacity for freezing tolerance and to further elucidate the underlying molecular mechanisms of low temperature survival in N. parkeri. We investigated the capability of freezing tolerance (time and ice content tolerated) and the effect of freezing exposure on the water and glycogen contents in liver and muscle. The underlying metabolite responses to freezing exposure were analyzed using ultra high performance liquid chromatography coupled to quadrupole-orbitrap high resolution mass spectrometry (UHPLC-QE-MS). This technology showed high resolution and could produce accurate and precise quantitative data (Sturm et al. 2016). An improved understanding of the molecular mechanisms that are responsible for freezing tolerance of amphibians may have applications in preservation technologies for drugs, foods, cells, and tissues.
Materials and methods
Experimental animals
All experiments were carried out according to protocols approved by the Ethics Committee of Animal Experiments at Lanzhou University and in accordance with guidelines from the China Council on Animal Care. Adult male frogs Nanorana parkeri (n = 48) were collected from ponds by hand in Damxung County (30.28° N, 91.05° E, 4280 m above sea levels), Xizang Autonomous Region, China, in mid-December 2017. They were transferred to the laboratory (in Lanzhou University) and placed into a wet plastic box, then they were held in a 4 °C refrigerator for 48 h before freezing experiments were conducted. This temperature was chosen on the basis of the daytime microhabitat temperature recorded in the ponds during sampling in the field. The mean snout-vent length of frogs was 3.91 ± 0.03 cm (n = 48), and the mean body mass of frogs was 4.43 ± 0.11 g (n = 48).
Freezing experiment
Experimental freezing was performed according to our previously reported method (Niu et al. 2018b) with some modifications. Briefly, frogs were placed individually into 50 mL plastic centrifuge tubes with damp cotton, and the tubes were then placed into a refrigerated circulating ethanol bath. The initial temperature of the cold bath was set to 4 °C and temperature was cooled down at a speed of 1 °C d−1 until to − 2 °C was reached. Then, frogs were inoculated by addition of a small ice crystal to trigger body freezing. During the freezing experiment, the body temperature of each frog was recorded in real time by a thermocouple connected to a Multiplex Temperature Tester (SH-8, ShenZhen ShenHwa Technology Co., Ltd.). After the exotherm was observed from each frog, freezing exposure lasted 12 h. Then, seven frogs were returned to a constant-temperature incubator (4 °C) for a week to assess survival, which was judged by righting response and capacity to locomote. Another seven frogs were used to determine ice content in the whole body by calorimetry. A final group of frogs (n = 17) was euthanized by decapitation and the liver and hind leg skeletal muscle tissues were dissected. These tissues were immediately fast-frozen in liquid nitrogen to assess the water content, glycogen concentration, and metabolome. The control group was sampled directly from the 4 °C incubator.
Ice, water, glycogen, glycerol and lactate content
The percentage of body water as ice, as well as water and glycogen contents in the liver and muscle were determined using our previously reported methods (Niu et al. 2018b). Briefly, for ice determination, individual frozen frogs (n = 7) were immediately transferred into a calorimeter (F factor = 1.06 ± 0.01) that contained 50 mL of deionized water at 20 °C, allowing the frozen frog to thaw. The decrease in the water temperature was positively correlated with ice content in the frog. Liver and muscle samples from other frogs (n = 6 for each tissue) were assayed for glycogen concentration using commercial assay kits (Nanjing Jiancheng Ltd. Co., Nanjing, China), whereas a remaining portion of each sample (n = 6 for each tissue) was rapidly weighed and then desiccated for 1 week at 65 °C until they were thoroughly dried. Tissue weights were recorded before and after drying and used to determine the water content of tissues. Moreover, liver and muscle samples (n = 7 for each tissue) were assayed for glycerol and lactate content using commercial assay kits (Nanjing Jiancheng Ltd. Co., Nanjing, China).
Untargeted metabolomics
Metabolites extraction
Liver and muscle samples (n = 11 for each group) were extracted (1:20; w:v) in ice-cold acetonitrile:methanol:water (2:2:1 v:v:v, containing L-2-chlorophenylalanine as an internal standard) via homogenate at 45 Hz for 4 min using a tissuelyser (Tissuelyser-24L, Shanghai Jingxin Industrial Development Co., LTD) and via sonication for 5 min in an ice-water bath followed by vortex-mixing for 30 s. The homogenizing and sonicating processes were repeated three times, and subsequently the mixture was incubated at − 20 °C for 1 h and then centrifuged at 13,400 g, 4 °C for 15 min. The supernatant was transferred to LC–MS vials and used for UHPLC-QE Orbitrap/MS analysis. Moreover, an equal amount of supernatant from all samples was mixed and used as the quality control (QC) sample.
UHPLC-QE Orbitrap/MS analysis
Chromatographic separation was performed using a UHPLC system (1290, Agilent Technologies) with a UPLC HSS T3 column (2.1 × 100 mm, 1.8 μm) coupled to a Q Exactive (Orbitrap MS, Thermo). Formic acid (0.1%) and ammonium acetate in water (5 mM) were used as the mobile phase A for positive and negative ion modes, respectively. Acetonitrile was used as the mobile phase B. The injection volume was 1 μL and the flow rate was 500 μL min−1 through a 12 min elution gradient (0 min, 1% B; 1 min, 1% B; 8 min, 99% B; 10 min, 99% B; 10.1 min, 1% B; 12 min, 1% B). The analysis parameters of mass spectrometry were set as follows: the scan range was from 70 to 1000 m/z, the spray voltages were 3.8 kV (positive) and 3.1 kV (negative), sheath gas flow rate was 45 Arb, aux gas flow rate was 15 Arb, and capillary temperature was 320 °C, respectively. The primary and secondary mass spectrometry data were continuously collected using the acquisition software (Xcalibur 4.0.27, Thermo) at 20/40/60 eV collision energy in NCE model.
Data processing and metabolite identification
ProteoWizard was used to convert the MS raw data (.raw) files to the mzML format, which was further processed by R package XCMS (version 3.2) and converted into a matrix including retention time (RT), mass to charge ratio (m/z) and peak intensity. Then peak identification was carried out using OSI-SMMS (version 1.0, Dalian Chem Data Solution Information Technology Co. Ltd.) on the basis of an in-house MS/MS database (Biotree Biotech Co., Ltd. Shanghai, China). The internal standard normalization method was employed for the relative quantitative analysis.
Statistical analysis
Prior to statistical analysis, the data from the freezing experiment, water, glycogen, glycerol and lactate content were examined for normality of distribution and homogeneity of variance, and then were analyzed by one-way ANOVA using IBM SPSS 20.0 software (SPSS Inc., Chicago, USA). All data are shown as means ± s.e.m., and a significance level of 0.05 (P < 0.05) was accepted.
The metabolomics data were treated as follows. After denoising via the interquartile range method and normalizing the original data against an internal standard, the metabolomics data were imported into the SIMCA 14.1 software (MKS Data Analytics Solutions, Umea, Sweden) for multivariate statistical analyses including principal component analysis (PCA) and orthogonal projections to latent structures discriminant analysis (OPLS-DA). Afterwards, permutation tests with 200 iterations were applied to assess the OPLS-DA model. Screening of differential metabolites was based on the variable importance for the projection (VIP > 1) values from OPLS-DA and the P value from Student’s t-test (P < 0.05) or the Kruskal–Wallis test (P < 0.05). The metabolic pathways analysis was performed combining KEGG database (https://www.kegg.jp/) with Metaboanalyst 3.0 (https://www.metaboanalyst.ca) (Xia et al. 2015).
Results
Freezing survival and ice content
Thermal equilibrium was attained about 4–6 h after the onset of freezing. After freezing for 12 h at − 2.0 °C, body ice content was 39.9% ± 5.4% (n = 7) for frogs thawed in the calorimeter. Of other frozen frogs returned to 4 °C and allowed to thaw and recover over time, three of seven frogs were assessed as having survived the freezing episode after 1 week in a 4 °C incubator, as judged by righting response and movement. Frozen frogs presented stiff and brittle limbs, and no ventilation or heartbeat were observed. Moreover, the abdominal cavity was filled with ice crystals and these were also found surrounding the leg muscles upon dissection.
Changes in water, glycogen, glycerol and lactate content
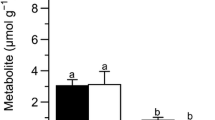
After being frozen for 12 h at − 2.0 °C, water content showed no significant change in the liver (P > 0.05), but a 32% decrease was found in the muscle (F1,11 = 9.334, P = 0.012; Fig. 1a). Compared with the control group, the concentration of hepatic and muscle glycogen in frozen frogs decreased significantly, by about 43% (F1,11 = 12.278, P = 0.006) and 28% (F1,11 = 5.648, P = 0.039), respectively (Fig. 1b). In liver, glycerol and lactate content showed 230% (F1,13 = 137.489, P < 0.001; Fig. 2a) and 97% (F1,13 = 85.938, P < 0.001; Fig. 2b) increase in frozen frogs, respectively, but no significant changes were found in the muscle.
Overall changes of metabolites in the liver and muscle after freezing exposure
In positive ion mode (POS), 4407 and 4005 peaks were detected in the liver and muscle, respectively. Of these, 155 and 123 metabolites were identified in the liver and muscle using the self-built database from Biotree Biotechnology Co., Ltd. (Shanghai, China), respectively. In negative ion mode (NEG), 2782 and 2722 peaks were acquired in the liver and muscle, respectively, and of these, 115 and 99 metabolites were identified in the liver and muscle, respectively.
In positive and negative ion modes, both PCA (Supplementary Fig. 1a, b) and OPLS-DA (Supplementary Fig. 2a, b) score plots for liver showed an obvious separation between the control group and the freezing exposure group, suggesting that there was a significant change in the metabolic profiles of liver after freezing exposure. The OPLS-DA model was applied to maximize the distinction between different samples from two groups and to obtain a better understanding of the metabolites responsible for the separation. The estimated goodness of fit of R2Y was 98.9% (POS) and 99.4% (NEG), respectively, and the goodness of prediction of Q2Y was 94.8% (POS) and 95.1% (NEG) which underlines the robustness of the model. A permutation test was conducted to assess the significance of the predictive ability and showed no overfitting (POS: R2Y-intercept of 0.864 and Q2-intercept of − 0.384; NEG: R2Y-intercept of 0.918 and Q2-intercept of − 0.411) (Supplementary Fig. 2c, d).
In positive and negative ion modes, both PCA (Supplementary Fig. 1c, d) and OPLS-DA (Supplementary Fig. 3a, b) score plots for muscle showed clear discrimination between the freezing exposure and control groups, indicating that the metabolites exhibited substantial changes between the two states. The R2Y values of the OPLS-DA model were 99.9% (POS) and 99.7% (NEG), respectively. The Q2 values of the OPLS-DA model were 86.6% (POS) and 95% (NEG), respectively. The values for R2Y-intercept and Q2-intercept in the permutation test were 0.991 and − 0.251 in positive ion mode (Supplementary Fig. 3c), respectively, and 0.929 and − 0.415 in negative ion mode (Supplementary Fig. 3d), respectively. These results suggest that the OPLS-DA model possessed good stability and was not over-fitting, which can be exploited in the subsequent analyses.
Differential metabolites in liver and muscle tissues after freezing exposure
Combining the t-test (P < 0.05) and OPLS-DA model (VIP > 1), a total of 33 differentially expressed metabolites were identified in the liver, including 29 up-regulated metabolites and 4 down-regulated metabolites in the frozen group compared to controls (Supplementary Table 1). A total of 36 differentially expressed metabolites were identified in skeletal muscle, including 25 up-regulated metabolites and 11 down-regulated metabolites in frozen frogs, compared with controls (Supplementary Table 2). To further intuitively reveal the tendencies in the variation of metabolite concentrations, heat maps (one column per sample and one row per metabolite) were plotted according to the relative quantities of differently expressed metabolites between the two groups; Fig. 3 shows data for liver and Fig. 4 for muscle. Mean values for differentially expressed metabolites in control and frozen samples as well as fold changes during freezing are also shown in Supplementary Tables 1 and 2.
Metabolic pathway analysis
Using both pathway impact scores of > 0.05 and -lnp-values of > 1.0, the metabolic pathways affected by freezing exposure were visually displayed by a bubble diagram. The most relevant metabolic pathways were involved in linoleic acid metabolism (ko00591), alanine, aspartate and glutamate metabolism (ko00250), pantothenate and CoA biosynthesis (ko00770), arginine and proline metabolism (ko00330), D-glutamine and D-glutamate metabolism (ko00471), aminoacyl-tRNA biosynthesis (ko00970), citrate cycle (ko00020) and pentose phosphate pathway (ko00030) in the liver (Fig. 5a). In the muscle, freezing exposure significantly affected the glycine, serine and threonine metabolism (ko00260), alpha-linolenic acid metabolism (ko00592), alanine, aspartate and glutamate metabolism (ko00250), arginine and proline metabolism (ko00330), riboflavin metabolism (ko00740), aminoacyl-tRNA biosynthesis (ko00970), D-glutamine and D-glutamate metabolism (ko00471), citrate cycle (ko00020) and cysteine and methionine metabolism (ko00270) (Fig. 5b).
Pathway analysis of differential metabolites in the liver (a) and muscle (b) of N. parkeri between control and freezing exposure. The -ln(p-value) represents the enrichment score. The impact score (0−1) indicates the pathway topological importance of the metabolites. The color and size of each circle are based on p values and pathway impact values, respectively (color figure online)
Discussion
Here we present a first assessment of freeze tolerance in the frog N. parkeri and profile the metabolite changes that occur in liver and muscle in response to freezing. These results provide clues to understanding the molecular mechanisms of survival strategies in the cold winter environments of the Tibetan Plateau.
Freeze tolerance capability, water, glycogen, glycerol and lactate content
In the present study, three of the seven frogs that were frozen at − 2.0 °C for 12 h fully recovered and were apparently unharmed after 1 week at 4 °C as judged by righting ability and locomotion. This suggests that overwintering Nanorana parkeri can tolerate a brief and partial freezing of their body. These findings coincide with ecological observations that overwintering N. parkeri experience relatively brief exposures to sub-zero temperatures in caves within shallow ponds at night (unpublished data), and thus it is advantageous for frogs to express a modicum of freeze tolerance to be able to survive when temperatures infrequently fall below 0 °C. Similarly, Hyla regilla could survive 6 h of freezing at − 2 °C (Croes et al. 2000). The freeze tolerance capability exhibits significant interspecific, intraspecific and seasonal differences among amphibian species (Larson et al. 2014; Storey and Storey 2017; Voituron et al. 2009). These differences could derive from a variety of variables: the freezing temperature, exposure time, sampling season, acclimation conditions, genetic variation, and the preferred natural type of overwintering site.
During the freezing process, partial dehydration can contribute to regulating osmotic pressure, reducing the equilibrium freezing point and ice content, concentrating the cryoprotectants inside cells, and easing the mechanical pressure caused by tissue freezing (Lee and Costanzo 1998). In the present study, freezing exposure induced partial dehydration in the muscle, which could limit ultrastructural damage to tissues, but also concentrate protective solutes within the muscle. Similarly, the water content in the muscle of Rana sylvatica decreased significantly after 5 h freezing exposure at − 8 °C (Costanzo et al. 2015). However, no significant changes in water content were found in the liver, which may contribute to preserving metabolic activity since liver is a main metabolic organ that is crucial to freezing survival. Liver might stay hydrated for longer than muscle for two reasons: (1) it is typically the organ that is actively making cryoprotectant and thereby raising its intracellular osmolality even though it is also exporting cryoprotectant, (2) ice growth moves from the periphery of the body (skin, muscle) inwards to the core organs. The core organs are also bathed in a significant amounts of abdominal fluid that would need to freeze substantially before creating the osmotic gradient to draw water out of liver and start dehydrating cells. Previous studies showed that liver was always the last organ to freeze in wood frogs (R. sylvatica) and this is likely the same in all frogs (Rubinsky et al. 1994).
It is interesting that the hepatic and muscle glycogen content of unfrozen frogs was similar to that of winter-collected Alaskan wood frogs (Costanzo et al. 2013), and the catabolism of these reserves did not translate into an increase in glucose. This suggests that glucose was not a cryoprotectant in N. parkeri. A similar result was observed in R. ridibunda with no significant augmentation of glucose concentration occurring in liver during freezing (Voituron et al. 2003). This high glycogen content and its catabolism likely led to a hyperhydration of the tissue (due to release of water bound to the glycogen) thus resulting in no change in liver water content (Costanzo et al. 2013). Moreover, frozen frogs with 39.9% ice content would expect to be relying on anaerobic metabolism for ATP that comes from glycogen breakdown. Therefore, anaerobic use of glycogen may be detected by increasing levels of lactate in the liver. Indeed, lactate content in liver showed a significant increase in frozen frogs.
Carbohydrates
Glycerol-3-phosphate, a main precursor for glycerol synthesis, accumulated significantly in the liver of N. parkeri after freezing exposure. Similarly, glycerol-3-phosphate content showed a comparable increase at − 5 °C (1.59 ± 0.15 μmol g−1 wet weight) compared with 15 °C (0.19 ± 0.03 μmol g−1 wet weight) in larvae of freeze tolerant Eurosta solidaginis during the seasonal acquisition of glycerol cryoprotectant (Storey et al. 1981). In liver of the marine fish, Osmerus mordax, the content of glycerol-3-phosphate also increased significantly as temperature declined from 5° to − 0.5 °C, as did enzyme activities of glyceraldehyde-3-phosphate dehydrogenase and glycerol-3-phosphate dehydrogenase; this fish is known to produce large amounts of glycerol as a winter antifreeze (Driedzic et al. 2006; Lewis et al. 2004). We found that glycerol content showed a significant increase in liver. However, such level of glycerol would not be particularly cryoprotective for frozen frogs since freezing exposure induced a remarkable augument in the hepatic glycerol content of Cope’s gray treefrog, Dryophytes chrysoscelis, up to 155 ± 27 μmol g−1 dry tissue (do Amaral et al. 2018).
Although glucose is the main cryoprotectant of three North American frog species (wood frogs R. sylvatica, spring peepers Pseudacris crucifer and chorus frogs P. triseriata) (Storey and Storey 2017), a lack of cryoprotection by glucose in erythrocytes was found in Cope’s gray treefrog, Dryophytes chrysoscelis (Geiss et al. 2019). Monosaccharides (such as glucose and galactose) showed almost no protective effect on mouse sperm over freeze–thaw cycles, regardless of their concentration. However, mouse sperm showed a higher survival rate after the treatment of disaccharides (such as sucrose and trehalose) and trisaccharides (such as raffinose and melezitose) (An et al. 2000). Moreover, maltotriose can effectively protect ATPase activity, actomyosin-SH and solubility during freezing (Ooshiro et al. 1976). In the present study, the melezitose and maltotriose content also increased significantly in the liver of N. parkeri after freezing exposure, suggesting that these may have cryoprotective effect. The actual concentrations of these carbohydrates in vivo is unknown, thus their cryoprotective effect needs to be further confirmed in freezing N. parkeri. α-Ketoglutarate, a Krebs cycle intermediate, was significantly upregulated in the muscle tissue after freezing exposure, suggesting that the flux through the Krebs cycle was reduced due to a substantial metabolic depression in frozen frogs.
Amino acids and derivatives
Our results showed that amino acids and derivatives significantly accumulated in the liver and muscle of N. parkeri. Similarly, alanine, glycine, ammonium (NH4+), serine, glutamine, ornithine, and lysine increased in the liver of R. sylvatica after being frozen for 48 h at − 2.5 °C (Storey and Storey 1986). Moreover, previous studies have shown that amino acids with the length of hydroxyl side chain less than three carbons function as cryoprotectants in the presence of sodium chloride, which could enhance the solubility of amino acids such as glycine, alanine, 2-aminobutyric acid, valine, leucine, and isoleucine. A cryoprotective effect was also observed for amino acids with charged amino groups in side chains owing to the interactions between positively charged side chains and negatively charged phosphatidyl groups, such as ornithine, lysine, arginine and histidine (Anchordoguy et al. 1988). Therefore, we speculated that the accumulation of these amino acids in the liver and muscle may play a cryoprotective role in N. parkeri during freezing.
In the frozen state, ectothermic vertebrates mainly rely on endogenous energy reserves (creatine phosphate, ATP) and glycolysis to produce ATP (leading to lactate and alanine accumulation as end products) and help sustain tissue/organ viability (Storey and Storey 1984, 1986, 1988). In the present study, creatine content of muscle increased markedly after freezing exposure indicating creatine-P use to buffer ATP levels. Similarly, creatine content showed a significant increase (from 12.5 ± 1.41 to 24.9 ± 1.75 μmol g−1 wet weight) in the muscle of R. sylvatica after 4 days frozen at − 4 °C (Storey and Storey 1984). In addition, the activity of creatine kinase was significantly higher (35%) in muscle of frozen R. sylvatica than in the control group (acclimated for 2 weeks at 5 °C) (Dieni and Storey 2009). Hence, elevated creatine content in N. parkeri can be ascribed to the enhancement of creatine kinase activity in the muscle during freezing and a breakdown of creatine phosphate reserves to support muscle ATP needs. Guanidinosuccinic acid is an upregulated metabolite in the liver, which is a well-known marker of uremia (Cohen 1970). Similarly, winter-acclimated R. sylvatica (Alaskan population) achieved a high uremia under moist conditions (Costanzo et al. 2013), which may result from heightened proteolysis.
Lipids and fatty acids
Consistent with the findings of other studies, freezing exposure induced an obvious increase in the content of unsaturated fatty acids in the liver and muscle of N. parkeri. Such changes are essential for maintaining cell physiology and function (Li et al. 2006), and thus promoting freezing survival in ectothermic animals (Bennett et al. 1997; Koštál et al. 2003; Pruitt and Lu 2008). In freeze-tolerant Chilo suppressalis, the content of unsaturated fatty acids in tissues from hibernating larvae was significantly higher than that in tissues of non-hibernating insects (Izumi et al. 2009). Reynolds et al. (2014) found no significant change in the lipid composition of wood frog liver after freezing exposure, and they ascribed this to the short exposure (48 h). However, Williams and Hazel (1995) considered that a change in lipid composition induced by freezing could occur within hours. Additionally, linoleic acid, as a necessary polyunsaturated fatty acid for organisms, can affect the hibernation cycle and the metabolic rate of hibernating animals. Specifically, the lack of linoleic acid results in shortening the hibernation cycle, leading to increased energy consumption, and even reducing survival rate during hibernation (Florant 1998). Therefore, the accumulation of linoleic acid noted in the present study may play an important role in the freezing tolerance and hibernation of N. parkeri.
Oxidative stress
Freeze–thaw cycles are usually accompanied by ischemia and reperfusion in ectothermic vertebrates, which stimulate the production of reactive oxygen species (ROS) such as O2− and H2O2 (McCord 1985). In the present study, the content of hypoxanthine showed a notable increase in the liver of N. parkeri after freezing exposure, suggesting increased oxidative stress. This inference could be supported by the elevation of 8-hydroxy-2-deoxyguanine in the muscle, which is a marker of oxidative DNA damage (Shi et al. 1994). Moreover, there was no doubt that elevated unsaturated fatty acids could also increase the risk of oxidative damage in tissues since free fatty acids and membrane phospholipids are among the main targets of reactive oxygen species (ROS) damage (Willmore and Storey 1997). 7-Ketocholesterol, the most upregulated substance in muscles, is a non-enzymatically formed toxic compound found in many diseases and is believed to be formed as a result of oxidative stress (Seet et al. 2010). Oxidative stress damage was also indicated in R. sylvatica; after being frozen for 24 h at − 2.5 °C, the level of oxidized glutathione and the ratio of GSSG/GSH increased significantly, suggesting that freezing induced oxidative stress (Joanisse and Storey 1996). Moreover, the red-sided garter snake (Thamnophis sirtalis parietalis) also showed indicators of oxidative stress after freezing (Hermes-Lima and Storey 1993).
In conclusion, overwintering N. parkeri showed a weak freeze tolerance, accompanied by partial dehydration of muscle tissues, which contributed to decreasing the amount of freezable water within the muscle and could be protective for the myocytes themselves. Metabolomics successfully identified a number of metabolic changes, especially among amino acids and fatty acids that may improve the survival of N. parkeri during freezing. Moreover, freezing exposure induced oxidative stress in N. parkeri. These findings provide new insights into the molecular physiology of freeze tolerance in ectothermic vertebrates and contribute to revealing the survival mechanism of amphibians in the Tibetan Plateau, as well as to conserving these species.
References
An TZ, Iwakiri M, Edashige K, Sakurai T, Kasai M (2000) Factors affecting the survival of frozen–thawed mouse spermatozoa. Cryobiology 40:237–249
Anchordoguy T, Carpenter JF, Loomis SH, Crowe JH (1988) Mechanisms of interaction of amino acids with phospholipid bilayers during freezing. BBA-Biomembranes 946:299–306
Angelcheva L, Mishra Y, Antti H, Kjellsen TD, Funk C, Strimbeck RG, Schröder WP (2014) Metabolomic analysis of extreme freezing tolerance in Siberian spruce (Picea obovata). New Phytol 204:545–555
Bennett VA, Pruitt NL, Lee RE (1997) Seasonal changes in fatty acid composition associated with cold-hardening in third instar larvae of Eurosta solidaginis. J Comp Physiol B 167:249–255
Cohen BD (1970) Guanidinosuccinic acid in uremia. Arch Intern Med 126:846–850
Costanzo JP (2019) Overwintering adaptations and extreme freeze tolerance in a subarctic population of the wood frog, Rana sylvatica. J Comp Physiol B 189:1–15
Costanzo JP, do Amaral MCF, Rosendale AJ, Lee RE, (2013) Hibernation physiology, freezing adaptation and extreme freeze tolerance in a northern population of the wood frog. J Exp Biol 216:3461–3473
Costanzo JP, Lee RE (2008) Urea loading enhances freezing survival and postfreeze recovery in a terrestrially hibernating frog. J Exp Biol 211:2969–2975
Costanzo JP, Lee RE (2013) Avoidance and tolerance of freezing in ectothermic vertebrates. J Exp Biol 216:1961–1967
Costanzo JP, Baker PJ, Lee RE (2006) Physiological responses to freezing in hatchlings of freeze-tolerant and -intolerant turtles. J Comp Physiol B 176:697–707
Costanzo JP, Reynolds AM, do Amaral MCF, Rosendale AJ, Lee RE (2015) Cryoprotectants and extreme freeze tolerance in a subarctic population of the wood frog. PLoS ONE 10:e0117234
Croes SA, Thomas RE (2000) Freeze tolerance and cryoprotectant synthesis of the Pacific tree frog Hyla regilla. Copeia 2000:863–868
D’Alessandro A, Nemkov T, Bogren LK, Martin SL, Hansen KC (2016) Comfortably numb and back: plasma metabolomics reveals biochemical adaptations in the hibernating 13-lined ground squirrel. J Proteome Res 16:958–969
Dieni CA, Storey KB (2009) Creatine kinase regulation by reversible phosphorylation in frog muscle. Comp Biochem Physiol B 152:405–412
do Amaral MCF, Frisbie J, Goldstein DL, Krane CM (2018) The cryoprotectant system of Cope’s gray treefrog, Dryophytes chrysoscelis: responses to cold acclimation, freezing, and thawing. J Comp Physiol B 188:611–621
Driedzic WR, Clow KA, Short CE, Ewart KV (2006) Glycerol production in rainbow smelt (Osmerus mordax) may be triggered by low temperature alone and is associated with the activation of glycerol-3-phosphate dehydrogenase and glycerol-3-phosphatase. J Exp Biol 209:1016–1023
Duman JG (2015) Animal ice-binding (antifreeze) proteins and glycolipids: an overview with emphasis on physiological function. J Exp Biol 218:1846–1855
Dunn WB, Ellis DI (2005) Metabolomics: current analytical platforms and methodologies. TrAC Trends Anal Chem 24:285–294
Florant GL (1998) Lipid metabolism in hibernators: the importance of essential fatty acids. Am Zool 38:331–340
Geiss L, do Amaral MCF, Frisbie J, Goldstein DL, Krane CM (2019) Postfreeze viability of erythrocytes from Dryophytes chrysoscelis. J Exp Zool A 331:308–313
Hermes-Lima M, Storey KB (1993) Antioxidant defenses in the tolerance of freezing and anoxia by garter snakes. Am J Physiol Regul Integr Comp Physiol 265:R646–R652
Izumi Y, Katagiri C, Sonoda S, Tsumuki H (2009) Seasonal changes of phospholipids in last instar larvae of rice stem borer Chilo suppressalis Walker (Lepidoptera: Pyralidae). Entomol Sci 12:376–381
Joanisse DR, Storey KB (1996) Oxidative damage and antioxidants in Rana sylvatica, the freeze-tolerant wood frog. Am J Physiol Regul Integr Comp Physiol 271:R545–R553
Koštál V, Berková P, Šimek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol B 135:407–419
Koštál V, Korbelová J, Rozsypal J, Zahradníčková H, Cimlová J, Tomčala A, Šimek P (2011) Long-term cold acclimation extends survival time at 0 °C and modifies the metabolomic profiles of the larvae of the fruit fly Drosophila melanogaster. PLoS ONE 6:e25025
Lardon I, Eyckmans M, Vu TN, Laukens K, De Boeck G, Dommisse R (2013) 1H-NMR study of the metabolome of a moderately hypoxia-tolerant fish, the common carp (Cyprinus carpio). Metabolomics 9:1216–1227
Larson DJ, Middle L, Vu H, Zhang W, Serianni AS, Duman J, Barnes BM (2014) Wood frog adaptations to overwintering in Alaska: new limits to freezing tolerance. J Exp Biol 217:2193–2200
Lee RE, Costanzo JP (1998) Biological ice nucleation and ice distribution in cold-hardy ectothermic animals. Annu Rev Physiol 60:55–72
Lewis JM, Ewart KV, Driedzic WR (2004) Freeze resistance in rainbow smelt (Osmerus mordax): seasonal pattern of glycerol and antifreeze protein levels and liver enzyme activity associated with glycerol production. Physiol Biochem Zool 77:415–422
Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, Vance DE (2006) The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 3:321–331
Lu X, Ma X, Fan L, Hu Y, Lang Z, Li Z, Fang B, Guo W (2016) Reproductive ecology of a Tibetan frog Nanorana parkeri (Anura: Ranidae). J Nat Hist 50:2769–2782
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312:159–163
Michaud MR, Benoit JB, Lopez-Martinez G, Elnitsky MA, Lee RE, Denlinger DL (2008) Metabolomics reveals unique and shared metabolic changes in response to heat shock, freezing and desiccation in the Antarctic midge, Belgica antarctica. J Insect Physiol 54:645–655
Nelson CJ, Otis JP, Martin SL, Carey HV (2009) Analysis of the hibernation cycle using LC-MS-based metabolomics in ground squirrel liver. Physiol Genomics 37:43–51
Nelson CJ, Otis JP, Carey HV (2010) Global analysis of circulating metabolites in hibernating ground squirrels. Comp Biochem Physiol D 5:265–273
Niu Y, Cao W, Zhao Y, Zhai H, Zhao Y, Tang X, Chen Q (2018a) The levels of oxidative stress and antioxidant capacity in hibernating Nanorana parkeri. Comp Biochem Physiol A 219:19–27
Niu Y, Wang J, Men S, Zhao Y, Lu S, Tang X, Chen Q (2018b) Urea and plasma ice-nucleating proteins promoted the modest freeze tolerance in Pleske’s high altitude frog Nanorana pleskei. J Comp Physiol B 188:599–610
Ooshiro Z, Hironaka Y, Hayashi S (1976) Preventive effect of sugars on denaturation of fish protein during frozen storage. Mem Fac Fish Kagoshima Univ 25:91–99
Pruitt NL, Lu C (2008) Seasonal changes in phospholipid class and class-specific fatty acid composition associated with the onset of freeze tolerance in third-instar larvae of Eurosta solidaginis. Physiol Biochem Zool 81:226–234
Reynolds AM, Lee RE, Costanzo JP (2014) Membrane adaptation in phospholipids and cholesterol in the widely distributed, freeze-tolerant wood frog, Rana sylvatica. J Comp Physiol B 184:371–383
Rochfort S (2005) Metabolomics reviewed: a new “omics” platform technology for systems biology and implications for natural products research. J Nat Prod 68:1813–1820
Rubinsky B, Wong ST, Hong JS, Gilbert J, Roos M, Storey KB (1994) 1H magnetic resonance imaging of freezing and thawing in freeze-tolerant frogs. Am J Physiol Regul Integr Comp Physiol 266:R1771–R1777
Schiller TM, Costanzo JP, Lee RE (2008) Urea production capacity in the wood frog (Rana sylvatica) varies with season and experimentally induced hyperuremia. J Exp Zool A 309:484–493
Seet RC, Lee C-YJ, Lim EC, Tan JJ, Quek AM, Chong W-L, Looi W-F, Huang S-H, Wang H, Chan Y-H (2010) Oxidative damage in Parkinson disease: measurement using accurate biomarkers. Free Radical Bio Med 48:560–566
Shi X, Mao Y, Knapton AD, Ding M, Rojanasakul Y, Gannett PM, Dalal N, Liu K (1994) Reaction of Cr (VI) with ascorbate and hydrogen peroxide generates hydroxyl radicals and causes DNA damage: role of a Cr (IV)-mediated Fenton-like reaction. Carcinogenesis 15:2475–2478
Storey KB (2015) Regulation of hypometabolism: insights into epigenetic controls. J Exp Biol 218:150–159
Storey KB, Storey JM (1984) Biochemical adaption for freezing tolerance in the wood frog, Rana sylvatica. J Comp Physiol B 155:29–36
Storey KB, Storey JM (1986) Freeze tolerant frogs: cryoprotectants and tissue metabolism during freeze-thaw cycles. Can J Zool 64:49–56
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev 68:27–84
Storey KB, Storey JM (2013) Molecular biology of freezing tolerance. Compr Physiol 3:1283–1308
Storey KB, Storey JM (2017) Molecular physiology of freeze tolerance in vertebrates. Physiol Rev 97:623–665
Storey KB, Baust JG, Storey JM (1981) Intermediary metabolism during low temperature acclimation in the overwintering gall fly larva, Eurosta solidaginis. J Comp Physiol B 144:183–190
Sturm RM, Jones BR, Mulvana DE, Lowes S (2016) HRMS using a Q-Exactive series mass spectrometer for regulated quantitative bioanalysis: how, when, and why to implement. Bioanalysis 8:1709–1721
Sun Y-B, Xiong Z-J, Xiang X-Y, Liu S-P, Zhou W-W, Tu X-L, Zhong L, Wang L, Wu D-D, Zhang B-L (2015) Whole-genome sequence of the Tibetan frog Nanorana parkeri and the comparative evolution of tetrapod genomes. Proc Natl Acad Sci 112:E1257–E1262
Vesala L, Salminen TS, Koštál V, Zahradníčková H, Hoikkala A (2012) Myo-inositol as a main metabolite in overwintering flies: seasonal metabolomic profiles and cold stress tolerance in a northern drosophilid fly. J Exp Biol 215:2891–2897
Viant MR (2008) Recent developments in environmental metabolomics. Mol BioSyst 4:980–986
Voituron Y, Eugene M, Barré H (2003) Survival and metabolic responses to freezing by the water frog (Rana ridibunda). J Exp Zool A 299:118–126
Voituron Y, Barré H, Ramløv H, Douady CJ (2009) Freeze tolerance evolution among anurans: frequency and timing of appearance. Cryobiology 58:241–247
Vr K, Berková P, Šimek P (2003) Remodelling of membrane phospholipids during transition to diapause and cold-acclimation in the larvae of Chymomyza costata (Drosophilidae). Comp Biochem Physiol B 135:407–419
Wang G-D, Zhang B-L, Zhou W-W, Li Y-X, Jin J-Q, Shao Y, Yang H-c, Liu Y-H, Yan F, Chen H-M (2018) Selection and environmental adaptation along a path to speciation in the Tibetan frog Nanorana parkeri. Proc Natl Acad Sci 115:E5056–E5065
Williams E, Hazel J (1995) Restructuring of plasma membrane phospholipids in isolated hepatocytes of rainbow trout during brief in vitro cold exposure. J Comp Physiol B 164:600–608
Willmore W, Storey KB (1997) Antioxidant systems and anoxia tolerance in a freshwater turtle Trachemys scripta elegans. Mol Cell Biochem 170:177–185
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res 43:W251–W257
Acknowledgements
Funding for this research was provided by the National Natural Science Foundation of China (no. 31971416 and no. 32001110), the Open Project of State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University (2020-KF-002) and the Project of Scientific Research Foundation of Dezhou University (2019xjrc315). We thank Biotree Biotechnology Co., Ltd. for assistance in the metabolomics analysis of samples.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Niu, Y., Cao, W., Wang, J. et al. Freeze tolerance and the underlying metabolite responses in the Xizang plateau frog, Nanorana parkeri. J Comp Physiol B 191, 173–184 (2021). https://doi.org/10.1007/s00360-020-01314-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01314-0