Abstract
Chemical signals can provide useful information to potential mates and rivals. The production mechanisms of these signals are poorly understood in birds, despite emerging evidence that volatile compounds from preen oil may serve as chemosignals. Steroid hormones, including testosterone (T), may influence the production of these signals, yet variation in circulating T only partly accounts for this variation. We hypothesized that odor is a T-mediated signal of an individual’s phenotype, regulated in part by androgen sensitivity in the uropygial gland. We quantified natural variation in chemosignals, T, uropygial gland androgen sensitivity, and aggressive behavior in dark-eyed juncos (Junco hyemalis). The interaction between circulating T and androgen receptor transcript abundance significantly correlated with volatile concentrations in male, but not female, preen oil. In both sexes, odorant variables correlated with aggressive response to an intruder. Our results suggest that preen oil volatiles could function as signals of aggressive intent, and, at least in males, may be regulated by local androgen receptor signaling in the uropygial gland. Because these behavioral and chemical traits have been linked with reproductive success, local regulation of androgen sensitivity in the periphery has the potential to be a target of selection in the evolution of avian olfactory signaling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Information communicated chemically plays an important role in mate choice and competition. In addition to signaling whether an individual is an appropriate mate (e.g., same species, opposite sex), chemical cues and signals can provide a great deal of information about a potential mate’s quality (Johansson and Jones 2007). For example, chemical compounds may be honest indicators of immunocompetence, disease, parasite load, nutrition, reproductive status, and circulating hormone levels (Penn and Potts 1998). Thus, information acquired via chemical signals may be useful to the receiver in either intersexual mate choice (e.g., a female evaluating a potential mate) or intrasexual competition (e.g., a male determining whether to challenge a potential rival).
In songbirds, volatile compounds in preen oil secreted by the uropygial gland contain information about an individual’s species (Mardon et al. 2010; Soini et al. 2013), sex (Mardon et al. 2010; Whittaker et al. 2010), breeding condition (Whittaker et al. 2011b), and kinship to the receiver (Krause et al. 2012), and are even predictive of individual reproductive success (Whittaker et al. 2013). Male house finches (Carpodacus mexicanus) can assess a potential rival’s quality on the basis of its odor, and choose to approach or avoid the scent of another male accordingly (Amo et al. 2012b). In birds, natural variation in the ability to produce testosterone (T) is correlated with territorial aggression towards same-sex intruders (McGlothlin et al. 2007), and thus is a likely candidate for influencing chemical signals assessed by potential mates and rivals. Although chemical communication is relatively well explored in some animal groups, such as mammals and insects, the mechanisms behind chemosignaling are currently less well understood in birds (Caro et al. 2015).
In reptiles and birds, chemosignals originate from the skin and glands in the skin, and several studies have linked the production of these signals to seasonal variation in steroid hormones such as T and estradiol (Kolattukudy et al. 1987b; Reneerkens et al. 2002; Whittaker et al. 2011b; Parker and Mason 2014). In many species of lizards, males secrete reproductive semiochemicals from femoral glands, the production and attractiveness of which are affected by seasonal changes in androgens (Martín and López 2015). In garter snakes (Thamnophis sirtalis), the secretion of female sex pheromones is stimulated by estradiol (Parker and Mason 2012) and inhibited by T (Parker and Mason 2014). The uropygial gland, a sebaceous gland located above the base of the tail (Jacob and Ziswiler 1982), is the primary source of chemosignals in most birds. This gland contains androgen receptors (AR) (Daniel et al. 1977; Shanbhag and Sharp 1996), and shows increases in AR synthesis in response to experimentally increased T (Maiti and Ghosh 1972; Amet et al. 1986). A few studies have also suggested relationships between seasonal changes in steroid hormones and volatile compounds in preen oil. For example, mallard duck (Anas platyrhynchos) preen oil chemical signals are induced by seasonal changes in estradiol (Kolattukudy et al. 1987a, b; Bohnet et al. 1991). Seasonal differences in preen oil volatile compounds have been observed in a number of songbird species as well (Soini et al. 2007; Amo et al. 2012a; Tuttle et al. 2014), and these patterns appear to mimic seasonal changes in T in both males and females (Whittaker et al. 2011b). However, in dark-eyed juncos, exogenous T treatment did not recapitulate these effects on volatile profiles in a dose-dependent manner (Whittaker et al. 2011b), suggesting that circulating levels of T cannot fully account for variation in avian chemosignal production.
This disconnect between hormone levels and trait expression is frequently observed in behavioral endocrinology, and is typically thought to relate to functional variation in other components of the endocrine system that regulate T production or response (Adkins-Regan 2005). For example, one particularly well studied component of the pathway by which T influences phenotype is variation in abundance of steroid hormone receptors in different tissues (Ball and Balthazart 2008), which can change in relation to seasonal (Wacker et al. 2010) or social (Fuxjager et al. 2010) cues from the environment. Steroid hormone receptors play a key role in mediating the phenotypic effects of circulating hormones, because the hormone–receptor complex transcriptionally regulates other genes that ultimately affect physiology, morphology, and behavior. For example, target tissue variation in the expression of steroid binding receptors and converting enzymes has been shown to predict functional individual and population variation in behavioral phenotypes, even when hormone levels do not (Gonçalves et al. 2010; Rosvall et al. 2012; Bergeon Burns et al. 2013; Horton et al. 2014). In dark-eyed juncos, circulating T levels predicted aggressive behavior in males but not females, while steroid hormone receptor abundance in the brain (specifically, the medial amygdala, hypothalamus, and song control regions) predicted aggression in both males and females (Rosvall et al. 2012).
Thus, variation in steroid sensitivity in key tissues may be an important factor regulating expression of many components of the phenotype; however, most research on this issue focuses on androgen sensitivity in the brain, despite the fact that androgen receptors are expressed widely throughout the body (Staub and DeBeer 1997), including the uropygial gland (Daniel et al. 1977; Shanbhag and Sharp 1996). As a consequence, the degree to which individual variation in chemical signals is related to uropygial gland androgen sensitivity, testosterone levels, or both is unclear. Resolving this unknown has important implications for our understanding of the degree to which avian olfactory signals can or cannot encode unique or redundant information about an individual, e.g., because local endocrine control would allow more independent signal content, whereas systemic control via circulating T levels would integrate signaling across the organism (Ketterson et al. 2009).
In this study, we set out to test the hypothesis that songbird chemical signals are hormone-mediated signals of an individual’s phenotype by focusing on the relationships among preen oil volatile compounds, uropygial gland AR gene expression, and aggressive behavior. We approached this research by examining natural variation in chemosignals, T, androgen sensitivity, and aggressive behavior in male and female dark-eyed juncos (Junco hyemalis). We predicted that androgen signaling and sensitivity would relate to signal production, and that chemosignals would convey information about an individual’s aggressiveness. Because males and females are known to differ in preen oil volatile compounds and androgen signaling, we further predicted sex-specific relationships between chemosignals and behavior.
Methods
Behavioral analysis and sample collection
All subjects were subjected to a short behavioral trial, and then captured, on their breeding territories in the Jefferson National Forest near Mountain Lake, Virginia, USA (37°22′N, 80°32′W) in spring 2010 (1 May–5 June). Each male (n = 17) and female (n = 16) underwent a 6-min simulated territorial intrusion (STI), the results of which have been previously published, in relation to androgen sensitivity in the brain (Rosvall et al. 2012). All males were in breeding condition, as determined by observation of enlarged gonads. The females were all in the incubation stage of reproduction, determined by locating the female’s nest prior to the day of capture.
During the STI, we placed a live, caged, same-sex, junco in the approximate center of the territory (for male subjects) or 1 m from the nest (for female subjects). To simulate natural aggressive encounters, the male decoy (but not the female decoy) was accompanied by a conspecific song playback at a natural rate and amplitude (six songs per minute, 85–90 dB at 1 m). We measured the following well-established measures of songbird aggression (McGlothlin et al. 2007; Searcy and Beecher 2009; Cain and Ketterson 2012): the number of times the focal bird attacked the decoy by swooping (“flyovers”), focal bird’s distance from the decoy, number of songs sung by focal bird (for males), and amount of time focal bird spent responding to the decoy (for females, i.e., time spent responding to the intruder instead of incubating). One male’s behavior was excluded from these analyses because two unmarked males were present at the end of the STI, and we could not be sure that the captured male was the same one whose behavior was recorded. One female was excluded because we did not collect the preen gland or preen oil.
Immediately after the STI, each focal individual was captured in a mist net (4.5 ± 0.5 min after trial) and euthanized with an overdose of isoflurane, followed by rapid decapitation (2.2 ± 0.6 min after capture). Preen oil samples were collected immediately thereafter by rubbing a glass capillary tube against the tip of the uropygial gland, as described in Whittaker et al. (2010). We stored the preen oil at − 20 °C for later gas chromatography–mass spectrometry (GC-MS) analysis. Uropygial glands were then dissected from the body and flash frozen on dry ice within 15 min, a post-mortem interval that is short enough to ensure high quality RNA (Cheviron et al. 2011). We collected trunk blood (241 ± 16 µL, range 90–410 µL) and added 50 µL water-based heparin to prevent clotting. Whole blood was centrifuged, and the plasma was drawn off and stored at − 20 °C for hormone analysis. Brains were dissected and analyzed in a previous study (Rosvall et al. 2012).
Gas chromatography–mass spectrometry (GC-MS) of preen oil samples
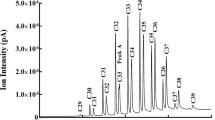
We measured GC–MS peak areas for 14 volatile compounds, selected as compounds of interest because they are elevated during the breeding season (Soini et al. 2007; Whittaker et al. 2011b) and have been shown to correlate with multiple aspects of individual identity and quality (Whittaker et al. 2010, 2013). We used a Twister® stir bar to extract volatile compounds from the preen oil samples, and then quantified them with an Agilent 6890N gas chromatograph connected to a 5973i MSD mass spectrometer (Agilent Technologies, Inc., Wilmington, DE, USA) with a Thermal Desorption Autosampler and Cooled Injection System (TSDA-CIS 4 from Gerstel, Mülheim and der Ruhr, Germany) following previously established protocols (Soini et al. 2013). During sample processing, we added 8 ng of 7-tridecanone (Aldrich, Milwaukee, WI, USA) in 5 μL of methanol to each vial as an internal standard. We identified major compounds by comparing their TIC (total ion current) spectra to the retention times and spectra of standard compounds from Sigma-Aldrich (St. Louis, MO, USA). We normalized peak areas of the compounds of interest by dividing each peak area by that of the internal standard in corresponding runs, yielding relative concentrations (i.e., relative amounts per 1 mg of preen oil).
Assessing androgen receptor transcript abundance
We assessed androgen receptor (AR) mRNA abundance using quantitative PCR on cDNA made from total RNA extracted from each uropygial gland. Briefly, we mechanically homogenized frozen glands in Trizol as they thawed (VWR VDI-12 homogenizer, speed 4), followed by immediate RNA extraction using the phenol-chloroform-based Trizol method according to the manufacturers instructions (Invitrogen, Carlsbad, CA, USA). All samples yielded a high quantity of high-quality RNA (108 ± 10 µg, range 49–210 µg, all 260/280 = 2.0). We then treated 1 µg of RNA with DNAse (Promega, Madison, WI, USA) and RNAse inhibitor (Promega #N2111), and used Superscript III (Invitrogen, Carlsbad, CA, USA) to synthesize cDNA for each sample.
We quantified expression of AR mRNA relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) using the 2− ΔΔC t method (Livak and Schmittgen 2001), in which AR abundance is normalized to an internal reference gene (GAPDH) for each sample and then quantified relative to that of a pooled standard run on each plate. We report the relative amount of transcript as the fold difference relative to this pooled standard (derived from junco neural tissue in another experiment), normalized via the amount of GAPDH mRNA in each sample. Each sample for each gene was run in duplicate using SYBR green low ROX on a Stratagene MX3000P Real-Time PCR System (Agilent Technologies, Santa Clara, CA, USA). Each well included 2.5 µL of cDNA diluted 1:10, with primers at 0.3 µM in a total volume of 25 µL. Both primer sets had nearly 100% efficiency (93.3–116.4%), indicating an approximate doubling of material in each PCR cycle. We controlled for slightly unequal efficiencies using MxPro software (v.4.10, Agilent). Primers for both genes were based on sequences from zebra finch (Taeniopygia guttata) and high sequence identity in these regions (95–98%) was confirmed in a previous study (Rosvall et al. 2012). AR forward primer: ATGAGTACCGCATGCACAAA; reverse primer: AACTCCTGGGGTGTGATCTG, GenBank #NM_001076688.1, 100 bp amplicon. GAPDH forward primer: TGACCTGCCGTCTGGAAAA, reverse primer: CCATCAGCAGCAGCCTTCA, GenBank # AF255390, 70 bp amplicon. All plates included no template controls that showed no amplification, and all reactions used the following thermal profile: 10 min at 95 °C, 40 cycles of 30 s at 95 °C, 1 min at 60 °C, 30 s at 70 °C, and a final dissociation phase, which confirmed product specificity for all wells (1 min at 95 °C, 30 s at 55 °C, and 30 s at 95 °C).
Hormone assays
We quantified circulating levels of T as a part of another study (Rosvall et al. 2012), using an enzyme immunoassay (Enzo, 901-065; assay sensitivity = 5.67 pg/mL). This kit has already been validated for use in the junco (Clotfelter et al. 2004), and the manufacturer reports high specificity for testosterone (100%), alongside limited cross-reactivity with 19-hydroxytestosterone (14.6%) and androstenedione (7.20%) and negligible cross-reactivity (< 1%) with other steroids. To summarize these methods briefly, we added water and radioactive T to each plasma sample, extracted plasma twice with diethyl ether, reconstituted in 350 µL assay buffer, and used extraction efficiencies to correct for incomplete recoveries (efficiencies 90.6 ± 0.5%). We used a logistic standard curve and curve-fitting program (Microplate Manager, Bio-Rad Laboratories) to obtain final T concentrations, and mathematically corrected for the 50 µL of water-based heparin solution added to each sample in the field. Samples were run on two plates, with intra-assay variability of 2.3 and 6.3%. Inter-plate variability was 19.7%, and so we used a plate correction factor based upon standards that were distributed multiple times on each plate (Jawor et al. 2007).
Statistical analyses
After checking for normality using Shapiro–Wilk tests, we transformed transcript abundance (log2), plasma T levels (natural log) and flyovers (square-root) to achieve normality. Testing hypotheses about chemical signals require different measures depending on whether the question is about signal production or odor perception. To test the hypotheses about the influence of hormones and receptors on the production of volatile compounds, we natural-log transformed the concentration of each volatile compound to produce a normalized “Odorant Abundance” measurement. However, odor is perceived by the recipient as a single blend of multiple components, and small differences in single components relative to the rest of that individual’s overall blend can significantly affect perception (Laing and Willcox 1983; Livermore and Laing 1998). Therefore, to examine how chemical signals might communicate information about aggression, we also transformed the absolute measurements of volatile compound concentrations into an “Odorant Proportion” measurement by dividing the peak area of each compound by the total peak area of all of the compounds measured in the sample. For both Odorant Abundance and Odorant Proportion measurements of volatile compounds, we used a principal components analysis with varimax rotation to reduce correlated data into a smaller number of variables, retaining PC scores with an Eigenvalue greater than 1.
We tested for sex differences in AR expression, T levels, and volatile compounds using unpaired T tests. Because male and female junco volatile profiles exhibit different relationships with many traits, sometimes in opposite directions (e.g., Whittaker et al. 2013), we subsequently analyzed the sexes separately.
To test for the relationship between volatile compound measurements and the endocrine and behavioral traits, we first used a stepwise regression to identify the most informative predictive variables. For initial models exploring the endocrine predictors of chemical signals, we asked whether plasma T levels, AR mRNA abundance, or their interaction predicted each Odorant Abundance PC. For initial models exploring whether volatile compounds may serve as a signal of aggression, we asked whether Odorant Proportion PCs predicted each measure of aggressive response to STI. We chose the model with the lowest Bayesian information criterion (BIC), which performs well in datasets with correlation among candidate variables and heterogeneity in the dataset (Brewer et al. 2016). We then constructed standard least-squares regression models using the variables identified in the stepwise regressions, and we report results of initial models in the Supplementary Material. All analyses were conducted in JMP 12 (SAS Institute, Cary, NC, USA).
Results
Principal component analysis of odorant measurements
Two principal components emerged from the Odorant Abundance measurements, explaining 54.7 and 28.7% of the variation (Table 1a). Odorant Abundance PC1 was strongly influenced by linear alcohols 1-undecanol through 1-hexadecanol and the three methyl ketones 2-tridecanone, 2-tetradecanone, and 2-pentadecanone. Odorant Abundance PC2 reflected the methyl ketones 2-undecanone and 2-dodecanone and the carboxylic acids tetradecanoic acid and hexadecanoic acid most strongly.
Five principal components (Table 1b) cumulatively explain 83.9% of the variation in proportional odorant measurements. Proportion PC1 strongly reflects methyl ketones 2-tridecanone, 2-tetradecanone, and 2-pentadecanone. The other proportion PC scores were most heavily loaded by the following compounds: PC2, 1-undecanol, 1-dodecanol, and 1-hexadecanol; PC3, 1-tridecanol, 1-pentadecanol, and 2-undecanone; PC4, tetradecanoic acid and hexadecanoic acid; and PC5, dodecanoic acid.
Odorant and sex-specific relationships with endocrine parameters
We observed no sex differences in AR transcript abundance (Fig. 1a, males = 2.97 ± 0.26, n = 17; females = 3.03 ± 0.28, n = 15; p = 0.878). As expected and previously reported (Rosvall et al. 2012), males had significantly higher levels of circulating T than females (unpaired t test, mean ± SE: males, 0.84 ± 0.15, n = 16; females, − 0.80 ± 0.16; p < 0.0001). Testosterone levels were not correlated with AR mRNA abundance in either sex (Fig. 1b, males: r 2 adj = 0.02, p = 0.271; females: r 2 adj = − 0.08, p = 0.957). Odorant Abundance PC scores did not differ between the sexes (p > 0.1, all comparisons). Females showed a non-significant trend towards lower Odorant Proportion PC1 scores than males (Fig. 1c, males = 0.29 ± 0.23, n = 17; females = − 0.33 ± 0.25, n = 15; p = 0.076), but no differences were observed in the other Proportion PC scores (p > 0.1, all comparisons).
a No sex differences in uropygial gland androgen receptor expression (p = 0.878). b No correlation between androgen receptor expression and testosterone levels in either sex (females: p = 0.957; males: p = 0.271). c Marginally significant sex differences in Odorant Proportion PC1 score (p = 0.076). Box plots denote the median and upper and lower quartiles
Relationship between endocrine variables and odorant production
In males, Abundance PC1 was significantly related to the interacting effects of both T and AR (overall model: F 3,12 = 5.20, r 2 adj = 0.46, p = 0.016; Table 2). The main effects of T (F = 11.94, p = 0.005) and AR (F = 5.53, p = 0.037) were both significantly positive, with a non-significant positive interaction effect (F = 3.89, p = 0.072). The stepwise regression model identified a non-significant, positive relationship between T and Abundance PC2 (r 2 adj = 0.11, df = 1, p = 0.118). In females, neither Odorant Abundance PC score was related to either of the endocrine variables (see Supplementary Table 1 for all initial models).
Relationship between odorant blend and aggressive behavior
Several components of odorants predicted aggressive behavior (final models in Table 3, see initial models in Supplementary Table 2). In males, Odorant Proportion PC1 positively correlated with the number of flyovers performed by the focal male over the intruder’s cage (r 2 adj = 0.27, df = 1, p = 0.022) (Fig. 2a). The number of songs the focal male sang in response to the intruder was positively related to Odorant Proportion PC3 and PC4 (overall model: F 2,13 = 5.10, r 2 adj = 0.35, p = 0.023; Table 3), with PC3 having the strongest main effect (F = 8.05, p = 0.014) and PC4 having a non-significant main effect (F = 4.07, p = 0.065). No aspects of male odorants were related to time spent in proximity to the intruder.
Relationship between odor variables and aggression in response to a territorial intruder. a Odorant Proportion PC1 correlated positively with square-root transformed flyovers performed by males (r 2 adj = 0.274, p = 0.022). b Odorant Proportion PC1 had a non-significant negative relationship with square-root transformed flyovers performed by females (r 2 adj = 0.118, p = 0.114)
In females, Odorant Proportion PC1 was identified by the stepwise regression as negatively related to the number of flyovers performed; however, the relationship was not significant (r 2 adj = 0.12, df = 1, p = 0.114, Fig. 2b). Time spent within ¼m of the intruder was significantly related to four principal components: negatively with Odorant Proportion PC1 and PC5, and positively with PC2 and PC4 (overall model: F 4,10 = 5.51, r 2 adj = 0.58, p = 0.011, main effects reported in Table 3). The same four principal component scores were similarly related to the total amount of time the focal female spent responding to an intruder (overall model: F 4,10 = 5.51, r 2 adj = 0.58, p = 0.013, Table 3).
Discussion
Avian chemical signals—now studied in several species—are likely to play an important role in reproductive behavior, as they contain information about the sender’s individual identity, quality, and breeding condition (Mardon et al. 2010; Whittaker et al. 2010, 2013; Amo et al. 2012b), and recipients have demonstrated preferences for certain odors (Whittaker et al. 2011a; Amo et al. 2012a, b). However, few studies have yet investigated the mechanism by which these odors are produced. Steroid hormones, particularly androgens, are thought to play a role in regulating uropygial gland secretions (Maiti and Ghosh 1972; Amet et al. 1986), but prior research suggests that testosterone levels in circulation do not fully account for variation in preen oil volatile profiles (Whittaker et al. 2011b). In this study, we found sex-specific relationships among volatile compounds, circulating T, AR transcript abundance, and aggression. Our findings suggest that songbird chemosignals may be influenced by androgens, and that local variation in androgen receptor density in the uropygial gland may be an important factor regulating volatile compound production. Our data further reveal relationships between chemical signals and aggression, suggesting that the complex blend of volatile compounds produced via the preen gland may in turn provide information to a receiver about the sender’s aggression levels. Because chemosignals have been linked to reproductive success in these birds in the wild (Whittaker et al. 2013), these potentially androgen-mediated relationships may be relevant for sexual selection.
Sex, odorants, and androgen-mediated signaling
As expected, males had significantly higher T levels than females, but there was no difference in AR expression. Critically, within each sex, there was no correlation between T levels in circulation and AR mRNA abundance in the uropygial gland, suggesting that systemic T levels and local AR sensitivity in this behaviorally important peripheral tissue are independently controlled. As a consequence, it ought to be possible for an individual to down-regulate AR sensitivity in the preen gland, irrespective of T levels elsewhere in the body. Thus, to the degree that volatile compounds produced in preen oil are androgen-dependent (see below), this disconnect between T and AR may allow chemosignals to convey information that is unique from the information that might be conveyed via other steroid-dependent tissues, such as growing feather follicles, which may influence plumage color (Chen et al. 2016) or various neural tissues, which may influence behavior.
Odorant variables did not differ between the sexes, but Proportion PC1, which is most strongly influenced by three compounds typically found in higher proportions in males (2-tridecanone, 2-tetradecanone, and 2-pentadecanone, Whittaker et al. 2013) was somewhat higher in males. The lack of marked sex differences in chemical signals in our dataset was unexpected given that previous studies have found significant sex differences in preen oil volatile composition. However, in the previous studies, birds were sampled at the beginning of the breeding season (Whittaker et al. 2010, 2013), whereas in the current study, samples were collected during a narrow time-frame after birds had initiated their first clutch. Furthermore, incubating females are under different selection pressures than those seeking mates, which could result in breeding stage-related changes in chemosignals—for example, increased volatile production during incubation may attract predators to the nest. Our observation that female volatile compounds did not correlate with T or AR suggests that female chemosignal production may exhibit reduced androgen-dependence during this narrow stage of breeding.
Sex-specific androgen dependence of odorants
In male juncos, we observed a strong relationship between the two endocrine variables and volatile compound abundance. Males with higher T levels and higher AR mRNA in the uropygial gland had higher abundance PC1 scores, which includes all six linear alcohols and the three methyl ketones 2-tridecanone, 2-tetradecanone, and 2-pentadecanone. Notably, T and AR did not independently correlate with Abundance PC1, and the best-fitting model included the interaction variable (T × AR). This result suggests that circulating androgens, and their effects that operate via testosterone–AR binding, could stimulate the production of these compounds in males, and more androgenized males have a greater abundance of certain volatile compounds. The three methyl ketones were found in a previous study to predict individual reproductive success in juncos, with males that had higher proportions of these compounds having more genetic offspring (Whittaker et al. 2013). Although correlational, these results suggest that male volatile production may be androgen dependent and regulated both systemically and locally, via T levels and uropygial AR sensitivity, respectively. As a consequence, the overall abundance of male preen oil volatile compounds could serve as a signal of male quality, related to multiple components of an individual’s endocrine phenotype.
We were unable to detect any relationships among the endocrine variables and Odorant Abundance in female juncos. This negative result suggests that androgens may not be involved in stimulating odorant production in females, at least during this narrow breeding stage (incubating females). As described above, incubating females may experience selection for reduced detectability (i.e., to minimize predation), and we speculate that female volatile compound production may use androgen-independent mechanisms during this time frame. Regardless of the underlying explanation, our data clearly show that male and female chemosignal production may depend upon sex-specific endocrine mechanisms.
Odorants as potential signals of aggression
Aspects of aggressive response to an intruder were correlated with volatile compounds in males and females, suggesting that chemical signals may be potential signals of aggression. In males, individuals with a higher Proportion PC1 score, reflecting 2-tridecanone, 2-tetradecanone, and 2-pentadecanone, performed more flyovers or “dive-bombs” directed at the decoy. These compounds were among those that showed a significant positive relationship with T and AR in this study (Tables 1, 2), and were also related to reproductive success in a previous study (Whittaker et al. 2013). The number of songs males directed at intruders was related to Proportion PC3 (positive relationship with 1-tridecanol, negative relationship with 2-undecanone) and Proportion PC4 (tetradecanoic acid, hexadecanoic acid). Of these compounds, 1-tridecanol was also higher in males with higher T levels and AR expression, suggesting that this volatile compound may be a reliable indicator of a male’s androgen levels and aggression. Our observation that different components of junco chemosignals correlate with different aggressive behaviors (e.g., flyovers vs. songs) also suggest that the specific blend of volatile compounds may convey unique signal content.
The amount of time that females spent responding to an intruder, and the time spent in close proximity to an intruder, related to all of the Odorant Proportion PC scores except for Proportion PC3 (1-tridecanol and 2-undecanone). As noted above, however, we found no evidence of androgen-dependence of female odorants in this study. These relationships suggest that female preen oil volatile compounds have the potential to serve as a signal of aggression, but a different androgen-independent mechanism may underlie the chemical signaling of aggressive intent in incubating females.
Conclusions and implications for future research
Our results demonstrate that circulating testosterone and androgen receptor expression are correlated with chemical signal production in male juncos, suggesting that the androgen receptor signaling pathway may influence the production of preen oil compounds in male songbirds. We found that these chemosignals predicted aggressive behavior in both sexes, indicating a possible function as signals of aggressive intent. In females, we did not detect a relationship between androgens and volatile compound production, though it is not clear whether this result may be due to the breeding stage in which the females were sampled. By linking androgen sensitivity in the periphery to behavior and odor, these results complement and extend the findings of a prior study using the same individual birds (Rosvall et al. 2012), which reported that circulating T correlated with aggressive behavior only in males, but gene expression for steroid receptors in the brain predicted aggression in both sexes. Together these results suggest that steroid sensitivity in target tissues, including the brain and uropygial gland, may play an important role in regulating aggressive behavior and the chemical cues that signal aggression, respectively.
A pathway for the synthesis of preen oil volatile compounds has not yet been described, and so it is possible that T and AR may influence uropygial gland secretions via a more indirect mechanism that may operate in a sex-specific manner. For example, androgens can directly affect the odors produced by bacteria. Studies of human axillary gland volatile compounds determined that coryneform bacteria (family Corynebacteriaceae) present on the skin were able to metabolize androgens and produce 16-androstenes, an important component of human male axillary odor and a proposed human pheromone (Rennie et al. 1990, 1991; Gower et al. 1994). Like human skin, avian uropygial glands are home to a rich and diverse bacterial community, including those in the family Corynebacteriaceae and several genera that are known to produce some of the same volatile compounds found in preen oil (Soler et al. 2008; Pearce 2016; Whittaker et al. 2016; Whittaker and Theis 2016). Androgen receptors also may affect microbial communities by regulating the production of preen oil in their environment (Maiti and Ghosh 1972). Future work should investigate the biochemical pathways that produce preen oil volatile compounds, including the role of symbiotic bacteria, which may interact with hormone signaling to generate yet another layer of peripheral control of an individual’s phenotype, including chemosignals. Other hormones and their receptors are also likely to influence chemical signals (Maiti and Ghosh 1969; Maiti 1971; Daniel et al. 1977; Chakraborty et al. 1979; Bohnet et al. 1991; Asnani and Ramachandran 1993; Douglas et al. 2008), and more work is needed to investigate the mechanisms that directly produce these compounds and the physiological factors that regulate them.
Although many open questions remain, we have made significant progress in understanding the signal content and behavioral role of avian chemical signals in the past decade. Our study is the first to consider the role of both a hormone and local expression of its receptor in relation to an avian chemosignal and the information communicated by this signal. This sort of integrative consideration of both hormone levels and tissue sensitivity to those hormones has the potential to elucidate the complex relationships that influence chemical signaling, the information present in chemical signals, and the role that the periphery may play in regulating signal content. Chemical signals provide important information to potential mates and rivals, and can strongly affect individual reproductive success. Understanding how local hormonal control may tie signals to individual physiological state, reproductive condition, or behavior could lead to significant advances in the study of animal communication, as well as a greater appreciation of the diversity of roles that peripheral tissues play in mediating phenotypic variation.
Abbreviations
- AR:
-
Androgen receptor
- BIC:
-
Bayesian information criterion
- cDNA:
-
Complementary deoxyribonucleic acid
- GC–MS:
-
Gas chromatography–mass spectrometry
- mRNA:
-
Messenger ribonucleic acid
- PC:
-
Principal component
- PCR:
-
Polymerase chain reaction
- RNA:
-
Ribonucleic acid
- T:
-
Testosterone
References
Adkins-Regan E (2005) Hormones and animal social behavior. Princeton University Press, Princeton
Amet Y, Abalain JH, Daniel JY, Di Stefano S, Floch HH (1986) Testosterone regulation of androgen receptor levels in the uropygial gland of quails (Coturnix coturnix): a further proof for the androgen dependency of the uropygial gland. Gen Comp Endocrinol 62:210–216
Amo L, Avilés JM, Parejo D, Peña A, Rodríguez J, Tomás G (2012a) Sex recognition by odour and variation in the uropygial gland secretion in starlings. J Anim Ecol 81:603–613
Amo L, López-Rull I, Pagán I, Macías Garcia C (2012b) Male quality and conspecific scent preferences in the house finch, Carpodacus mexicanus. Anim Behav 84:1483–1489
Asnani MV, Ramachandran AV (1993) Roles of adrenal and gonadal steroids and season in uropygial gland function in male pigeons, Columba livia. Gen Comp Endocrinol 92:213–224
Ball GF, Balthazart J (2008) Individual variation and the endocrine regulation of behaviour and physiology in birds: a cellular/molecular perspective. Philos Trans R Soc Lond B Biol Sci 363:1699–1710
Bergeon Burns C, Rosvall KA, Ketterson ED (2013) Neural steroid sensitivity and aggression: comparing individuals of two songbird subspecies. J Evol Biol 26:820–831
Bohnet S, Rogers L, Sasaki G, Kolattukudy PE (1991) Estradiol induces proliferation of peroxisome-like microbodies and the production of 3-hydroxy fatty acid diesters, the female pheromones, in the uropygial glands of male and female mallards. J Biol Chem 266:9795–9804
Brewer MJ, Butler A, Cooksley SL (2016) The relative performace of AIC, AICc and BIC in the presence of unobserved heterogeneity. Methods Ecol Evol 7:679–692
Cain KE, Ketterson ED (2012) Competitive females are successful females: phenotype, mechanism, and selection in a common songbird. Behav Ecol Sociobiol 66:241–252
Caro SP, Balthazart J, Bonadonna F (2015) The perfume of reproduction in birds: chemosignaling in avian social life. Horm Behav 68:25–42
Chakraborty S, Maiti BR, Bhattacharyya SP (1979) Action of prolactin on the uropygial gland of chicks. Gen Comp Endocrinol 38:461–465
Chen CC, Plikus MV, Tang PC, Widelitz RB, Chuong C-M (2016) The modulatable stem cell niche: tissue interactions during hair and feather follicle regeneration. J Mol Biol 428:1423–1440
Cheviron ZA, Carling MD, Brumfield RT (2011) Effects of postmortem interval and preservation method on RNA isolated from field-preserved avian tissues. Condor 113:483–489
Clotfelter ED, O’Neal DM, Gaudioso JM, Casto JM, Parker-Renga IM, Snajdr E, Duffy DL, Nolan V Jr, Ketterson ED (2004) Consequences of elevating plasma testosterone in females of a socially monogamous songbird: evidence of constraints on male evolution? Horm Behav 46:171–178
Daniel JY, Vignon F, Assenmacher I, Rochefort H (1977) Evidence of androgen and estrogen receptors in the preen gland of male ducks. Steroids 30:703–709
Douglas HD, Kitaysky III, Kitaiskaia AS EV (2008) Seasonal variation in progesterone and odorant emissions among breeding crested auklets (Aethia cristatella). Horm Behav 54:325–329
Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA (2010) Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA 107:12393–12398
Gonçalves D, Saraiva J, Teles M, Teodósio R, Canário AVM, Oliveira RF (2010) Brain aromatase mRNA expression in two populations of the peacock blenny Salaria pavo with divergent mating systems. Horm Behav 57:155–161
Gower DB, Holland KT, Mallet AI, Rennie PJ, Watkins WJ (1994) Comparison of 16-androstene steroid concentrations in sterile apocrine sweat and axillary secretions: interconversions of 16-androstenes by the axillary microflora—a mechanism for axillary odour production in man? J Steroid Biochem Mol Biol 48:409–418
Horton BH, Hudson WH, Ortlund EA, Shirk S, Thomas JW, Young ER, Zinzow-Kramer WM, Maney DL (2014) Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. Proc Natl Acad Sci USA 111:1443–1448
Jacob JP, Ziswiler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology. Academic Press, New York, pp 199–324
Jawor JM, McGlothlin JW, Casto JM, Greives TJ, Snajdr EA, Bentley GE, Ketterson ED (2007) Testosterone response to GnRH in a female songbird varies with stage of reproduction: implications for adult behaviour and maternal effects. Funct Ecol 21:767–775
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289
Ketterson ED, Atwell JW, McGlothlin JW (2009) Phenotypic integration and independence: hormones, performance, and response to environmental change. Integr Comp Biol 49:365–379
Kolattukudy PE, Bohnet S, Roberts E, Rogers L (1987a) Peroxisomes in sebaceous glands: biosynthetic role and hormonal regulation. In: Fahimi HD, Sies H (eds) Peroxisomes in biology and medicine. Springer, Berlin, pp 18–31
Kolattukudy PE, Bohnet S, Rogers L (1987b) Diesters of 3-hydroxy fatty acids produced by the uropygial glands of female mallards uniquely during the mating season. J Lipid Res 28:582–588
Krause ET, Krüger O, Kohlmeier P, Caspers BA (2012) Olfactory kin recognition in a songbird. Biol Lett 8:327–329
Laing DG, Willcox ME (1983) Perception of components in binary odor mixtures. Chem Senses 7:249–264
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta c(t)) method. Methods 25:402–408
Livermore A, Laing DG (1998) The influence of odor type on the discrimination and identification of odorants in multicomponent odor mixtures. Physiol Behav 65:311–320
Maiti BR (1971) Influence of estrogen on the histophysiology of the uropygial gland. Arch Histol Jpn 33:371–380
Maiti BR, Ghosh A (1969) Effect of cortisone on mitotic activity and cell loss in the uropygial gland of male pigeons. Acta Anat 74:97–103
Maiti BR, Ghosh A (1972) Probable role of androgen in the regulation of the uropygial gland. Gen Comp Endocrinol 19:527–536
Mardon J, Saunders SM, Anderson MJ, Couchoux C, Bonadonna F (2010) Species, gender, and identity: cracking petrels’ sociochemical code. Chem Senses 35:309–321
Martín J, López P (2015) Condition-dependent chemosignals in reproductive behavior of lizards. Horm Behav 68:14–24
McGlothlin JW, Jawor JM, Ketterson ED (2007) Natural variation in a testosterone-mediated trade-off between mating effort and parental effort. Am Nat 170:864–875
Parker MR, Mason RT (2012) How to make a sexy snake: estrogen activation of female sex pheromone in male red-sided garter snakes. J Exp Biol 215:723
Parker MR, Mason RT (2014) A novel mechanism regulating a sexual signal: the testosterone-based inhibition of female sex pheromone expression in garter snakes. Horm Behav 66:509–516
Pearce DS (2016) Physiology and genetics shape the microbiome of a seabird species (Oceanodroma leucorhoa) more than environmental and social factors. Dissertation, Western Michigan University
Penn D, Potts WK (1998) Chemical signals and parasite-mediated sexual selection. Trends Ecol Evol 13:391–396
Reneerkens J, Piersma T, Sinninghe Damste JS (2002) Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc R Soc B 269:2135–2139
Rennie PJ, Gower DB, Holland KT, Mallet AI, Watkins WJ (1990) The skin microflora and the formation of human axillary odour. Int J Cosmet Sci 12:197–207
Rennie PJ, Gower DB, Holland KT (1991) In-vitro and in-vivo studies of human axillary odour and the cutaneous microflora. Br J Dermatol 124:596–602
Rosvall KA, Bergeon Burns CM, Barske J, Goodson JL, Schlinger BA, Sengelaub DR, Ketterson ED (2012) Neural sensitivity to sex steroids predicts individual differences in aggression: implications for behavioural evolution. Proc R Soc B Biol Sci 279:3547–3555
Searcy WA, Beecher MD (2009) Song as an aggressive signal in songbirds. Anim Behav 78:1281–1292
Shanbhag BA, Sharp PJ (1996) Immunocytochemical localization of androgen receptor in the comb, uropygial gland, testis, and epididymis in the domestic chicken. Gen Comp Endocrinol 101:76–82
Soini HA, Schrock SE, Bruce KE, Wiesler D, Ketterson ED, Novotny MV (2007) Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J Chem Ecol 33:183–198
Soini HA, Whittaker DJ, Wiesler D, Ketterson ED, Novotny M (2013) Chemosignaling diversity in songbirds: chromatographic profiling of preen oil volatiles in different species. J Chromatogr A 1317:186–192
Soler JJ, Martín-Vivaldi M, Ruiz-Rodríguez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M, Peralta-Sánchez JM, Méndez M (2008) Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct Ecol 22:864–871
Staub NL, DeBeer M (1997) The role of androgens in female vertebrates. Gen Comp Endocrinol 108:1–24
Tuttle EM, Sebastian PJ, Posto AL, Soini HA, Novotny MV, Gonser RA (2014) Variation in preen oil composition pertaining to season, sex, and genotype in the polymorphic white-throated sparrow. J Chem Ecol 40:1025–1038
Wacker DW, Wingfield JC, Davis JE, Meddle SL (2010) Seasonal changes in aromatase and androgen receptor, but not estrogen receptor mRNA expression in the brain of the free-living male song sparrow, Melospiza melodia morphna. J Comp Neurol 518:3819–3835
Whittaker DJ, Theis KR (2016) Bacterial communities associated with junco preen glands: Preliminary ramifications for chemical signaling. In: Schulte BA, Goodwin TE, Ferkin MH (eds) Chemical signals in vertebrates 13. Springer International Publishing Switzerland, Cham, pp 105–117
Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny MV, Ketterson ED (2010) Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav Ecol 21:608–614
Whittaker DJ, Richmond KM, Miller AK, Kiley R, Bergeon Burns C, Atwell JW, Ketterson ED (2011a) Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav Ecol 22:1256–1263
Whittaker DJ, Soini HA, Gerlach NM, Posto AL, Novotny MV, Ketterson ED (2011b) Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. J Chem Ecol 37:1349–1357
Whittaker DJ, Gerlach NM, Soini HA, Novotny MV, Ketterson ED (2013) Bird odour predicts reproductive success. Anim Behav 86:697–703
Whittaker DJ, Gerlach NM, Slowinski SP, Corcoran KP, Winters AD, Soini HA, Novotny MV, Ketterson ED, Theis KR (2016) Social environment has a primary influence on the microbial and odor profiles of a chemically signaling songbird. Front Ecol Evol 4:90
Acknowledgements
This research was supported by the National Institutes of Health (T32HD049336 and F32HD068222), the Indiana Academy of Sciences, the Biology Department at Indiana University, the National Science Foundation (IOS-0820055), and NSF Cooperative Agreement DBI-0939454. All work was conducted in compliance with the Bloomington Institutional Animal Care and Use Committee guidelines (BIACUC protocol 09-037) and with permission from the US Department of Fish and Wildlife, the Virginia Department of Game and Inland Fisheries, and the US Forest Service. We thank Mountain Lake Biological Station, University of Virginia, as well as Mountain Lake Hotel and the Dolinger family for allowing research on their grounds. We thank N. Tonge for assistance in the field and S. P. Jayaratna and C. M. Bergeon Burns for assistance with qPCR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Whittaker and Rosvall are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Whittaker, D.J., Rosvall, K.A., Slowinski, S.P. et al. Songbird chemical signals reflect uropygial gland androgen sensitivity and predict aggression: implications for the role of the periphery in chemosignaling. J Comp Physiol A 204, 5–15 (2018). https://doi.org/10.1007/s00359-017-1221-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1221-5