Abstract
Intraspecific chemical communication may play a significant role in avian behavior. Preen oil secreted by the uropygial gland emits volatile compounds that vary with many aspects of bird biology and may figure prominently in mate choice. Many of these compounds are known end products of bacterial metabolism in other environments. The fermentation hypothesis for chemical recognition suggests that symbiotic bacteria in mammalian scent glands produce volatile odorants that are used as recognition cues by the host animals and that variation in these bacterial communities contributes to variation in the animal scents. We preliminarily evaluated whether this hypothesis could apply to birds by sampling and sequencing bacterial communities associated with the preen gland of adult breeding dark-eyed juncos (Junco hyemalis). The most common operational taxonomic units (OTUs), defined by the V4 region of the 16S rRNA gene, belonged to the phyla Actinobacteria, Firmicutes, and Proteobacteria. Most of the identified bacterial genera contain species that are known odor producers. Most notably, Burkholderia and Pseudomonas species consistently produce 9 of the 17 volatile compounds whose production is either upregulated during the breeding season or differs between the sexes in juncos. Other genera have documented antifungal or antibacterial properties and may provide other valuable services to the birds. We found no effect of host sex on bacterial community composition or structure. Instead, paired males and females clustered together, suggesting that individuals that have frequent contact may develop similar microbial communities quickly. Our study suggests that the fermentation hypothesis for chemical recognition, originally formulated for mammals, may apply to birds as well, opening new pathways for avian-microbial research.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Renewed interest in the role of symbiotic bacteria in animal behavior—and particularly in producing chemical signals—has led to new insights and questions about the evolution of animal communication and host-symbiont coevolution (Archie and Theis 2011). The fermentation hypothesis for chemical recognition, first proposed in the 1970s (Albone et al. 1974; Gorman et al. 1974), suggests that symbiotic bacteria in mammalian scent glands produce volatile odorants that are used as recognition cues by the host animals and that variation in these bacterial communities contributes to variation in the animal scents. Support for this hypothesis has been demonstrated in several mammalian species, including spotted hyenas (Theis et al. 2012, 2013), meerkats (Leclaire et al. 2014), European badgers (Sin et al. 2012), North American porcupines (Roze et al. 2010), and greater sac-winged bats (Voigt et al. 2005). This hypothesis has rarely been applied outside of mammals and insects, although studies in birds and other taxa suggest that bacteria are present in glands related to chemical signaling (Ezenwa and Williams 2014). In this chapter, we explore whether symbiotic bacteria may play a role in manufacturing chemical signals in a songbird, the dark-eyed junco.
Chemical communication among birds, especially songbirds, is a long-neglected area of study due primarily to the persistent belief that most birds have little to no sense of smell. However, recent studies have revealed a potentially significant role for intraspecific chemical communication in avian behavior (Caro and Balthazart 2010). The best-studied source of volatile odors in birds is preen oil secreted by the uropygial or “preen” gland, the largest exocrine gland in most birds (Jacob and Ziswiler 1982). While preening, birds spread preen oil on their feathers, which protects them in a number of ways, including enhancing their insulative capacity, maintaining feather condition, and defense against pathogenic bacteria and fungi (Jacob and Ziswiler 1982; Moyer et al. 2003; Giraudeau et al. 2010). Symbiotic bacteria associated with the gland appear to provide some of these services, by producing antimicrobial compounds that help protect against feather-degrading bacteria and other pathogenic microbes (Shawkey et al. 2003; Martín-Vivaldi et al. 2009, 2010; Soler et al. 2010). Preen oil also emits volatile and semivolatile compounds that contribute to a bird’s odor profile and thereby potentially function in communication (Mardon et al. 2010; Whittaker et al. 2010). These compounds vary among bird species (Soini et al. 2013), and within species, they can vary with individual identity (Mardon et al. 2010; Whittaker et al. 2010), sex (Soini et al. 2007; Whittaker et al. 2010), age (Shaw et al. 2011), and breeding condition (Whittaker et al. 2011b). In some bird species, they also reliably predict genetic and social reproductive success (Whittaker et al. 2013) and provide information about the relative quality of male rivals (Amo et al. 2012). Therefore, preen oil compounds may figure prominently in avian mate competition and choice. Importantly, many of these compounds, which include linear alcohols, methyl ketones, and carboxylic acids, are known to be end products of bacterial metabolism in other environments (Madigan et al. 2010; Agler et al. 2011), suggesting that the fermentation hypothesis for chemical recognition could explain the presence of these compounds in preen oil.
To our knowledge, no studies have yet tested whether bacteria play a role in avian chemical communication. However, several researchers have described relationships between preen glands and bacteria, including the effects of preen oil on feather-degrading bacteria, for example, in house finches (Shawkey et al. 2003), and beneficial bacteria found in breeding European hoopoe (Martín-Vivaldi et al. 2010) and wood hoopoe (Law-Brown and Meyers 2003) preen glands. Experimental work demonstrated that symbiotic bacteria in hoopoe uropygial glands produce preen oil volatile compounds with potent antimicrobial properties, though that study did not identify the symbiotic bacteria (Martín-Vivaldi et al. 2010). Shawkey and colleagues (2006) characterized the bacterial assemblages found on the plumage of several bird species and suggested that bacteria unique to the crested auklet could be a potential source for that species’ distinctive tangerine odor, but did not explore potential links between auklet bacteria and odors (Hagelin et al. 2003; Shawkey et al. 2006). In this chapter, we bring together data on bacteria and preen oil volatile compounds in the context of chemical communication in a songbird.
One of the best characterized songbird chemical communication systems is that of the dark-eyed junco (Junco hyemalis), a widespread North American sparrow whose behavior, ecology, and physiology are well understood (Nolan et al. 2002). Thirty-nine volatile and semivolatile compounds have been identified in junco preen oil, 17 of which varied seasonally or differed in relative concentration between the sexes and were selected for further research in subsequent studies: linear alcohols 1-decanol through 1-octadecanol, methyl ketones 2-undecanone through 2-pentadecanone, and carboxylic acids dodecanoic acid, tetradecanoic acid, and hexadecanoic acid (Soini et al. 2007; Whittaker et al. 2010). These 17 compounds vary with many aspects of junco biology, including population of origin, sex, hormone levels, and reproductive success (Soini et al. 2007; Whittaker et al. 2010, 2011b, 2013). Furthermore, juncos are able to detect and differentiate among preen oil odors from different individuals, sexes, and species (Whittaker et al. 2009, 2011a). These qualities make preen oil volatiles reliable candidate cues for mate assessment and choice, yet their mechanism of production remains unknown.
To begin evaluating whether the fermentation hypothesis for chemical recognition can account for variation in junco odor profiles, here we characterize the bacterial communities associated with the preen glands of breeding adult dark-eyed juncos and determine whether the taxa present would logically contribute to junco chemical signals. We compare diversity in bacterial community composition and structure among nesting pairs and between sexes. Finally, we consider the ramifications of our findings for future studies of chemical communication, mate choice, and kin recognition in birds.
2 Methods
We sampled the preen gland bacterial communities of wild adult Carolina dark-eyed juncos (J. h. carolinensis) at Mountain Lake Biological Station in Pembroke, VA. In this region of the Appalachian Mountains, juncos are primarily altitudinal migrants, moving down into the valleys during the winter and returning to higher elevations to breed. Males typically arrive on the breeding grounds in March, with females arriving about 2 weeks later (Nolan et al. 2002). Juncos are socially monogamous, typically forming pair bonds that are maintained throughout the breeding season (May to August). Juncos also demonstrate appreciable levels of extra-pair fertilization, with about 28 % of all offspring being sired by a male other than their mother’s social mate (Ketterson et al. 1997; Gerlach et al. 2012). Juncos display biparental care, with females incubating the eggs and brooding the hatchlings, and both pair mates provisioning the young (Nolan et al. 2002).
Throughout May 2012, we identified junco nests during egg laying or incubation and monitored them through the nestling phase to fledging (day 11 or 12 post-hatching). On the morning of fledging day, we captured the adult female and male at the nest using mist nets. For this study, we captured 25 juncos from 13 nests (one nest did not have an attendant male). From each junco, we sampled the bacterial communities associated with the preen gland by rubbing the tip of the gland using a sterile cotton swab. This rubbing motion mimics the birds’ own preen oil collection behavior when preening and is similar to that used to stimulate preen oil secretion for collection in capillary tubes (Whittaker et al. 2010). This collection method ensured that our samples included a small amount of preen oil and microbes from inside and outside the gland, which represent the mixture that birds collect on their bills in preparation for preening. We stored the samples at −80 °C until analysis.
We extracted DNA from the bacteria on swabs using MO BIO PowerSoil® DNA isolation kits (MO BIO Laboratories, Inc., Carlsbad, CA). We followed the manufacturer’s recommended protocol, except we added an initial 10 min saturation step during which the swab bathed in bead solution within the bead tube, and we subsequently vigorously vortexed the bead tube for 1 min before removing the swab and proceeding to the step in which solution 1 is added. Each DNA extraction yielded a discernible band, consistent with 16S rDNA, on an agarose gel following PCR amplification of the 16S rRNA gene. Aliquots of the original DNA extractions were provided to the Michigan State University Research Technology Support Facility’s Genomics Core, where the V4 region of the 16S gene was targeted for sequencing on the Illumina MiSeq platform. Sample preparation, sequencing, and preliminary quality filtering were completed using previously published protocols (Caporaso et al. 2011, 2012).
We processed the MiSeq run files using mothur software, v. 1.31.2 (Schloss et al. 2009; Kozich et al. 2013). Specifically, we removed all sequences that (1) contained any ambiguous base calls, (2) had homopolymer runs longer than eight bases, (3) did not start and end at our specific V4 primer positions when aligned to the Silva bacterial database, (4) were deemed chimeric by mothur’s uchime tool, or (5) were classified as originating from mitochondria, chloroplasts, archaea, eukaryotes, or other nonbacterial sources using the Ribosomal Database Project’s trainset9_032012 (Wang et al. 2007; Claesson et al. 2009). This process revealed that the preen sample of one male junco was not successfully sequenced, so data from this sample were discarded. Each of the 24 remaining samples were subsampled to a depth of 6000 sequences, and these sequences were binned into operational taxonomic units (OTUs) using mothur’s average neighbor split-clustering algorithm and a 97 % sequence similarity cutoff. We then removed all singleton and doubleton OTUs from the data set and derived a consensus taxonomy for each of the remaining OTUs using a conservative 80 % confidence threshold (Claesson et al. 2009).
We generated a Clearcut cladogram, v 1.0.9, in mothur to illustrate the phylogenetic and taxonomic relationships among the prominent (i.e., widespread) OTUs in samples (Sheneman et al. 2006). We then used this information in conjunction with data on the typical production of volatiles by bacterial taxa, available through the mVOC database (Lemfack et al. 2014), to determine whether the prominent OTUs associated with junco preen glands are likely to manufacture any of the 17 volatile compounds of interest. Variation in the OTU profiles of samples among nests and between sexes was visualized via two-dimensional, principle coordinates analyses (PCoA) and statistically evaluated using nonparametric MANOVA with 10,000 permutations (Anderson 2001). We conducted these analyses using Dice and Bray-Curtis similarity indices, reflecting similarities in bacterial community membership and structure, respectively (Hammer 2011). Community membership addresses the shared presence or absence of OTUs, while community structure further considers similarities in their relative abundances. Prior to conducting community structure analyses, OTU abundance data were log10 (x + 1) transformed (Ramette 2007). All analyses were completed using PAST software, v 2.17 (Hammer et al. 2001; Hammer 2011).
3 Results and Discussion
Our preliminary bacterial survey suggests that junco preen glands, like mammalian scent glands, harbor diverse communities of symbiotic odor-producing bacteria. Figure 8.1 shows a cladogram of the most common OTUs found in our samples. Sixteen OTUs were unclassified, while 18 were assigned to 16 genera in the phyla Actinobacteria, Firmicutes, and Proteobacteria (classes Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria). Several of these genera were previously found on the plumage of seabirds, chickens, and songbirds, including Staphylococcus, Enterococcus, Pseudomonas, and Acinetobacter; Burkholderia was found on the plumage of chickens and auklets (Shawkey et al. 2005, 2006). Enterococcus was also found in the preen glands and secretions of hoopoes and wood hoopoes (Martín-Vivaldi et al. 2009; Law-Brown and Meyers 2003). These findings suggest that these genera may be commonly found on birds, as they are on mammals.
Clearcut, relaxed neighbor-joining cladogram of the most common operational taxonomic units (OTUs) associated with junco preen glands. All OTUs listed were found in at least 50 % of birds sampled; those marked with asterisks were found in at least 75 % of the birds sampled. Taxonomic assignments were made using the Ribosomal Database Project’s classifier tool in mothur, with an 80 % confidence threshold
Most of the bacterial genera associated with junco preen glands contain species that are known odor producers (Balkwill et al. 2006; Ezaki et al. 2006; Towner 2006; López del Castillo-Lozano et al. 2008; Blom et al. 2011; Latorre-Moratalla et al. 2011; Filipiak et al. 2012; Spraker et al. 2014). Most notably, Burkholderia and Pseudomonas, which can survive in a wide variety of habitats and utilize diverse nutrients, including oils (Haas and Défago 2005; Mahenthiralingam et al. 2005), can produce 9 of the 17 volatile compounds of interest (53 %) in juncos. Most of these nine volatile compounds are known to be produced by multiple species within each genus (e.g., nine species of Burkholderia and five species of Pseudomonas produce 2-tridecanone, Table 8.1). Thus, even without species-level identification in this study, the available data suggest that Burkholderia and Pseudomonas are strong candidates for the production of volatile compounds involved in junco chemical communication. We have begun efforts to successfully cultivate and metabolically characterize these bacteria from junco preen oil to directly test this hypothesis.
Not all of the identified genera contain odor producers, or at least they are not known to produce volatile compounds described in junco preen oil. However, while mammalian scent glands are specialized for a single purpose, secretions from avian preen glands perform diverse functions in addition to chemical signal production, including parasite defense, feather protection, and thermoregulation. Several genera associated with junco preen glands have documented antifungal (Arthrobacter, Burkholderia, Pseudomonas) or antibacterial (Methylobacterium, Enterococcus, Pseudomonas) effects (Fernando et al. 2005; Haas and Défago 2005; Green 2006; Jones and Keddie 2006; Soler et al. 2008; Groenhagen et al. 2013), and Burkholderia and Pseudomonas species are also used for biocontrol in agriculture and in bioremediation to clean up pollutants (Haas and Défago 2005; Mahenthiralingam et al. 2005). Therefore, these bacteria may be providing other valuable services to their junco hosts.
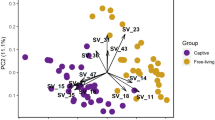
Although male and female juncos have significantly different volatile profiles (Whittaker et al. 2010), we did not find a consistent effect of sex on bacterial community composition or structure (NPMANOVA, N = 22, df = 1, Dice: F = 0.858, p = 0.7713, Bray-Curtis: F = 0.8024, p = 0.8511). Instead, paired males and females clustered together (Fig. 8.2). Similarly, in a study of captive zebra finches (Taeniopygia guttata), bacteria applied to the feathers of one zebra finch were found in the cloaca of its pair mate 24 h later, most likely transmitted via copulation (Kulkarni and Heeb 2007). Humans—and their pet dogs—living in the same household have significantly more similar skin microbiomes than those living in different households (Song et al. 2013), and individual human microbiomes quickly colonize the individual’s surroundings (Lax et al. 2014). Since we collected our samples in May, near the beginning of the breeding season, our data suggest that adult birds that spend time in close physical proximity or have frequent contact may develop similar microbial communities in a fairly short period of time.
Previous studies have suggested that preen oil volatile compounds may be important for mate recognition and assessment, as they vary with species, sex, and individual identity (Soini et al. 2007; Mardon et al. 2010; Whittaker et al. 2010), and they predict reproductive success in juncos (Whittaker et al. 2013). These volatiles may also be important for recognizing kin (Leclaire et al. 2012) and one’s home nest or burrow (Bonadonna and Bretagnolle 2002; Caspers and Krause 2010). Cross infection of symbiotic microbes between mates and between parents and offspring may contribute to the development of a recognizable, “signature” home scent (Archie and Theis 2011).
4 Conclusion
To our knowledge, this is the first study to examine avian microbiomes in the context of chemical signaling. Previous studies of symbiotic bacteria on birds’ skin, feathers, or glands have focused on microbes that produce beneficial, antibacterial substances (e.g., Martín-Vivaldi et al. 2010) or on pathogenic microbes, especially feather-degrading bacteria (e.g., Shawkey et al. 2003; Saranathan and Burtt 2007; Saag et al. 2011). Our study suggests that the fermentation hypothesis for chemical recognition, originally formulated for mammals, may apply to a much broader range of taxa and opens new pathways for research. Future studies should evaluate covariance between preen gland bacterial communities and odors, the effect of manipulating bacterial communities on odor profiles, and the extent to which subsequent changes in odor profiles influence birds’ behavioral responses to preen oil. They should also further elucidate the effect of social behavior on the transmission of preen gland bacterial communities and determine the extent to which preen gland bacterial communities differ from those associated with other avian organs.
References
Agler MT, Wrenn BA, Zinder SH, Angenent LT (2011) Waste to bioproduct conversion with undefined mixed cultures: the carboxylate platform. Trends Biotechnol 29:70–78
Albone ES, Eglinton G, Walker JM, Ware GC (1974) The anal sac secretion of the red fox (Vulpes vulpes); its chemistry and microbiology. A comparison with the anal sac secretion of the lion (Panthera leo). Life Sci 14:387–400
Amo L, López-Rull I, Pagán I, Macías Garcia C (2012) Male quality and conspecific scent preferences in the house finch, Carpodacus mexicanus. Anim Behav 84:1483–1489
Anderson M (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Archie EA, Theis KR (2011) Animal behaviour meets microbial ecology. Anim Behav 82:425–436
Balkwill DL, Fredrickson JK, Romine MF (2006) Sphingomonas and related genera. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 605–629
Blom D, Fabbri C, Connor EC, Schiestl FP, Klauser DR, Boller T, Eberl L, Weisskopf L (2011) Production of plant growth modulating volatiles is widespread among rhizosphere bacteria and strongly depends on culture conditions. Environ Microbiol 13:3047–3058
Bonadonna F, Bretagnolle V (2002) Smelling home: a good solution for burrow-finding in nocturnal petrels? J Exp Biol 205:2519–2523
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6(8):1621–1624
Caro SP, Balthazart J (2010) Pheromones in birds: myth or reality? J Comp Physiol A 196:751–766
Caspers BA, Krause ET (2010) Odour-based natal nest recognition in the zebra finch (Taeniopygia guttata), a colony-breeding songbird. Biol Lett 7:184–186
Claesson MJ, O’Sullivan O, Wang Q, Nikkilä J, Marchesi JR, Smidt H, de Vos WM, Ross RP, O’Toole PW (2009) Comparative analysis of pyrosequencing and a phylogenetic microarray for exploring microbial community structures in the human distal intestine. PLoS One 4(8):e6669
Ezaki T, Li N, Kawamura Y (2006) The anaerobic gram-positive cocci. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 795–808
Ezenwa VO, Williams AE (2014) Microbes and animal olfactory communication: where do we go from here? Bioessays 36:847–854
Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC (2005) Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964
Filipiak W, Sponring A, Baur MM, Filipiak A, Ager C, Wiesnhofer H, Nagl M, Troppmair J, Amann A (2012) Molecular analysis of volatile metabolites released specifically by Staphylococcus aureus and Pseudomonas aeruginosa. BMC Microbiol 12:113
Gerlach NM, McGlothlin JW, Parker PG, Ketterson ED (2012) Promiscuous mating produces offspring with higher lifetime fitness. Proc R Soc B 279:860–866
Giraudeau M, Duval C, Guillon N, Bretagnolle V, Gutierrez C, Heeb P (2010) Effects of access to preen gland secretions on mallard plumage. Naturwissenschaften 97:577–581
Gorman ML, Nedwell DB, Smith RM (1974) An analysis of the contents of the anal and scent pockets of Herpestes auropunctatus (Carnivora: Viverridae). J Zool 172:389–399
Green PN (2006) Methylobacterium. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 257–265
Groenhagen U, Baumgartner R, Bailly A, Gardiner A, Eberl L, Schultz S, Weisskopf L (2013) Production of bioactive volatiles by different Burkholderia ambifaria strains. J Chem Ecol 39:892–906
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Hagelin JC, Jones IL, Rasmussen LEL (2003) A tangerine-scented social odour in a monogamous seabird. Proc R Soc B 270:1323–1329
Hammer O (2011) PAST: PAleontological STatistics manual, 2.07 edn. Natural History Museum, Oslo, Norway
Hammer O, Harper DAT, Ryan PD (2001) PAST: PAleontological STatistics software package for education and data analysis. Palaeontol Electron 4:1–9
Jacob JP, Ziswiler V (1982) The uropygial gland. In: Farner DS, King JR, Parkes KC (eds) Avian biology, vol 6. Academic, New York, pp 199–324
Jones D, Keddie RM (2006) The genus Arthrobacter. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 945–960
Ketterson ED, Parker PG, Raouf SA, Nolan V Jr, Ziegenfus C, Chandler CR (1997) The relative impact of extra-pair fertilizations on variation in male and female reproductive success in dark-eyed juncos (Junco hyemalis). Ornithol Monogr 1997:81–101
Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120
Kulkarni S, Heeb P (2007) Social and sexual behaviours aid transmission of bacteria in birds. Behav Proc 74:88–92
Latorre-Moratalla ML, Bosche-Fusté J, Bover-Cid S, Aymerich T, Vidal-Carou MC (2011) Contribution of enterococci to the volatile profile of slightly-fermented sausages. LWT Food Sci Technol 44:145–152
Law-Brown J, Meyers PR (2003) Enterococcus phoeniculicola sp. nov., a novel member of the enterococci isolated from the uropygial gland of the red-billed woodhoopoe, Phoeniculus purpureus. Int J Syst Evol Microbiol 53:683–685
Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, Gibbons SM, Larsen P, Shogan BD, Weiss S, Metcalf JL, Urselll LK, Vázquez-Baeza Y, Van Treuren W, Hasan NA, Gibson MK, Colwell R, Dantas G, Knight R, Gilbert JA (2014) Longitudinal analysis of microbial interaction between humans and the indoor environment. Science 345:1048–1052
Leclaire S, Merkling T, Raynaud C, Mulard H, Bessière J-M, Lhuillier É, Hatch SA, Danchin É (2012) Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc R Soc B 279:1185–1193
Leclaire S, Nielsen JF, Drea CM (2014) Bacterial communities in meerkat anal scent secretions vary with host sex, age, and group membership. Behav Ecol 25:996–1004
Lemfack MC, Nickel J, Dunkel M, Preissner R, Piechulla B (2014) mVOC: a database of microbial volatiles. Nucleic Acids Res 42(1):D744–D748
López del Castillo-Lozano M, Mansour S, Tåche R, Bonnarme P, Landaud S (2008) The effect of cysteine on production of volatile sulphur compounds by cheese-ripening bacteria. Int J Food Microbiol 122:321–327
Madigan MT, Martinko JM, Stahl DA, Clark DP (2010) Brock biology of microorganisms, 13th edn. Benjamin Cummings, San Francisco
Mahenthiralingam E, Urban TA, Goldberg JB (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3:144–156
Mardon J, Saunders SM, Anderson MJ, Couchoux C, Bonadonna F (2010) Species, gender, and identity: cracking petrels’ sociochemical code. Chem Senses 35:309–321
Martín-Vivaldi M, Ruiz-Rodríguez M, Soler JJ, Peralta-Sánchez JM, Méndez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M (2009) Seasonal, sexual and developmental differences in hoopoe Upupa epops preen gland morphology and secretions: evidence for a role of bacteria. J Avian Biol 40:191–205
Martín-Vivaldi M, Peña A, Peralta-Sánchez JM, Sánchez L, Ananou S, Ruiz-Rodríguez M, Soler JJ (2010) Antimicrobial chemicals in hoopoe preen secretions are produced by symbiotic bacteria. Proc R Soc B 277:123–130
Moyer BR, Rock AN, Clayton DH (2003) Experimental test of the importance of preen oil in rock doves (Columba livia). Auk 120:490–496
Nolan V Jr, Ketterson ED, Cristol DA, Rogers CM, Clotfelter ED, Titus R, Schoech SJ, Snajdr E (2002) Dark-eyed junco (Junco hyemalis), vol 716, The birds of North America. The Birds of North America, Inc., Philadelphia, PA
Ramette A (2007) Multivariate analyses in microbial ecology. FEMS Microbiol Ecol 62:142–160
Roze U, Leung KT, Nix E, Burton G, Chapman DM (2010) Microanatomy and bacterial flora of the perineal glands of the North American porcupine. Can J Zool 88:59–68
Saag P, Tilgar V, Mänd R, Kilgas P, Mägi M (2011) Plumage bacterial assemblages in a breeding wild passerine: relationships with ecological factors and body condition. Microbiol Ecol 61:740–749
Saranathan V, Burtt EH (2007) Sunlight on feathers inhibits feather-degrading bacteria. Wilson J Ornithol 119:239–245
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541
Shaw CL, Rutter JE, Austin AL, Garvin MC, Whelan RJ (2011) Volatile and semivolatile compounds in gray catbird uropygial secretions vary with age and between breeding and wintering grounds. J Chem Ecol 37:329–339
Shawkey MD, Pillai SR, Hill GE (2003) Chemical warfare? Effects of uropygial oil on feather-degrading bacteria. J Avian Biol 34:345–349
Shawkey MD, Mills KL, Dale C, Hill GE (2005) Microbial diversity of wild bird feathers revealed through culture-based and culture-independent techniques. Microbiol Ecol 50:40–47
Shawkey MD, Hussain J, Hagelin JC, Vollmer AC, Hill GE (2006) Use of culture-independent methods to compare bacterial assemblages on feathers of crested and least auklets (Aethia cristatella and Aethia pusilla) with those of passerines. Waterbirds 29:507–510
Sheneman L, Evans J, Foster JA (2006) Clearcut: a fast implementation of relaxed neighbor joining. Bioinformatics 22:2823–2824
Sin YW, Buesching CD, Burke T, MacDonald DW (2012) Molecular characterization of the microbial communities in the subcaudal gland secretion of the European badger (Meles meles). FEMS Microbiol Ecol 81:648–659
Soini HA, Schrock SE, Bruce KE, Wiesler D, Ketterson ED, Novotny MV (2007) Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J Chem Ecol 33:183–198
Soini HA, Whittaker DJ, Wiesler D, Ketterson ED, Novotny M (2013) Chemosignaling diversity in songbirds: chromatographic profiling of preen oil volatiles in different species. J Chromatogr A 1317:186–192
Soler JJ, Martín-Vivaldi M, Ruiz-Rodríguez M, Valdivia E, Martín-Platero AM, Martínez-Bueno M, Peralta-Sánchez JM, Méndez M (2008) Symbiotic association between hoopoes and antibiotic-producing bacteria that live in their uropygial gland. Funct Ecol 22:864–871
Soler JJ, Martín-Vivaldi M, Peralta-Sánchez JM, Ruiz-Rodríguez M (2010) Antibiotic-producing bacteria as a possible defence of birds against pathogenic microorganisms. Open Ornithol J 3:93–100
Song SJ, Lauber CL, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, Gordon JI, Fierer N, Knight R (2013) Cohabiting family members share microbiota with one another and with their dogs. eLife 2:e00458
Spraker JE, Jewell K, Roze LV, Scherf J, Ndagano D, Beaudry R, Linz JE, Allen C, Keller NP (2014) A volatile relationship: profiling an inter-kingdom dialogue between two plant pathogens, Ralstonia solanacearum and Aspergillus flavus. J Chem Ecol 40:502–513
Theis KR, Schmidt TM, Holekamp KE (2012) Evidence for a bacterial mechanism for group-specific social odors among hyenas. Sci Rep 2:615
Theis KR, Venkataraman A, Dycus JA, Koonter KD, Schmitt-Matzen EN, Wagner AP, Schmidt TM, Holekamp KE (2013) Symbiotic bacteria appear to mediate hyena social odors. Proc Natl Acad Sci U S A 110:19832–19837
Towner K (2006) The genus Acinetobacter. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 746–758
Voigt C, Caspers B, Speck S (2005) Bats, bacteria, and bat smell: sex-specific diversity of microbes in a sexually selected scent organ. J Mammal 86:745–749
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Whittaker DJ, Reichard DG, Dapper AL, Ketterson ED (2009) Behavioral responses of nesting female dark-eyed juncos Junco hyemalis to hetero- and conspecific passerine preen oils. J Avian Biol 40:579–583
Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny MV, Ketterson ED (2010) Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav Ecol 21:608–614
Whittaker DJ, Richmond KM, Miller AK, Kiley R, Bergeon Burns C, Atwell JW, Ketterson ED (2011a) Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav Ecol 22:1256–1263
Whittaker DJ, Soini HA, Gerlach NM, Posto AL, Novotny MV, Ketterson ED (2011b) Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. J Chem Ecol 37:1349–1357
Whittaker DJ, Gerlach NM, Soini HA, Novotny MV (2013) Bird odour predicts reproductive success. Anim Behav 86:697–703
Acknowledgments
This work was supported by the BEACON Center for the Study of Evolution in Action (NSF Cooperative Agreement DBI-0939454). Fieldwork was supported by a Mountain Lake Biological Station Early Career Fellowship to DJW. All work was conducted in compliance with the Bloomington Institutional Animal Care and Use Committee guidelines (BIACUC protocol 09-037) and with permission from the US Department of Fish and Wildlife, the Virginia Department of Game and Inland Fisheries, and the US Forest Service. We thank Mountain Lake Biological Station, University of Virginia, as well as Mountain Lake Hotel and the Dolinger family for allowing research on their grounds. We thank Nicole Gerlach, Abby Kimmitt, Dustin Reichard, Samuel Slowinski, Sarah Wanamaker, and Joseph Welklin for assistance in the field, John Dover and Arvind Venkataraman for assistance with DNA extraction and sequence processing, and Ellen Ketterson for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Whittaker, D.J., Theis, K.R. (2016). Bacterial Communities Associated with Junco Preen Glands: Preliminary Ramifications for Chemical Signaling. In: Schulte, B., Goodwin, T., Ferkin, M. (eds) Chemical Signals in Vertebrates 13. Springer, Cham. https://doi.org/10.1007/978-3-319-22026-0_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-22026-0_8
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-22025-3
Online ISBN: 978-3-319-22026-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)