Abstract
Chemical signaling has been well studied in invertebrates and mammals but less so in birds, due to the longstanding misconception that olfaction is unimportant or even non-existent in this taxon. However, recent findings suggest that olfaction plays an important role in avian mate choice and reproductive behavior, similar to other taxa. The leading candidate source for compounds involved in avian chemical communication is preen oil, a complex mixture secreted from the uropygial gland. Preen oil contains volatile compounds and their potential wax ester precursors, and may act as a reproductive chemosignal. Reproductive signals are generally sexually dimorphic, age-specific, seasonally variable, and may also vary geographically. We tested whether preen oil meets these expectations by using gas chromatography to examine the wax ester composition of preen oil in song sparrows (Melospiza melodia). We found that the wax ester composition of preen oil was significantly different between sexes, age classes, seasons, and populations. Collectively, our results suggest that song sparrow preen oil meets the criteria of a chemical cue that may influence mate choice and reproduction. Our findings in song sparrows, which are sexually monomorphic in plumage, also parallel patterns described for dark-eyed juncos (Junco hyemalis), a closely related songbird with sexually dimorphic plumage. Behavioral tests are needed to confirm that song sparrows attend to the cues present in preen oil, but our findings support the increasingly accepted idea that chemical communication is common and widespread in birds as it is in other taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite early controversy surrounding avian olfaction, there is no longer any doubt that birds possess a fully functional olfactory system (Balthazart and Taziaux 2009; Caro and Balthazart 2010; Caro et al. 2015). Indeed, it is now widely accepted that birds use olfaction in a variety of contexts including navigation, food location, predator detection, nest location, and conspecific, kin, and mate recognition (Balthazart and Taziaux 2009; Bonadonna and Nevitt 2004; Caro and Balthazart 2010; Caspers and Krause 2013; Caro et al. 2015; Moreno-Rueda 2017). The role of olfaction in avian reproduction and mate choice is of particular interest, and a growing body of evidence supports the importance of this previously overlooked area of research (Caro et al. 2015).
In birds, the major source of body odor is preen oil, a complex mixture of waxy secretions produced by the uropygial gland and consisting of low boiling (low molecular) and high boiling (high molecular) components (Hagelin and Jones 2007; Caro et al. 2015). The chemical composition of preen oil can differ between the sexes, among individuals, and among species (Balthazart and Schoffeniels 1979; Jacob et al. 1979; Soini et al. 2007, 2013; Whittaker et al. 2010; Tuttle et al. 2014). Moreover, some birds exhibit sex, population, and conspecific odor preferences (Amo et al. 2012a; Bonadonna and Nevitt 2004; Whittaker et al. 2010, 2011;), indicating that birds can detect and respond to chemical information available in preen oil.
While avian chemical communication is enjoying a surge in research interest, much remains to be discovered (Hagelin and Jones 2007; Moreno-Rueda 2017), particularly concerning the role of chemical signaling in mate choice. In particular, although the order Passeriformes comprises over half of all extant bird species (Gill 2007) and has been well-represented in studies of avian mate choice (Andersson 1994; Andersson and Simmons 2006), we are just beginning to study reproductive chemosignaling in this diverse group (Caro et al. 2015; Whittaker et al. 2010). Reproductive chemosignals should differ between the sexes and may also vary seasonally, reflecting reproductive condition (Johansson and Jones 2007). Further, reproductive signals may vary geographically due to population differences in environment (e.g., diet), genotype, or their interaction (Johansson and Jones 2007; Whittaker et al. 2010).
We used gas chromatography with flame ionization detection (GC-FID) to characterize the wax ester composition of preen oil from song sparrows (Melospiza melodia), a sexually monomorphic and geographically widespread songbird. Wax esters are comprised of a fatty alcohol and fatty acid linked by an ester bond. We treated variation in the chemical composition of wax esters as a proxy for variation in the composition of preen oil-derived volatiles. That is, we expect variation in the wax ester composition to contribute to variation in odor.
We tested for differences between sexes, age classes, two geographically distinct populations, and between breeding- and post-breeding stages. We used gas chromatography-mass spectrometry (GC-MS) to identify the wax ester components of song sparrow preen oil and assessed which compounds likely contribute most to the group differences we observed. Here we report differences in wax ester composition of preen oil from breeding-stage males versus females, between post-breeding adults versus juveniles, between breeding populations, and between breeding versus post-breeding season. Our results show that preen oil wax esters in this species vary between sexes, age classes, populations, and seasons, and therefore could be precursors to volatiles that convey information salient to reproductive decision-making. Our findings provide the foundation for future behavioral experiments that will test whether passerine birds attend to the information available in preen oil.
Methods
Field Methods
Song sparrows were captured using seed-baited Potter traps and mist nets at two breeding locations in Ontario, Canada: a northeastern site on land owned by the Queen’s University Biological Station near Newboro (43.008°N, 81.291°W; hereafter Newboro) and a southwestern site at the rare Charitable Research Reserve near Cambridge (43.383°N, 80.357°W; hereafter Cambridge). These two sites are separated by 390 km, well beyond the mean range of juvenile dispersal for this species, which is estimated as about 6 km (Zink and Dittmann 1993). At each site, we captured song sparrows during the early part of the breeding season (hereafter breeding), which encompasses nest building and early egg laying (Newboro: April 12 – May 5 2016 and April 8 – May 3 2017; Cambridge: April 3 – May 1 2017), and during late summer (Newboro: July 15–28 2016; Cambridge: August 8–28 2016) after most chicks have fledged, and juveniles are largely independent (hereafter post-breeding).
In the field, we determined the age class and sex of each song sparrow captured. We used wing length, plumage, and gape characteristics to distinguish juveniles (hatch-year) from adults (after-hatch-year) and used wing length, together with the presence versus absence of a cloacal protuberance (male) or brood patch (female), to distinguish males from females. From each bird, we collected a small blood sample through brachial venipuncture for genetic analysis, and later confirmed sex for all birds using the P2-P8 PCR protocol described by Griffiths et al. (1998). Preen oil was collected by gently probing the uropygial gland with an unheparinized capillary tube until ~1–5 mg was expressed into the tube. Samples were kept on ice in the field and stored at −20 °C pending analysis. We fitted each bird with a numbered aluminum leg band (Canadian Wildlife Service: 10691).
In all, we collected 356 samples of preen oil. Breeding-stage samples were collected from 49 males and 41 females at Newboro in 2016; 48 males and 28 females at Newboro in 2017; and 48 males and 36 females at Cambridge in 2017. In 2016, we collected post-breeding samples from 26 adults (24 males, 2 females) and 28 juveniles (5 males, 18 females, and 5 that were not successfully sexed and were excluded from sex-specific analysis) at Newboro and from 16 adults (11 males, 5 females) and 36 juveniles (17 males, 17 females, and 2 that were not successfully sexed and were excluded from sex-specific analysis) at Cambridge.
Laboratory Methods
Preen oil samples were dissolved in 1–3 mL chloroform (CHCl3) and analyzed using an Agilent 7890A gas chromatograph with flame ionization detector (GC-FID), fitted with a 5% phenyl methyl siloxane column (Agilent Technologies DB-5, 30 m × 0.32 μm ID × 0.25 μm film thickness) as described previously (Slade et al. 2016). Briefly, 1 μL samples were injected with a 30 psi pressure pulse (1 min) and after an initial 1 min hold at 70 °C eluted with the following temperature profile: increase to 130 °C at 20 °C/min, then to 320 °C at 4 °C/min. The injector and FID temperatures were 200 °C and 310 °C, respectively. Hydrogen was used as a carrier gas at 2.5 mL/min. Each batch of GC-FID runs (typically 20–24) included a blank sample containing solvent only (CHCl3) and a sample of known composition (i.e., previously analyzed with both GC-FID and GC-MS (Slade et al. 2016)). Because the volume of preen oil collected varied across individuals, peak sizes were quantified based on the proportional peak size relative to total chromatogram peak area. Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero. Peaks were then standardized by total peak area per individual (Stoffel et al. 2015).
To provide preliminary identification of the wax esters present in preen oil, we performed GC-MS on a subset of 21 samples, balanced across groups (male versus female, adult versus juvenile, Newboro versus Cambridge, breeding versus post-breeding). GC-MS was performed on a Varian 3800 gas chromatograph connected to a Varian MS220 ion trap mass spectrometer. The GC parameters were the same as for GC-FID, except that helium (He) was used as a carrier gas at 1 mL/min. We identified monoesters based on the m/z of the protonated fatty acid fragments and parent ions (Thomas et al. 2010; Slade et al. 2016) and determined the fatty alcohol part by subtraction. We used GC-MS to compare the preen oil profiles of breeding males (N = 3) and females (N = 3), post-breeding males (N = 3) and females (N = 2), and post-breeding males, females, and juveniles (N = 4) from Newboro. To assess population differences, we also compared breeding males (N = 3) and females (N = 3) from Cambridge with those from Newboro (Supplementary Material, Figs S1 – S6). Due to low sample sizes per group, we did not perform statistical analyses but instead visually identified peaks that might contribute to the group differences found using GC-FID, and quantified peak sizes based on the proportional peak area relative to total chromatogram peak area. Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero.
Statistical Analyses
To prevent large chromatogram peaks from disproportionately affecting distance measures, we normalized chemical data using the ‘range’ method in the deconstand function in the R package VEGAN (Dixon and Palmer 2003) following previous studies (Leclaire et al. 2012; Slade et al. 2016). We then log(x + 1) transformed the GC-FID data on preen oil wax ester composition, and constructed pairwise matrices of Bray-Curtis dissimilarity. Chemical distances (i.e., Bray-Curtis dissimilarities) between samples were visualized using nonmetric multidimensional scaling (NMDS). This approach places each sample on a two-dimensional scatter plot, preserving ranked pairwise distances such that two points close together represent two individuals with relatively similar chemical composition while points further apart represent individuals that are more dissimilar (Clarke 1999; Stoffel et al. 2015).
To assess the statistical significance of differences between groups (males versus females, adults versus juveniles, Newboro versus Cambridge, breeding- versus post-breeding), we used nonparametric analysis of similarities (ANOSIM), implemented in the R package VEGAN (Dixon and Palmer 2003) with 10,000 iterations. This permutation approach does not make assumptions about the data’s distribution (Clarke 1999; Stoffel et al. 2015). To minimize effects of year-to-year variation within an analysis, our analyses of sex and population differences were restricted to samples from breeding-stage adults collected in 2017. Similarly, we tested for adult versus juvenile differences using samples collected during post-breeding 2016; and for breeding versus post-breeding differences using samples collected at Newboro in 2016.
All analyses were performed in R version 3.2.3 “Wooden Christmas-Tree” (R Development Core Team 2017). As noted above, for the GC-MS dataset we report qualitative rather than quantitative differences among groups.
Results
Our GC-MS and GC-FID analysis on 21 samples revealed that the wax esters comprising song sparrow preen oil represented at least two homologous series of differently methyl branched fatty alcohols (C18 – C25) and differently methyl branched fatty acids (C12 – C19) esterified in different combinations to form monoesters with a total carbon number of C30 – C38. We characterised 53 unique wax esters and detected a characteristic pattern of clearly separated doublet peaks (denoted A and B; Fig. 1) having the same total number of carbons. For a given carbon number and molecular weight, peaks of series A and B were largely comprised of distinct mixtures of up to 13 isomeric monoesters, with some variation in the proportions of each component (Supplementary Material, Table S1).
Sex Differences
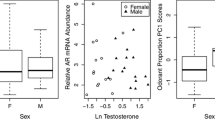
Among adult song sparrows sampled during breeding, we found significant sex differences in the wax ester composition of preen oil at both sites (ANOSIM; Newboro: global R = 0.313, P < 0.0001, Fig. 2; Cambridge: global R = 0.252, P < 0.0001; all samples collected in 2017). By contrast, sex differences were not detected in post-breeding adults at either site (ANOSIM; Newboro: global R = 0.200, P = 0.178; Cambridge: global R = 0.062, P = 0.269; all samples collected in 2016). However, it should be noted that post-breeding sample sizes for adult females were very low (N = 2 and 5 for Newboro and Cambridge respectively) and this reduces statistical power.
Wax ester composition of song sparrow preen oil from breeding-stage males and females. The figure shows a two-dimensional nonmetric multidimensional scaling (NMDS) plot indicating Bray-Curtis chemical similarity: each symbol represents an individual (sampled at Newboro, 2017), and points appearing closer together are more chemically similar. Axis scales are arbitrary. 2D stress represents the amount of disagreement between the 2D configuration and predicted values from the multivariate regression (values closer to zero are better)
In our qualitative GC-MS comparison of breeding-stage males versus females (samples collected at Newboro), we noted certain low-abundance compounds that appeared elevated in males relative to females. These compounds were C18 – C22 and C25 fatty alcohols esterified to C12 fatty acids (hereafter denoted by C#alcohol:C#acid; e.g., C18 – C22:C12, C25:C12). In contrast, relative to breeding-stage males, breeding-stage females appeared to have elevated C17:C13 – C15 wax esters. We also observed sex differences, albeit less dramatic, in C18 – C19:C13 esters (elevated somewhat in breeding-stage females relative to males) and C17:C13 and C17 – C18:C17 esters (elevated somewhat in breeding-stage males relative to females; Supplementary Material, Figs. S1, S2).
Age Differences
At both sites, preen oil wax ester composition was significantly different between adults and juveniles (ANOSIM; Newboro: global R = 0.069, P = 0.016, Fig. 3; Cambridge: global R = 0.253, P < 0.001; all samples collected during post-breeding, 2016). However, despite these statistically significant differences in preen oil composition, our review of GC-MS profiles showed no obvious candidate substances differing in relative abundance between adults and juveniles (Supplementary Material, Fig. S3).
Wax ester composition of song sparrow preen oil from post-breeding stage adults and juveniles (sexes pooled within each age class; sampled at Newboro). The figure shows a two-dimensional nonmetric multidimensional scaling (NMDS) plot indicating Bray-Curtis chemical similarity (see Fig. 2 for details). For complete wax ester composition, see Supplementary Material, Fig. S3
Population Differences
Comparing the wax ester composition of males and females from Newboro and Cambridge identified significant differences between these four groups (ANOSIM: global R = 0.267, P < 0.0001, Fig. 4; all samples collected from breeding-stage adults, 2017). To disentangle sex differences from site differences, we repeated this analysis on the same dataset but pooled sexes within each site. Again, we observed significant differences in wax ester composition of breeding-stage adults from Newboro versus Cambridge (ANOSIM: global R = 0.084, P < 0.0001; all samples collected in 2017). In contrast, population differences were not observed in adults sampled post-breeding (ANOSIM: global R = 0.008, P = 0.381; all samples collected in 2017).
Wax ester composition of song sparrow preen oil from different populations (breeding-stage adults sampled at Newboro and Cambridge, 2017). The figure shows a two-dimensional nonmetric multidimensional scaling (NMDS) plot indicating Bray-Curtis chemical similarity (see Fig. 2 for details)
In our qualitative GC-MS comparison of breeding stage samples for Newboro versus Cambridge, we noted that the C17:C13 ester was elevated in the Newboro females relative to Newboro males and both sexes from Cambridge. Similarly, a C19:C15 ester was elevated in the Cambridge females only. Cambridge males showed elevated levels of C18 – C19:C13 esters relative to all other groups but lacked a number of esters (C22:C13, C22:C15, C23:15) that were present at low abundance in Newboro males. The C17:C14 ester was elevated in males from both populations, relative to females (Supplementary Material, Figs. S4, S5).
Seasonal Differences
Breeding versus post-breeding stage adults differed significantly in wax ester composition of preen oil (sexes pooled, ANOSIM: global R = 0.840, P < 0.0001, Fig. 5; all samples collected in Newboro, 2016). In our qualitative GC-MS comparison of breeding versus post-breeding stage adults at the Newboro site, we noted the low abundance compounds that were elevated in males relative to females during breeding (C18 – C22:C12 and C25:C12 wax esters) were absent in both males and females post-breeding. Similarly, levels of the C17:C13 wax ester (elevated in females relative to males during breeding) were dramatically lower post-breeding, especially in females. Levels of the C17:C14 – C15 esters also decreased in females from breeding to post-breeding stages. In both sexes, levels of C20:C14, C20:C16, C19:C16, and C19:C18 wax esters were higher post-breeding than during breeding (Supplementary Material, Figs. S6, S7).
Wax ester composition of song sparrow preen oil from different times in the season (sexes pooled, breeding and post-breeding samples collected at Newboro, 2016). The figure shows a two-dimensional nonmetric multidimensional scaling (NMDS) plot indicating Bray-Curtis chemical similarity (see Fig. 2 for details)
Discussion
Preen oil wax ester profiles differed significantly between breeding-stage males and females, adults and juveniles, breeding populations, and between breeding and post-breeding stages.
Sex Differences
We observed significant sex differences in the wax ester profiles of song sparrows during the breeding season (April through early May), a time period corresponding at these sites to birds returning from spring migration, establishing territories, pairing, constructing nests, and laying eggs for their first brood. In particular, we noted an increase in shorter chain fatty acids (especially C12) in the wax esters of male song sparrows. These differences were replicated across two breeding sites, but were no longer evident in the post-breeding period (July to August), corresponding to the post-fledging period when juveniles are becoming independent. Sample sizes (particularly for females) were low during post-breeding, and this latter result should thus be interpreted with caution. That said, in a separate study conducted at a third breeding site and with a larger sample size, we similarly found no sex differences in preen oil wax ester composition for song sparrows sampled during late summer (Grieves et al. 2018).
Evidence for sex differences in preen oil chemical composition is mixed across bird species. Sex differences have been reported for domestic ducks, Anas platyrhynchos (Jacob et al. 1979); Sandpipers, Scolopacidae (Reneerkens et al. 2002); dark-eyed juncos, Junco hyemalis (Whittaker et al. 2010); budgerigars, Melopsittacus undulatus (Zhang et al. 2010); black-legged kittiwakes, Rissa tridactyla (Leclaire et al. 2011); spotless starlings, Sturnus unicolor (Amo et al. 2012a), and house finches, Carpodacus mexicanus (Amo et al. 2012b) but not for crested auklets, Aethia cristatella (Hagelin et al. 2003); rock pigeons, Columbia livia (Salibian and Montalti 2009); New Zealand silvereyes, Zosterops lateralis (Azzani et al. 2016); Cory’s shearwaters, Calonectris borealis and Scopoli’s shearwaters, C. diomedea (Gabirot et al. 2016). Our finding that the wax ester composition of song sparrow preen oil differs between the sexes during breeding but not post-breeding indicates that seasonal effects are important to consider. Moreover, with the exception of black-legged kittiwakes all of the species listed above for which sex differences in preen oil chemistry have been found are sexually dimorphic. In contrast, all of the species for which sex differences in preen oil chemistry have not been found are sexually monomorphic. While this is not an exhaustive survey of the literature, it does suggest that plumage dimorphism broadly predicts chemical dimorphism, and that our finding of sex differences in preen oil chemistry in a monomorphic songbird is novel.
Age Differences
We detected subtle but statistically significant differences in the wax ester profiles of post-breeding adults compared to juvenile song sparrows. However, GC-MS did not reveal any candidate compounds dramatically elevated in one age class relative to the other.
Population Differences
We detected significant differences in the wax ester profiles of two geographically distinct breeding populations of song sparrow. Chemical stimuli are important in maintaining reproductive isolation in many taxa, but evidence for this function in birds is lacking (Caro et al. 2015; Smadja and Butlin 2009). However, differences in preen oil chemistry have been detected between two recently diverged populations of dark-eyed junco (Whittaker et al. 2010), suggesting that chemical stimuli may function as isolating mechanisms in birds as in other taxa (LeMaster and Mason 2003; Martín and López 2006; Smadja and Butlin 2009; Whittaker et al. 2010).
Seasonal Differences
We found significant differences in preen oil when we compared samples collected during breeding versus post-breeding, consistent with several other studies: domestic duck, Anas platyrhynchos (Jacob et al. 1979); Sandpipers, Scolopacidae (Reneerkens et al. 2002); Emberizidae (7 species), Corvidae (2 species), Mimidae (1 species) (Haribal et al. 2005); dark-eyed junco, Junco hyemalis (Soini et al. 2007); white-throated sparrow, Zonotrichia albicollis (Tuttle et al. 2014); herring gull, Larus argentatus (Fischer et al. 2017). This result may also support our finding that sex differences diminish by the end of breeding and may help to explain why some studies have failed to detect sex differences in preen oil. Sex differences in preen oil may be driven by seasonal changes in female physiology (Jacob et al. 1979); however, findings from sandpipers (Scolopacidae) suggest that chemical changes in preen oil may be influenced by the incubating sex rather than females specifically (Reneerkens et al. 2002). Additionally, seasonal changes in preen oil composition may play a role in nest defense via chemical crypsis (Reneerkens et al. 2002, 2005).
Avian preen oil is commonly made up of mixtures of large monoester waxes comprised of straight chain and methyl branched fatty acids esterified to long-chain monohydroxy fatty alcohols (which can also be straight chain or branched). The diversity of carbon chain lengths and methylation patterns can lead to mixtures containing hundreds of compounds (Campagna et al. 2012). The wax esters we detected in song sparrows consisted of monoesters with both even and odd total carbon numbers (hereafter “even-numbered” and “odd-numbered”, respectively). Even-numbered waxes included both even-numbered alcohols esterified to even-numbered fatty acids and odd-numbered alcohols esterified to odd-numbered fatty acids. Conversely, odd-numbered waxes included even-numbered alcohols esterified to odd-numbered fatty acids, as well as odd-numbered alcohols esterified to even-numbered fatty acids. These patterns are similar to those reported by Thomas et al. (2010) for the closely related white-throated sparrow. In all, we characterized four even-numbered and six odd-numbered alcohols and four even-numbered and four odd-numbered fatty acids, esterified in the combinations described above.
The presence of odd-numbered alcohols and fatty acids suggests that the chemical components of song sparrow preen oil may contain multiple methyl branches. In some avian families, for example in the red knots (Calidris canutus, order Charadriiformes), odd-numbered esters are predominantly composed of even-numbered alcohols esterified to odd-numbered fatty acids (Dekker et al. 2000). In the case of even-numbered carbon waxes, odd- and even-numbered carbon alcohols were roughly equal in number, and various isomers of branched fatty acids were detected with increasing molecular mass (Dekker et al. 2000). In songbirds, branched alcohols have been found in several species, including northern mockingbirds (Mimus polyglottos) and Carolina chickadees (Poecile carolinensis) (Soini et al. 2013). Thus, our findings are not unprecedented for songbirds.
Due to the complexity of avian preen oil, the structural identification of each individual component is rarely achieved (Campagna et al. 2012). Consequently, it is common to instead qualitatively analyze chromatographic profiles (Campagna et al. 2012). While we were unable to pursue detailed structural analysis of each preen wax ester, we used GC-MS and proportional analysis to measure the chromatographic profiles of avian preen oil for a subset of our data. Because of sample size constraints, we did not perform any statistical analysis on these data.
Using thermal-desorption-cooled injection gas chromatography-mass spectrometric analysis of organic compounds extracted from preen oil by solid phase extraction, Soini et al. (2013) identified many of the same fatty acids and fatty alcohols we found in intact preen oil wax esters, but especially the lower molecular weight compounds (e.g., C12-C18 1-alkanols and C12, C14 and C16 fatty acids). Our analysis allowed us to measure intact preen oil using standard GC-FID equipment. The column and temperature profile we used are suitable for both volatile components and intact wax esters; however, we found no direct evidence for the presence of low-boiling components in our preen oil samples.
Conclusion
The wax ester composition of song sparrow preen oil differs between the sexes, between adults and juveniles, between populations, and breeding stages. This variation, together with the identification of specific compounds that vary among these groups, provides the foundation for future behavioral experiments on chemosignaling in this and other songbird species, particularly with respect to mate choice and reproduction.
References
Amo L, Avilés JM, Parejo D, Peña A, Rodríguez J, Tomás G (2012a) Sex recognition by odour and variation in the uropygial gland secretion in starlings. J Anim Ecol 81:605–613. https://doi.org/10.1111/j.1365-2656.2011.01940.x
Amo L, López-Rull I, Pagán I, Garcia CM (2012b) Male quality and conspecific scent preferences in the house finch, Carpodacus mexicanus. Anim Behav 84:1483–1489
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton.
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Azzani L, Rasmussen JL, Gieseg SP, Briskie JV (2016) An experimental test of the effect of diet on preen wax composition in New Zealand silvereyes (Zosterops lateralis). In: Schulte BA, Goodwin TE, Ferkin MH (eds) Chemical Signals in Vertebrates 12. Springer, New York, pp 511–525
Balthazart J, Schoffeniels E (1979) Pheromones are involved in the control of sexual behaviour in birds. Naturwissenschaften 66:55–56
Balthazart J, Taziaux M (2009) The underestimated role of olfaction in avian reproduction? Behav Brain Res 200:248–259. https://doi.org/10.1016/j.bbr.2008.08.036
Bonadonna F, Nevitt GA (2004) Partner-specific odor recognition in an Antarctic seabird. Science 306:835–835. https://doi.org/10.1126/science.1103001
Campagna S, Mardon J, Celerier A, Bonadonna F (2012) Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies. Chem Senses 37:3–25
Caro SP, Balthazart J (2010) Pheromones in birds: myth or reality? J Comp Physiol A 196:751–766. https://doi.org/10.1007/s00359-010-0534-4
Caro SP, Balthazart J, Bonadonna F (2015) The perfume of reproduction in birds: chemosignaling in avian social life. Horm Behav 68:25–42
Caspers BA, Krause ET (2013) Intraspecific olfactory communication in zebra finches (Taeniopygia guttata): potential information apart from visual and acoustic cues. In: East ML, Dehnhard M (eds) Chemical Signals in Vertebrates 12. Springer, New York, pp 341–351
Clarke KR (1999) Nonmetric multivariate analysis in community-level ecotoxicology. Environ Toxicol Chem 18:118–127. https://doi.org/10.1002/etc.5620180205
Dekker MH, Piersma T, Damsté JSS (2000) Molecular analysis of intact preen waxes of Calidris canutus (Aves: Scolopacidae) by gas chromatography/mass spectrometry. Lipids 35:533–541
Dixon P, Palmer MW (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930
Fischer I, Haliński LP, Meissner W, et al (2017) Seasonal changes in the preen wax composition of the Herring gull Larus argentatus. Chemoecology 27:127–139
Gabirot M, Raux L, Dell’Ariccia G et al (2016) Chemical labels differ between two closely related shearwater taxa. J Avian Biol 47:540–551
Gill FB (2007) Ornithology, 3rd edn. W. H. Freeman, New York
Grieves LA, Kelly TR, Bernards MA, MacDougall-Shackleton EA (2018) Malarial infection alters wax ester composition of preen oil in songbirds: results of an experimental study. Auk 135:767–776. https://doi.org/10.1642/AUK-17-242.1
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Hagelin JC, Jones IL (2007) Bird odors and other chemical substances: a defense mechanism or overlooked mode of intraspecific communication? Auk 124:741–761. https://doi.org/10.1642/0004-8038(2007)124[741:BOAOCS]2.0.CO;2
Hagelin JC, Jones IL, Rasmussen LEL (2003) A tangerine-scented social odour in a monogamous seabird. Proc R Soc Lond B Biol Sci 270:1323–1329
Haribal M, Dhondt AA, Rosane D, Rodriguez E (2005) Chemistry of preen gland secretions of passerines: different pathways to same goal? Why? Chemoecology 15:251–260
Jacob J, Balthazart J, Schoffeniels E (1979) Sex differences in the chemical composition of uropygial gland waxes in domestic ducks. Biochem Syst Ecol 7:149–153
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289. https://doi.org/10.1111/j.1469-185X.2007.00009.x
Leclaire S, Merkling T, Raynaud C, Giacinti G, Bessière JM, Hatch SA, Danchin É (2011) An individual and a sex odor signature in kittiwakes? Study of the semiochemical composition of preen secretion and preen down feathers. Naturwissenschaften 98:615–624
Leclaire S, Merkling T, Raynaud C, Mulard H, Bessiere JM, Lhuillier E, Hatch SA, Danchin E (2012) Semiochemical compounds of preen secretion reflect genetic make-up in a seabird species. Proc R Soc B Biol Sci 279:1185–1193. https://doi.org/10.1098/rspb.2011.1611
LeMaster MP, Mason RT (2003) Pheromonally mediated sexual isolation among denning populations of red-sided garter snakes, Thamnophis sirtalis parietalis. J Chem Ecol 29:1027–1043
Martín J, López P (2006) Interpopulational differences in chemical composition and chemosensory recognition of femoral gland secretions of male lizards Podarcis hispanica: implications for sexual isolation in a species complex. Chemoecology 16:31–38
Moreno-Rueda G (2017) Preen oil and bird fitness: a critical review of the evidence. Biol Rev 92:2131–2143
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reneerkens J, Piersma T, Damsté JSS (2002) Sandpipers (Scolopacidae) switch from monoester to diester preen waxes during courtship and incubation, but why? Proc R Soc Lond B Biol Sci 269:2135–2139
Reneerkens J, Piersma T, Damsté JSS (2005) Switch to diester preen waxes may reduce avian nest predation by mammalian predators using olfactory cues. J Exp Biol 208:4199–4202. https://doi.org/10.1242/jeb.01872
Salibian A, Montalti D (2009) Physiological and biochemical aspects of the avian uropygial gland. Braz J Biol 69:437–446
Slade JWG, Watson MJ, Kelly TR, Gloor GB, Bernards MA, MacDougall-Shackleton EA (2016) Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc R Soc B Biol Sci 283:20161966
Smadja C, Butlin RK (2009) On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102(77):77–97
Soini HA, Schrock SE, Bruce KE, Wiesler D, Ketterson ED, Novotny MV (2007) Seasonal variation in volatile compound profiles of preen gland secretions of the dark-eyed junco (Junco hyemalis). J Chem Ecol 33:183–198. https://doi.org/10.1007/s10886-006-9210-0
Soini HA, Whittaker DJ, Wiesler D, Ketterson ED, Novotny MV (2013) Chemosignaling diversity in songbirds: chromatographic profiling of preen oil volatiles in different species. J Chromatogr A 1317:186–192
Stoffel MA, Caspers BA, Forcada J, Giannakara A, Baier M, Eberhart-Phillips L, Müller C, Hoffman JI (2015) Chemical fingerprints encode mother–offspring similarity, colony membership, relatedness, and genetic quality in fur seals. Proc Natl Acad Sci 112:E5005–E5012
Thomas RH, Price ER, Seewagen CL et al (2010) Use of TLC-FID and GC-MS/FID to examine the effects of migratory state, diet and captivity on preen wax composition in white-throated sparrows Zonotrichia albicollis. Ibis 152:782–792
Tuttle EM, Sebastian PJ, Posto AL, Soini HA, Novotny MV, Gonser RA (2014) Variation in preen oil composition pertaining to season, sex, and genotype in the polymorphic white-throated sparrow. J Chem Ecol 40:1025–1038
Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny MV, Ketterson ED (2010) Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav Ecol 21:608–614. https://doi.org/10.1093/beheco/arq033
Whittaker DJ, Richmond KM, Miller AK, Kiley R, Bergeon Burns C, Atwell JW, Ketterson ED (2011) Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav Ecol 22:1256–1263. https://doi.org/10.1093/beheco/arr122
Zhang J-X, Wei W, Zhang J-H, Yang W-H (2010) Uropygial gland-secreted alkanols contribute to olfactory sex signals in budgerigars. Chem Senses 35:375–382
Zink RM, Dittmann DL (1993) Gene flow, refugia, and evolution of geographic variation in the song sparrow (Melospiza melodia). Evolution 47:717–729
Acknowledgments
We thank the rare Charitable Research Reserve and the Queen’s University Biological Station (QUBS) for access to their land and research facilities. We further acknowledge that the land we used is on the traditional territories of the Anishinabek and Haudenosauneega (Iroquois) peoples, Haldimand Treaty and Simcoe Patent Treaty lands (Cambridge) and on the traditional territories of the Anishinabek, Huron-Wendat, and Mohawk peoples and Crawford Purchase lands (Newboro). We thank Tosha Kelly and Ross Dickson for field assistance and sample collection. This research was supported by an Ontario Graduate Scholarship and a Vanier Scholarship to LAG, and NSERC Discovery Grants to MAB and EAMS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All birds were captured under permission from the Canadian Wildlife Service (banding subpermits 10691B, E, F). All animal procedures were approved by The University of Western Ontario Animal Use Subcommittee (protocol # 2016–017).
Electronic supplementary material
Table S1
(DOCX 42 kb)
Fig. S1
Complete wax ester composition of breeding stage adult song sparrow preen oil at Newboro (Nfemales = 3, Nmales = 3, mean ± SD). Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero (see text for details). (PNG 30 kb)
Fig. S2
Selected wax ester composition of breeding stage adult song sparrow preen oil at Newboro (Nfemales = 3, Nmales = 3, mean ± SD). For complete wax ester composition, see Supplementary Material, Fig. S1. (PNG 43 kb)
Fig. S3
Complete wax ester composition of song sparrow preen oil from post-breeding stage adults and juveniles (sexes pooled for juveniles; sampled at Newboro). Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero (see text for details). (PNG 25 kb)
Fig. S4
Complete wax ester composition of breeding-stage song sparrow preen oil at Newboro and Cambridge (NNewboro males = 3, NNewboro females = 3, NCambridge males = 3, NCambridge females = 3, mean ± SD). Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero (see text for details). (PNG 31 kb)
Fig. S5
Selected wax ester composition of breeding-stage song sparrow preen oil at Newboro and Cambridge (NNewboro males = 3, NNewboro females = 3, NCambridge males = 3, NCambridge females = 3, mean ± SD). For complete wax ester composition, see Supplementary Material, Fig. S4. (PNG 85 kb)
Fig. S6
Complete wax ester composition of adult song sparrow preen oil sampled during breeding and post-breeding stages at Newboro (Nearly-season females = 3, Nlate-season females = 2, Nearly-season males = 3, Nlate-season males = 3, mean ± SD). Peaks that were at least 0.1% of the total chromatogram area were retained for analysis, while peaks that were <0.1% were counted as zero (see text for details). (PNG 31 kb)
Fig. S7
Selected wax ester composition of adult song sparrow preen oil sampled during breeding and post-breeding stages at Newboro (Nearly-season females = 3, Nlate-season females = 2, Nearly-season males = 3, Nlate-season males = 3, mean ± SD). For complete wax ester composition, see Supplementary Material, Fig. S6. (PNG 101 kb)
Rights and permissions
About this article
Cite this article
Grieves, L.A., Bernards, M.A. & MacDougall-Shackleton, E.A. Wax Ester Composition of Songbird Preen Oil Varies Seasonally and Differs between Sexes, Ages, and Populations. J Chem Ecol 45, 37–45 (2019). https://doi.org/10.1007/s10886-018-1033-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-018-1033-2