Abstract
Visual and auditory signals are well-established components of avian courtship, but the role of chemical signaling remains poorly understood, particularly in mating systems with elaborate courtship displays. To test how chemical cues influence mating behavior we conducted two experiments in the lance-tailed manakin (Chiroxiphia lanceolata). First, in a field experiment, we tested the response of free-living males and females to manipulation of chemical cues at male display perches by cleaning the perches with ethanol or a dry cloth (control) and quantified a potential chemical signaling behavior (bill-wiping) in response to experimental manipulation. During bouts of dance perch maintenance and displays with a female present (i.e., activity relevant to female mate choice) males increased bill-wiping behavior during initial ethanol treatment periods. We also detected carryover effects of the ethanol treatment; in later treatment periods males bill-wiped more when the prior treatment was ethanol. The likelihood of a female either revisiting a display area or copulating with a male was unrelated to experimental treatment. Next, in captive trials, we assessed female preference for olfactory cues from males that differed in their genetic diversity, a trait previously identified as relevant to female mate choice. In contrast to similar trials in other bird species, females showed no clear preference. Together, these results provide some evidence for chemical signaling by males at display perches, but it remains unclear what information chemical cues convey.
Significance statement
Chemical communication is an understudied phenomenon in birds, and especially so when it occurs as part of complex courtship displays. We investigated chemical signaling in the mating behavior of a highly polygynous bird with elaborate courtship displays by experimentally altering the chemical cues at courtship sites that are actively maintained by males. Male lance-tailed manakins detect and respond to changes in the chemical environment on display perches, however we detected no response from females. Our results contrast with other studies that detected significant responses to chemical cues by both males and females and suggest that association time with chemical cues may be important to their detection in avian systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemical communication plays an important role in animal behavior across a wide variety of taxa from bacteria to mammals (Searcy and Nowicki 2005). Chemical cues may influence mating behavior by indicating species, sex, or readiness to mate, and can also provide information about genetic characteristics (Johansson and Jones 2007). In particular, information about individual genetic diversity or genetic compatibility gleaned from chemical cues may be influential in mating decisions (Penn 2002; Charpentier et al. 2008). For free-living organisms, chemical cues often co-occur with visual, auditory, or tactile information that can also influence mating behavior (Candolin 2003). Investigating chemical communication in natural settings provides an opportunity to understand its overall importance in behavioral interactions.
In many non-resource based polygynous mating systems, males maintain individual display areas, courts, or bowers that are visited by females (Andersson 1991; Borgia 1995; Schaedelin and Taborsky 2006), providing an excellent opportunity for testing hypotheses about chemosensory communication in natural mating contexts. Males spend much of their time maintaining these display areas and respond to manipulation of objects or disruption of clearings at the display area (Schaedelin and Taborsky 2006; Endler et al. 2010; Chiver and Schlinger 2017). As display courts are the location of displays for females, cues in a variety of signaling modalities at the display area may influence female mate choice decisions (Robson et al. 2005), and male court maintenance behaviors when females are not present may also be important. For example, in satin bowerbirds (Ptilonorhynchus violaceus) males add salivary “paint” to the inside of their bowers, and females taste these deposits while assessing males (Keagy et al. 2016). Experimental removal of bower paint reduces the likelihood that a female will revisit the display area or copulate with the male, suggesting that the paint is a chemical signal used by females for mate choice decisions (Hicks et al. 2013).
Until recently, olfactory chemical cues were considered an insignificant part of birds’ lives. Instead, the majority of research on avian sensory capacity has focused on auditory and visual communication (Searcy and Nowicki 2005). We now know that chemosensory abilities, including olfaction, can be important in avian foraging (Potier et al. 2019), nest identification (Bonadonna and Hesters 2003; Caspers and Krause 2011), and social contexts such as kin recognition (Bonadonna and Sanz-Aguilar 2012) and mate choice (Whittaker et al. 2011; Grieves et al. 2019a; Van Huynh and Rice 2019). The uropygial gland is a primary source of chemical cues in birds (Caro et al. 2015; Whittaker and Hagelin 2021). Most birds have a uropygial, or “preen”, gland located above their tail which secretes a waxy substance that is spread over feathers while preening. Compounds in preen oil function in feather maintenance, and volatile components in preen oil may serve as olfactory cues (Caro et al. 2015; Alves Soares et al. 2023).
Y-maze trials are a commonly used method for measuring avian scent preference and mating-relevant responses to preen gland volatiles. Such binary choice trials have demonstrated olfactory preferences based on species (Krause et al. 2014), sex (Amo et al. 2012), and genotype (Leclaire et al. 2017; Grieves et al. 2019b). For example, in song sparrows (Melospiza melodia), the chemical composition of preen oil covaries with individual Major Histocompatibility Complex (MHC) genotype (Slade et al. 2016), and both males and females show preferences in binary choice trails for preen oil samples from individuals with more diverse and dissimilar MHC (Grieves et al. 2019b). Most research on avian chemical communication has focused on socially monogamous species, and less is known about how chemical cues influence mating behavior in highly polygynous species. Chemical cues may provide information about genetic characteristics of males, which may be important in highly polygynous systems where females receive no direct benefits from males.
Lance-tailed manakins (Chiroxiphia lanceolata) are a highly polygynous passerine bird species that ranges from southern Costa Rica to northern Venezuela. Males of this species maintain display areas that are within auditory, but not visual, contact of other display areas, cryptic females visit prospective mates which exhibit brightly colored plumage and conspicuous displays, and females raise young with no paternal assistance (DuVal 2007a). Male lance-tailed manakins are highly social and form long-term cooperative partnerships, consisting of a dominant “alpha” male and a subordinate “beta” male (DuVal 2007b). Alpha males perform the solo components of the courtship display that immediately precede copulation and sire almost all offspring in the population (DuVal and Kempenaers 2008). In addition to the alpha-beta partners, display areas are also frequently attended by “non-territorial” adult males and juvenile males, however these males do not participate in courtship displays when females are present at the display area (DuVal 2007b). Previous research in this system found no obvious link between specific display components and mate choice, highlighting the need to consider traits that are less salient to human observers in systems characterized by elaborate courtship displays (Vanderbilt 2019). In lance-tailed manakins, male age and experience are the best known predictors of male reproduction (DuVal and Kempenaers 2008) and male multi-locus heterozygosity also correlates with genetic siring success and offspring survival (Sardell et al. 2014). The relationship between male heterozygosity and reproductive success appears to result from active female choice for males based on heterozygosity or traits associated with heterozygosity. Male heterozygosity does not predict which males in the population a female will visit while searching for a mate, but females tend to mate with the most heterozygous male out the set of males they assess (EHD, unpubl. data). Olfactory cues from preen oil volatile compounds may provide close-range information on male genetic characteristics relevant to female mate choice in this system, as the composition of volatile compounds in male preen oil is correlated with male multi-locus heterozygosity (Whittaker et al. 2019a).
Much male activity and all courtship displays in this species center on a “dance perch,” a low horizontal branch that is the candidate venue for chemical communication such as the transmission of olfactory cues. Males maintain their preferred dance perches by clearing surrounding vegetation and frequently wiping their bill on or pecking at the perch (DuVal 2007a). Bill-wiping may transfer uropygial gland secretions (Whittaker et al. 2015), or other potential chemical cues such as saliva, to the perch and may also manipulate chemical cues already on the dance perch, providing an olfactory cue that could be used by females when assessing males. Female lance-tailed manakins perch on the dance perch while males perform many of the components of the highly complex courtship display, and copulation occurs on the dance perch (DuVal 2007a), therefore display perches are a likely candidate region for the deposition and reception of chemical cues in this system. Mate choice is an important component of reproduction in this system: females visit multiple males prior to choosing a mate and often repeatedly visit a particular male before copulating (DuVal and Kapoor 2015). Females are also observed regularly wiping their bills on the display perch as they attend male displays. Female bill-wiping could function in manipulating the preen oil that males have applied to dance perches to release volatile compounds, allowing the female to “smell” the males she is assessing (Whittaker et al. 2015). Females may also be adding preen oil or other chemical signaling compounds to the display perch for male assessment. Alternatively, bill-wiping by both females and males could instead or also be an indication of general excitement that is unrelated to the scent profile of the perch (Clark 1970).
To understand the role of chemical, and specifically olfactory, signaling in mating behavior in this species, we conducted two controlled field experiments. We first hypothesized that males manipulate the olfactory environment at display perches for visits by females, and chemical cues at the display perch influence female mate choice. We experimentally manipulated potential chemical cues at male display perches by cleaning them with ethanol and monitored male behavior in response to the manipulations. Cleaning the display perch with ethanol should remove compounds applied to the display perch by males, and more generally alter the chemical environment of the perch. We predicted that if males are adding or manipulating scent cues on the display perch, then they would increase scent communication behaviors after the ethanol treatment. We also predicted that if chemical cues primarily function in intersexual signaling, then male responses would be more pronounced in contexts relevant to courtship than during male-male interactions. Finally, we predicted that if females are attending to chemical cues at the display perch, then they would be less likely to revisit a male or copulate with a male after the ethanol treatment. In the second experiment, we tested the hypothesis that females use olfactory cues from preen oil to assess male genetic diversity, as previous work in this system suggests that females prefer males with greater multi-locus heterozygosity (Sardell et al. 2014) and that scent cues may indicate male heterozygosity (Whittaker et al. 2019a). We presented wild-caught female lance-tailed manakins with samples of male preen gland secretions from high- versus low- heterozygosity males in a Y-maze, an approach that has been applied with success in previous studies of avian olfactory preferences (Whittaker et al. 2011; Grieves et al. 2019a, b). We predicted that females would spend more time with the preen oil from the high-heterozygosity male, as heterozygosity is positively related to male reproductive success. By testing key predictions about the effects of chemical cues on male and female behavior, this study tests the importance of olfactory communication in mating contexts.
Materials and methods
We conducted two experiments investigating chemical communication in a population of lance-tailed manakins on a 46 ha section of dry tropical forest on Isla Boca Brava, Chiriquí Province, Panama (8°12’45"N 82°12’47"W). This population has been monitored annually during the peak breeding season (~ February to June) since 1999 and the majority of individuals in the population are color-banded, allowing for individual recognition in the field (DuVal 2007a). The work presented here was conducted in 2019–2022, with the scent manipulation experiment taking place in March-June 2019, and Y-maze trials conducted throughout the breeding season in 2021-22.
Experiment 1: scent manipulation experiment
To test whether display perches are a source of chemical cues used in mate choice contexts by lance-tailed manakins, we manipulated chemical cues at male display perches during the 2019 field season (March-June). To record male and female behavior and response to the manipulation, we used continuous video monitoring at one display perch at each of 14 display areas. A camera (Canon Vixia HF500 high-definition cameras) was set up on a tripod placed perpendicular to and 3–5 m from each dance perch. Recording started between 0700 and 0800 daily, and cameras recorded continuously for approximately 9.5 h each day, capturing most daylight hours and the majority of manakin activity. Each monitored display perch was attended by a different alpha male. Males use up to four display perches within an area (Vanderbilt et al. 2015), and we placed the camera at the perch on which we observed the most activity during in-person behavioral observations at display areas prior to the start of the experiment. To confirm that we were monitoring an actively used display perch, we video-recorded baseline behavior at dance perches for a minimum of 5 consecutive days before any manipulation. Bouts of activity during the baseline period were not included in analyses.
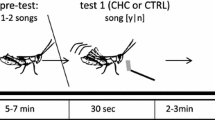
After the baseline monitoring period, we randomly assigned each display perch to a manipulation sequence (Fig. 1). The manipulation phase consisted of three sequential 5-day treatment periods, in which one treatment was repeated either at the beginning or the end of the manipulation sequence. This experimental design allowed us to use each display area as its own control and test for order and period effects without confounding these two potential contributors to behavioral responses (Kunert and Stufken 2002). Here, “order” refers to the sequence in which experimental treatments were applied (e.g., control first, ethanol second, ethanol third) and “period” refers to the point in the sequence during which an experimental treatment was applied (i.e., first treatment, second treatment, third treatment). With this experimental design each treatment was preceded equally by all treatment options (i.e. control treatment periods were preceded by both ethanol and control treatment periods) meaning that we could test for prior treatment effects without these effects being confounded with order effects. Additionally, because monitoring and manipulations were started at different dates among display areas, treatment and baseline periods overlapped at different sites and allow for tests of time-of-year decreases in mating or other activity. Activity at display perches peaked in the middle of the field season (Supplemental information; Fig. S1).
Diagram of the four treatment orders applied to video-monitored display perches. We used a strongly balanced design to account for carry-over effects, which included treatment orders in which each treatment type was preceded by itself and the other treatment type. The experimental design consisted of 3 consecutive 5-day treatment periods. To investigate male response to experimental manipulation, we recorded all bouts of activity on the first day of each treatment period (indicated by the green square). To investigate female response to experimental manipulation we recorded all displays for females throughout the experiment, i.e., on all 15 days of experimental manipulation
Manipulation occurred once daily immediately after each day’s video recording was started. During the treatment (or “ethanol”) condition, display perches were wiped with an autoclaved 100% cotton cloth with 2-3mL of 95% ethanol added to remove chemical signaling compounds. To control for daily human interaction with the display perch, the “control” condition consisted of wiping the display perch with a dry cloth. A fresh cloth was used at each display perch on each day of the experimental treatment. During both treatment conditions a researcher wiped the display perch by wrapping the cloth around the perch and wiping firmly across the perch 5 times. The manipulation took approximately 45 s to 1 min to complete. Ethanol is a universal solvent that readily dissolves hydrophobic compounds and is often used to clean behavioral testing equipment between trials to remove chemical compounds (Whittaker et al. 2011; Krause et al. 2014; Grieves et al. 2019a, b). The small amount of ethanol used to clean the dance perches evaporated quickly and it is reasonable to assume that it left no residue.
Video analysis
To quantify behaviors recorded at the dance perch during the experiment we used Movie Studio Platinum (v13.0; Sony Creative Software Inc., 2013) video-editing software to view the recordings. All video processing was conducted blind to treatment. To quantify male response to the experimental manipulation, we recorded all bouts of activity longer than 5 s at the display perch on the first day of each treatment period. To quantify female response to the experiment, we recorded all displays with a female present across the 15 days of manipulation (5 days of each treatment period) at each display perch. Females frequently return to display perches over the course of several days (DuVal and Kapoor 2015) and recording displays for females over several days was necessary to capture the female search process.
For each bout of activity, we recorded duration as the start and end time of activity on the display perch. The bout start time was recorded to the nearest second of a bird landing on the display perch. A bout ended when all individuals left the dance perch and did not land on it again for at least 15 s. The bout end time was recorded to the nearest second of the final bird’s feet leaving the dance perch.
We categorized bouts of activity into four categories: displays for a female (DFF), displays with no female (DNF), maintenance, and perching. Displays for females were characterized by the presence of a female on or visible near the display perch and the presence of one or two males performing display components. Displays for females often started with a female outside of the video frame, however we used male behavioral cues (e.g. males directing attention at a point out of frame while performing a display component that is used to attract females to the display perch, “up and downs”, “quick-downs”, etc.) and the presence of a female within frame later on in the bout of activity to label a bout of activity as a display for female. Displays with no female present were characterized by one or more males present and performing components of the courtship display (e.g. leapfrog, slow flight, etc.; DuVal 2007) with no female present on or visible near the display perch. During bouts categorized as “maintenance”, most behaviors observed were those manipulating the perch or its immediate surroundings, such as clearing of leaves on or around the display perch. Finally, bouts were categorized as “perching” if one or more males spent most of the bout stationary on the display perch with no maintenance or display behaviors, though the males could be singing the male duet song. These four categories of behavior can be grouped into behaviors most relevant to female mate choice (displays for females and maintenance bouts) and behaviors most relevant to male-male competition and maintenance of social bonds (displays with no female present and perching bouts). During maintenance bouts, we expected that males are preparing the display perch for visits by females, and behaviors performed during these bouts of activity may function in adding or manipulating chemical cues on the display perch. During displays with no female present and during perching bouts (both of which could include duet singing), we expected that males were more focused on establishing and maintaining social status with other males. These assumptions about the primary functions of these four types of behavior are not mutually exclusive; for example, perch maintenance also prepares the display perch for displays with no female present and displays with no female present likely influence the performance of display components when females visit a display area.
To quantify behavior that may function to add and/or manipulate chemical cues on the display perch, we recorded the total number of bill-wipes and pecks performed by all males that landed on the display perch during a bout of activity. Bill-wiping is a common behavior that may serve a variety of functions, including cleaning food or debris off the bill (Clark 1970), honing the shape of the bill for food handling (Cuthill et al. 1992), and chemical communication (Whittaker et al. 2015). A bill-wipe is a movement in which the bird lowers its head and draws the side of its beak across the perch; pecks involved striking the perch with the closed beak or opening the beak to grasp a part of the perch. We recorded the number and, where possible, the identity of all individuals that landed on the display perch during the bout of activity. Because we were interested in the response to the experimental manipulation of the chemical environment on the display perch, the number of individuals involved in an activity bout did not include birds that did not land on the display perch. We excluded two bouts in which no males were recorded landing on the display perch; in these two bouts a female was observed perching on the display perch, but no male was seen on or near the display perch.
During displays with a female present, we recorded the number of females present, the identity of all females that landed on the display perch (where possible), the number of bill-wipes or pecks by all females that landed on the display perch, and whether there was a copulation.
Statistical analysis
To test the prediction that manipulating olfactory cues at display perches affects male behavior we constructed two separate generalized linear mixed effects models, using a negative binomial error distribution and a log link function in both cases. In both models, we used the total number of bill-wipes and pecks by all males during the bout as the response variable. In the first model, we tested for the initial effects of experimental manipulation on male behavior and included only bouts of activity from the first day of experimental manipulation in the first treatment period at each display area. This first model included bout type (display for female, display with no female present, maintenance, or perching), treatment (ethanol or control), and their interaction as predictor variables. To test for carryover effects of the experimental manipulation, our second model included bouts of activity from the second and third treatment periods at each display area. This model included bout type, current treatment, prior treatment (i.e., the treatment in the immediately preceding treatment period: ethanol or control), and the interaction between bout type and current treatment as predictor variables. To test whether the effect of the experimental manipulation diminished over the course of the day, we included the time since the treatment was applied (in minutes) in all models. To correct for the logical expectation that longer bouts and bouts with more males present should include more total bill-wipes, we included bout length (seconds) and a categorical variable indicating the number of males present (one, two, or three + males) as fixed effects in both models of male bill-wiping behavior. Bout length was strongly right-skewed, so we used a natural-log transformation to improve model diagnostics. Finally, display area was included as a random effect in all models to account for repeated measures at each display area. While each monitored display area was attended by only one alpha male, we do note that non-alpha individuals were sometimes observed at more than one display area.
To test the prediction that experimental manipulation of chemical cues at display perches would make females less likely to revisit a display area, we constructed a generalized linear mixed effects model with a binomial error distribution and a logit link function. The response variable was a binomial (Y/N) variable indicating whether a female returned to the display area on a subsequent day. We included two key predictor variables: (1) the experimental treatment that was applied on the day of the female’s initial visit to the display perch, and (2) the time since treatment on the day of the female’s initial visit to the display perch (minutes) to test for diminishing effects of the experimental treatment over the course of the day. We also included the number of monitoring days after the female’s first visit (i.e., 14 − 0) as a fixed effect to control for the logically decreased detectability of repeated visits when first visits occurred late in the monitoring period. Female ID and display area were included as random effects to account for repeated sampling.
Finally, to test the prediction that experimental manipulation of chemical cues at display perches would make females less likely to copulate with a male, we constructed a generalized linear mixed effects model with a binomial error distribution and a logit link function. We used the binomial outcome (copulation Y/N) of display for a female as the response variable, indicating whether a female copulated at least once on that day. We included experimental treatment (ethanol or control) and the time since the treatment was applied (minutes) as predictor variables. We included display area and female ID as random effects to account for repeated sampling. Displays for females frequently had breaks where no individual was on the display perch for > 15 s, but where the same female returned after a break of several minutes, so we grouped all bouts with the same female together by day to generate the total display time for a female. Of these, 88% of 121 grouped bouts occurred over a period of less than one hour, and so likely represented a continuous display. However, 8% of 121 grouped bouts occurred over a period of two or more hours, possibly indicating multiple visits by the female on the same day. All analyses of female response to experimental manipulation only included visits to display perches where female identity was confidently assigned: 41% of 206 displays for females were excluded from the analysis because the female was unbanded or could not be clearly identified. To assess the effect of this analytical decision, we also conducted the analysis including all females.
All models were fit with restricted maximum likelihood and were constructed using the ‘glmmTMB’ function in the glmmTMB package (Brooks et al. 2017). We tested for predictor significance using the ‘Anova’ function in the car package (Fox and Weisberg 2019) and conducted post-hoc comparisons for significant predictors using the emmeans package (Lenth 2021). Estimated marginal means calculate the mean value of the response variable for each level of a categorical predictor, accounting for other variables included in the model. All analyses were performed in RStudio (v2023.3.1.446; RStudio Team 2023) using R Statistical Software (v4.3.0; R Core Team 2023).
Experiment 2: scent discrimination aviary trials
To test whether females use olfactory cues alone to distinguish between individual males differing in heterozygosity, we conducted scent discrimination trials in a Y-maze (Fig. 2). Trials were conducted on the Isla Boca Brava field site during the 2021 and 2022 field seasons.
Preen oil sample collection
We collected preen oil samples from adult males representing the upper and lower quantiles of heterozygosity in the population (Low = 0.42–0.60, High = 0.70–0.83) during the breeding season. In the first few weeks of the 2021 field season, we identified banded adult males during behavioral observations at display areas. We defined the heterozygosity cutoffs based on these observations of individuals that had been previously genotyped at 20 microsatellite loci using established protocols for this population (DuVal and Kempenaers 2008), and we used the same cutoffs in both the 2021 and 2022 field seasons. We calculated multi-locus heterozygosity (MLH) as the proportion of typed loci that were heterozygous (Sardell et al. 2014). In 2021 and 2022, respectively, 87% (26 of 30) and 84% (26 of 31) of adult males captured were previously banded individuals (i.e., of known heterozygosity). We then set up mist-nets in areas of high manakin activity and areas where males of known high- or low- heterozygosity had been observed during behavioral observations.
Upon capture of a previously banded male of known heterozygosity, we collected small preen gland secretion samples by gently rubbing the uropygial gland with the open end of a capillary tube. Birds were handled with nitrile gloves during sample collection. Samples were wrapped in aluminum foil, sealed in a plastic o-ring vial, and stored at -20˚C. Uropygial gland secretion samples from 19 males were used in Y-maze trials. Only one sample per male was used in a trial, except for one male from which preen oil samples were collected during two separate capture events and each preen oil sample was used in one trial.
Y-maze trials
Females were captured for Y-maze trials using mist nets placed in areas of manakin activity that were not directly adjacent to active nests. Upon capture, we assessed breeding condition by examination of the brood patch. To minimize disturbance to females with active nests, only birds with a pre-laying (n = 3 females) or a post-incubation (n = 7 females) brood patch were used in Y-maze trials. Lance-tailed manakin females regularly renest within a breeding season (Norton and DuVal 2023), and the minimum number of days between nest failure and egg-laying in a re-nesting attempt is 8 days (DuVal and Kapoor 2015). Females with a post-incubation brood patch may be reasonably expected to be in breeding condition. Females used in the behavioral trials were transported in a cloth drawstring bag to a central test site for the Y-maze trials.
Behavioral trials were conducted in a modified Y-maze aviary (Fig. 2). Each arm of the maze measured 40 cm x 20 cm x 20 cm (length x width x height). The sides and base of the Y-maze were constructed from plexiglass to prevent the scent cues from mixing. The sides of the Y-maze were covered with opaque material to minimize visual distraction to the bird in the Y-maze, and to reduce attempts by the focal birds to escape through clear plastic walls. The top of the Y-maze was constructed from black plastic mesh screen, which allowed for airflow and regulated temperature while discouraging focal birds from attempting to escape through a clear plastic top. Small computer fans attached at the end of the choice arms (Fig. 2) were placed to blow over the preen oil sample placed at each end to circulate the scent through the arm. Because most passerine birds are more comfortable on perches than on flat surfaces and lance-tailed manakins do not normally walk on the ground, we placed 6 wooden perches in the Y-maze. Each perch was a 9.5 mm diameter wood dowel placed 10 cm from one end of an arm, and 5 cm up from the floor of the Y-maze. This allowed the females to hop or make short flights between the perches. The Y-maze was placed under a string of “daylight” white LED strip lights (Jun Wen, 6500–7000 K, 1500 lm) to provide standardized lighting for the females. A similar Y-maze design has been used in other studies investigating preferences for olfactory cues in passerine birds (Whittaker et al. 2011, 2015; Grieves et al. 2019a, b).
Trials lasted 45 min. Immediately before each trial, preen oil samples from one low- and one high-heterozygosity male were brought to room temperature. Each sample was then dissolved in a small amount of acetone in either a plastic (n = 2 trials) or glass (n = 8 trials) screw-top vial, and then transferred to a small aluminum foil tray. The trays were placed on the floor of the Y-maze at the ends of the two choice arms, directly in front of the fans. During the first trial each year, we flipped a coin to decide on which side to place the sample from the low-heterozygosity male and alternated the placement of the samples in subsequent trials. A control tray with no sample was placed at the end of the starting arm to allow the female to habituate to the tray and to prevent the female from remaining in the starting arm to avoid a novel object. After allowing the acetone to evaporate, we placed the female in the Y-maze on the perch at the end of the “no choice” arm (Fig. 2). While previous studies investigating olfactory preferences in passerine birds have allowed the focal birds time to habituate in the Y-maze prior to adding the scent samples, we decided to place the bird and the scent samples in the Y-maze at the same time to minimize disturbance to the bird. When held in captivity, lance-tailed manakins typically remain motionless when disturbed and may remain motionless for > 30 min after disturbances such as disruption by humans (EHD, personal observation). We therefore decided that it was crucial to minimize disturbance to the focal female during the trial.
Female behavior throughout the trial was recorded with a video camera. At the end of the trial the female was released at the capture location. Females were held for no longer than 2 h after initial capture time. Between trials, the Y-maze was cleaned thoroughly with 90% ethanol.
Diagram of the plexiglass Y-maze used for preen oil preference trials. Preen oil samples from one high- and one low- heterozygosity male were placed at the ends of the left and right “choice arms” of the Y-maze. Females were placed at the end of the “No choice” arm at the beginning of the trial. Small computer fans at the ends of the choice arms were used to circulate olfactory cues through the choice arms during the trials
Video analysis
To quantify female choice, we recorded the time that the female spent in each arm of the Y-maze (right, left, no choice arm) during the final 10 min of the trial (i.e., beginning 35 min after the trial started). We chose the final 10 min of the trial as the choice period to allow females time to acclimate to the Y-maze and investigate both scent samples, while also standardizing the time point at which we measured female preference. All females visited both choice arms of the Y-maze prior to the 10-minute choice period. Videos were scored in Movie Studio Platinum (v13.0; Sony Creative Software Inc., 2013) by marking each time the female moved from one arm to another arm.
Statistical analysis
To test for female preference based on male heterozygosity, we constructed a linear mixed effects model using the ‘lmer’ function in the lme4 package (Bates et al. 2015). We included time spent in an arm of the maze as the response variable, log transformed to achieve a normal error distribution. To test whether females spent more time in the arm with the preen oil sample from the high- or low-heterozygosity male, we included a categorical predictor variable for male heterozygosity (high or low). Female ID was included as a random effect to account for the paired nature of the response variable. Residuals passed visual inspection for normality. We tested for predictor significance using the ‘Anova’ function in the car package (Fox and Weisberg 2019).
Results
Experiment 1: scent manipulation experiment
Male response to manipulation
We recorded 744 bouts of male activity across 42 days of recording at 14 display areas. During bouts of activity recorded on the first day of experimental manipulation at each display area (n = 274 bouts), male bill-wiping behavior differed by the type of activity (χ2 = 36.65, df = 3, p < 0.0001), the experimental treatment applied (χ2 = 6.63, df = 1, p = 0.01), and the interaction between these predictors (χ2 = 8.91, df = 3, p = 0.03; Fig. 3, Table S1). Time since the treatment was applied was not a significant predictor of male bill-wiping in the initial treatment period (χ2 = 1.35, df = 1, p = 0.25). During bouts of maintenance behavior males bill-wiped on average 3.3 times more at display perches that initially received the ethanol treatment compared to the control treatment (ethanol mean ± SE = 9.47 ± 2.53 bill-wipes, control mean ± SE = 2.89 ± 0.71 bill-wipes, pairwise contrast p < 0.0001; Table S2). During displays for females, males tended to bill-wipe more at display perches that initially received the ethanol treatment compared to the control, however this difference was not significant (ethanol mean ± SE = 4.75 ± 2.02 bill-wipes, control mean ± SE = 1.92 ± 0.67 bill-wipes, pairwise contrast p = 0.08; Table S2). During displays with no female present and perching bouts, experimental treatment did not influence bill-wiping behavior (Fig. 3, Table S1, S2).
Effect of experimental treatment on the number of bill-wipes by males during the different types of activity in the first experimental treatment period. Boxplots indicate median, quartiles, and range of model predicted values from a negative binomial generalized linear mixed effects model that also includes the number of males present and bout length as fixed effects, and display area as a random effect; points are raw data values from 274 bouts of activity at 14 display areas. DFF indicates displays for females, and DNF indicates displays with no female present. Asterisks indicate the level of significance in the pairwise comparison of ethanol and control treatments within the different bout types: ‘***’ indicates p < 0.001
We found evidence for carryover effects during bouts of activity recorded on the first days of the second and third treatment periods (n = 470 bouts): there was a significant effect of prior treatment on male bill-wiping behavior (χ2 = 5.46, df = 1, p = 0.02). Males bill-wiped on average 1.4 times more when the display perch had received an ethanol treatment in the immediately preceding treatment period, compared to males at display perches that had previously received the control treatment (prior ethanol mean ± SE = 2.77 ± 0.46 bill-wipes, prior control mean ± SE = 1.96 ± 0.33 bill-wipes, pairwise contrast p = 0.02, Fig. 4B). In these later treatment periods, male bill-wiping behavior also depended on the type of activity (χ2 = 66.41, df = 3, p < 0.0001), the experimental treatment applied (χ2 = 4.87, df = 1, p = 0.03), and the interaction between these predictors (χ2 = 23.40, df = 3, p < 0.0001; Fig. 4A). During displays for females and bouts of maintenance behavior, males bill-wiped significantly less on the ethanol treatment day compared to the control treatment day, a reverse of the pattern found in the initial testing period (Table S3, S4). During displays with no female present and perching bouts, experimental treatment again did not influence bill-wiping behavior (Fig. 4A, Table S3, S4). In addition to the effects of treatment and bout type, we also found a significant negative effect of time since the treatment was applied on male bill-wiping behavior in these later treatment periods (χ2 = 10.34, df = 1, p 0.001; Table S3): males bill-wiped less later in the day.
(A) Effect of experimental treatment on the number of bill-wipes by males during the different types of activity in the second and third experimental treatment periods. DFF indicates displays for females, and DNF indicates displays with no female present. (B) Effect of prior treatment on the number of male bill-wipes. In both panels the boxplots indicate median, quartiles, and range of model predicted values from a negative binomial generalized linear mixed effects model that also includes the number of males present and bout length as fixed effects, and display area as a random effect; points are raw data values from 470 bouts of activity at 14 display areas. Asterisks indicate the level of significance in the pairwise comparison of ethanol and control treatments either within the different bout types in (A) or for the comparison of prior ethanol and prior control in (B): ‘***’ indicates p < 0.001, ‘*’ indicates p < 0.05
Female response to manipulation
We scanned 210 days of recording at 14 display areas and recorded 76 initial visits and 121 total visits to display perches for 49 confidently identified females at 10 of the display areas. Whether a female revisited a male after an initial visit was unrelated to experimental treatment (χ2 = 2.07, df = 1, p = 0.15; n = 76 initial visits; Fig. 5A, Table S5). However, time since treatment was a significant positive predictor of whether a female revisited a male (χ2 = 7.78, df = 1, p = 0.005; Fig. 5B, Table S5). Females were more likely to ever revisit a male if they first visited the male later in the day (i.e., longer since the treatment was applied). Neither experimental treatment (χ2 = 1.29, df = 1, p = 0.26; Fig. 5C, Table S6) nor time since treatment (χ2 = 0.90, df = 1, p = 0.34; Table S6) were significant predictors of whether a female copulated with a male on any given day in which she visited a display area. When we included all banded females in the copulation model, the effect of treatment on the probability of copulation remained not significant (χ2 = 2.62, df = 1, p = 0.11). The duration of displays for females and female bill-wiping behavior were both unrelated to either the experimental treatment applied or the time since the treatment was applied (Supplemental Information).
(A and B) Relationship between the experimental treatment applied (A) or time since the treatment was applied (B) on the day of a female’s initial visit to a display perch and whether she revisited the perch. (C) Relationship between the experimental treatment applied and the likelihood of copulation. Both binomial generalized mixed effects models included Female ID and display area as random effects. Box plots show median, quartiles, and range of predicted values in (A) and (C), while (B) displays model-predicted values (black line) and confidence intervals (gray lines) with raw binomial outcomes as points in all figures
Experiment 2: scent discrimination aviary trials
In 8 of 10 Y-maze trials the focal female spent more time in the arm of the Y-maze with the preen oil sample from the high heterozygosity male compared to the arm with the preen oil sample from the low heterozygosity male. However, on average there was no significant difference in the amount of time females spent with the sample from the high or low heterozygosity male (χ2 = 1.16, DF = 1, p = 0.28; Fig. 6).
Time that each female spent in the choice arm with the preen oil sample from the either the high or low multi-locus heterozygosity male. Boxplots indicate median, quartiles, and range of model predicted values from linear mixed effects model that included female identity as a random effect. Gray points represent individual trials, with lines connecting points from the same female
Discussion
Using two controlled field experiments, we tested key predictions of the hypothesis that chemical cues on display perches play a role in the mating behavior of lance-tailed manakins. We expected to find evidence of chemical signaling in this system because the volatile composition of male preen oil covaries with individual multi-locus heterozygosity (Whittaker et al. 2019a) and heterozygosity is correlated with reproductive success in males (Sardell et al. 2014). Additionally, the relationship between specific components of the courtship display and reproductive success remains unclear, highlighting the need to consider traits that are less obvious to human observers (Vanderbilt 2019). In the first experiment we found that manipulating the chemical environment on male display perches influenced male behavior in contexts expected to be relevant to female mate choice. However, we measured no female response to these experimental changes in the chemical environment at male display perches. Furthermore, females did not show a preference for olfactory cues indicating male genetic diversity in aviary trials. The results presented here add to the growing body of literature demonstrating avian chemical signaling, an understudied signaling modality in birds, and underscore complexity in assessing behavioral responses to chemical cues.
We hypothesized that bill wiping by males was a chemical signaling behavior: bill-wiping may function to add or manipulate chemical cues at perches (Whittaker et al. 2015). Alternatively, bill-wiping could be a displacement behavior when birds are excited (Clark 1970; Whittaker et al. 2015) or an aggressive signal in male-male competitive contexts (Magdaleno et al. 2022). These alternative functions would predict that males should bill-wipe the most during displays for females and displays with no females present, and that there would be no difference in bill-wiping behavior between the ethanol and control treatments. Finally, bill-wiping may function in cleaning the bill and honing the shape of the bill for better food handling (Clark 1970; Cuthill et al. 1992), which would predict that males should bill-wipe consistently across behavioral contexts, or primarily when perched or performing perch maintenance. Instead, we observed the most bill-wipes during bouts of perch maintenance, and the second most bill-wipes during displays for females. We also observed a significant effect of treatment in initial and later treatment periods. Maintenance behaviors likely prepare the display perch for visits by females, and bill-wipes and pecks may add preen oil and other olfactory cues to the dance perch, while bill-wipes during displays for females may additionally function to release the volatile components for the visiting female (Whittaker et al. 2015). Bill-wiping in lance-tailed manakins may serve other functions, and future research in this system will investigate individual differences in bill-wiping behavior among alpha, beta, and other males to test for potential male-male signaling. Together, these results support our hypothesis that chemical signaling occurs during reproduction in this system.
The effect of experimental manipulation of chemical cues at display perches carried over across treatment periods. Prior ethanol treatment led to significantly more bill-wipes in the subsequent treatment period compared to prior control treatment (Fig. 4B). However, during displays for females and bouts of perch maintenance in these second and third treatment periods, males bill-wiped less during current ethanol treatment periods compared to the control treatment (Fig. 4A). The effect of the experimental manipulation changed over time, suggesting that chemical cues are not easily replaced or returned to their baseline conditions. One possible explanation for this result is that males are habituating to the change in the chemical environment on the display perch from the ethanol treatment, and thus the ethanol treatment in the second and third periods is eliciting a weaker response compared to the ethanol treatment in the first period. On average, males bill-wipe less during later ethanol treatment periods, compared to initial ethanol treatment periods, but the average number of bill-wipes during control treatment periods was relatively consistent across the experiment. Another possible explanation is that the males reduce their investment in bill-wiping during the later treatment periods and spend less time at display perches during the later treatment periods. We saw the opposite trend, however: we recorded more bouts of activity during final ethanol treatment periods (i.e. ethanol treatment applied in the third period, n = 177 bouts of activity) compared to initial ethanol treatment periods (n = 77 bouts of activity). Future analyses investigating the attenuation of bill-wiping over time would provide further insight into the delayed effects of the experimental manipulation.
The ethanol treatment may have influenced the bacterial community on the display perch, which could explain the carry-over effects of the ethanol treatment. The bacterial community on the uropygial gland directly influences the volatile components of preen oil in birds (Whittaker et al. 2019b), and the olfactory environment of lance-tailed manakin display perches may similarly be influenced by the bacterial community on the display perch. The microbiome can have a strong influence on host biology, including influencing reproductive traits (Rowe et al. 2020). Investigating how perch maintenance by multiple males influences the microbial community and the olfactory profile of the display perch, and how these characteristics influence mate choice, may provide insight into the evolution of cooperative display and sociality in this system.
We also found a significant effect of time since the treatment was applied on bill-wiping behavior in these combined later treatment periods: males bill-wiped less as the time since treatment increased. However, the effect size for time since treatment was very small. The distribution of different bout types over the course of the day is a likely explanation for bill-wiping decreasing with time of day. All different types of activity occurred throughout the day, however maintenance bouts and displays with no female present occurred more frequently in the morning while displays for females occurred more frequently in the afternoon (Fig. S2). On average, the most bill-wiping occurred during bouts of perch maintenance, therefore the slight decrease in bill-wiping over the course of the day could be explained by fewer bouts of perch maintenance occurring later in the day.
Repeated visits to male display areas by females have been observed in several non-resource based polygynous bird species (Trail and Adams 1989; Uy et al. 2001) and the factors influencing the decision to revisit a male may vary with female age and prior experience (Coleman et al. 2004). These repeat visits may be an important part of the search process and may function in narrowing down the mating options (Janetos 1980; Real 1990; Wiegmann et al. 2010). Despite evidence for chemical signaling behaviors by males, we found no evidence that females perceived or responded to changes in the chemical environment at the display perch. Experimental treatment was unrelated to the likelihood of females revisiting a display area or copulating on any given visit to a display area. Our results contrast with a similar study conducted with satin bowerbirds, where experimental removal of bower paint had a significant effect on the likelihood of females revisiting display areas (Hicks et al. 2013). However, in satin bowerbirds, females actively sample the paint on the bowers (Keagy et al. 2016), suggesting a role for taste rather than olfactory chemical communication. We did find that females were more likely to revisit a male when their initial visit was later in the day (Fig. 5B). One possible explanation for this result is that the effect of the experimental manipulation changed over the course of the day, i.e., that males were able to compensate for the removal of potential chemical cues as the day progressed. Because we only recorded male bill-wiping behavior on one day of each treatment period, but we recorded female response over the 15-day experimental period we were unable to directly test whether male bill-wiping prior to a visit by a female influenced her likelihood of copulation or of revisiting. The increased likelihood of a female revisiting when her initial visit occurred later in the day likely reflects other patterns of behavior in lance-tailed manakins. Displays for females that occur later in the day may be indicative of greater female interest in the male, and therefore the female may be more likely to revisit that male, compared to females that initially visit a male earlier in the day.
In contrast to other studies testing for avian olfactory preferences in Y-maze trials, we did not find evidence that female lance-tailed manakins can detect olfactory cues from male preen oil or use these cues to distinguish between males differing in multi-locus heterozygosity. In the Y-maze experiment, there was no significant difference in the amount of time that females spent with the preen oil sample from the high heterozygosity male, compared to the preen oil sample from the low heterozygosity male (Fig. 6). Other studies have successfully detected significant preferences for olfactory cues of sex, species, and genetic characteristics in birds using a modified Y-maze aviary (Whittaker et al. 2011; Krause et al. 2014; Leclaire et al. 2017; Grieves et al. 2019a, b; Van Huynh and Rice 2019). Our results are constrained by a limited sample size and the behavioral responses of female lance-tailed manakins to captivity. A post-hoc power analysis revealed that we had limited power to detect an effect of the magnitude found in a previous investigation of preference for cues of immune gene diversity (Supplemental Information). Additionally, we had moderate power to detect an effect size of the magnitude found in a previous analysis of cues of sex (Supplemental Information). Together, the results of the post-hoc analysis suggest that we may have detected a significant preference for cues of male heterozygosity with more y-maze trials. In addition to low power, we also had logistical constraints that may have influenced our ability to test for female preference for olfactory cues of male heterozygosity. Previous Y-maze experiments were able to allow females time to habituate to captivity in the Y-maze prior to the test period. However, lance-tailed manakins do not respond well to longer-term captivity and we therefore decided to place the odor samples in the Y-maze at the same time as the female was introduced, to minimize captive time and disturbance to the female. It is possible that the volatile components of the preen oil had dissipated by the final 10 min of the trial and female behavior in this period did not reflect their true choices. However, analysis of female behavior at an earlier time-point in the trials also showed no evidence of female preference for olfactory cues of male heterozygosity (Supplemental Information). Finally, we chose to use females that were captured with either a pre-laying or post-incubation brood patch in the Y-maze trials. While female lance-tailed manakins readily renest within a breeding season (Norton and DuVal 2023), and frequently renest rapidly after a nest failure (DuVal and Kapoor 2015), we cannot exclude the possibility that some of the females we tested in the Y-maze trials were not receptive to male cues. More research is needed to understand the information conveyed by chemical cues in lance-tailed manakins.
In this study we assumed that preen oil was the primary source of chemical cues in lance-tailed manakins. However, individual chemical profiles are also influenced by immune genetic characteristics (Slade et al. 2016; Jennings et al. 2022), as well as sex (Grieves et al. 2019a), and social affiliations (Whittaker et al. 2016). There are also alternative sources of chemical cues, such as saliva. If saliva functions in chemical signaling in lance-tailed manakins it could explain why we saw the male response to the chemical manipulation experiment but no clear female response in the y-maze trials where we used preen oil samples as the potential chemical cue. In satin bowerbirds males add a visible saliva-based paint to the inside of the bower that females can taste when visiting the bower (Keagy et al. 2016). We did not observe any visible clumps on the lance-tailed manakin display perch, suggesting that males are not adding large amounts of saliva to display perches, however future research will investigate other potential sources of chemical cues in this species.
While sexual selection through female choice is typically expected to be consistent over time, studies suggest that the magnitude and direction of female preferences can vary over time (Chaine and Lyon 2008). The lack a significant difference in the amount of time spent with the high- vs. low-heterozygosity male sample may be due to a lack of female preference for male heterozygosity in the breeding seasons during which the Y-maze trials were conducted. Previous research in this population has demonstrated female preference for male multi-locus heterozygosity (Sardell et al. 2014), however the magnitude of the effect of male heterozygosity on male reproductive success varies significantly over time (PRR and EHD, unpubl. data). Future research in this system will investigate how variation in population level preferences for male traits predict individual behavioral responses to male traits associated with reproductive success.
The results from the chemical manipulation experiment suggest that affiliation time with social partners or chemical cues might be important to chemical communication in birds. We found that males respond to the manipulation of chemical cues at display perches, but females did not. Males spend much more time at display perches compared to females, and thus may be more likely to detect changes in the chemical environment on the display perch. Close social affiliation between males and females may also be important for chemical communication in mate choice. Most of the research on the role of chemical communication in avian mating behavior has focused primarily on socially monogamous species (e.g. Whittaker et al. 2011; Krause et al. 2012; Leclaire et al. 2017; Grieves et al. 2019b; Jennings and Ebeler 2020) where males and females form close social bonds and may spend much of their time together, compared to non-resource based polygynous systems. Affiliative behaviors between mates, such as allogrooming, may facilitate detection of chemical cues, and these types of behaviors are not observed in lance-tailed manakins.
We demonstrated that male lance-tailed manakins respond to manipulation of chemical cues at their display perches and that the response is strongest when males are preparing their display perches for visits by females or during displays for females. Despite this evidence for male response to chemical cues present at display perches, we found no evidence that females distinguish between olfactory cues from males that differed in a mating-relevant genetic characteristic, or that females detected and responded to changes in the chemical environment on the display perch. Chemical signaling is found across the tree of life, and while it has historically been a neglected aspect of the rich perceptual world of birds, growing evidence indicates that it may be influential in avian mating and social behavior (Caro et al. 2015; Whittaker and Hagelin 2021). The results presented here indicate that the influence of chemical signaling in birds is nuanced, suggesting fruitful areas for research investigating the drivers of variation in chemical signaling across species.
Data availability
The datasets and R code generated during this study are included in this published article as supplementary information files.
References
Alves Soares T, Caspers BA, Loos HM (2023) Avian chemical signatures: an overview. In: Schaal B, Rekow D, Keller M, Damon F (eds) Chemical signals in vertebrates 15. Springer International Publishing, Cham, pp 113–137
Amo L, Avilés JM, Parejo D, Peña A, Rodríguez J, Tomás G (2012) Sex recognition by odour and variation in the uropygial gland secretion in starlings. J Anim Ecol 81:605–613. https://doi.org/10.1111/j.1365-2656.2011.01940.x
Andersson S (1991) Bowers on the savanna: display courts and mate choice in a lekking widowbird. Behav Ecol 2:210–218. https://doi.org/10.1093/beheco/2.3.210
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bonadonna F, Hesters F (2003) Scent of a nest: discrimination of own-nest odours in Antarctic prions, Pachyptila desolata. Behav Ecol Sociobiol 54:174–178. https://doi.org/10.1007/s00265-003-0610-7
Bonadonna F, Sanz-Aguilar A (2012) Kin recognition and inbreeding avoidance in wild birds: the first evidence for individual kin-related odour recognition. Anim Behav 84:509–513. https://doi.org/10.1016/j.anbehav.2012.06.014
Borgia G (1995) Complex male display and female choice in the spotted bowerbird: specialized functions for different bower decorations. Anim Behav 49:1291–1301. https://doi.org/10.1006/anbe.1995.0161
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9:378–400. https://doi.org/10.32614/rj-2017-066
Candolin U (2003) The use of multiple cues in mate choice. Biol Rev 78:575–595. https://doi.org/10.1017/S1464793103006158
Caro SP, Balthazart J, Bonadonna F (2015) The perfume of reproduction in birds: chemosignaling in avian social life. Horm Behav 68:25–42. https://doi.org/10.1016/j.yhbeh.2014.06.001
Caspers BA, Krause ET (2011) Odour-based Natal nest recognition in the zebra finch (Taeniopygia guttata), a colony-breeding songbird. Biol Lett 7:184–186
Chaine AS, Lyon BE (2008) Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science 319:459–462. https://doi.org/10.1126/science.1149167
Charpentier MJE, Boulet M, Drea CM (2008) Smelling right: the scent of male Lemurs advertises genetic quality and relatedness. Mol Ecol 17:3225–3233. https://doi.org/10.1111/j.1365-294X.2008.03831.x
Chiver I, Schlinger BA (2017) Clearing up the court: sex and the endocrine basis of display-court manipulation. Anim Behav 131:115–121. https://doi.org/10.1016/j.anbehav.2017.07.014
Clark GA Jr (1970) Avian bill-wiping. Wilson Bull 82:279–288
Coleman SW, Patricelli GL, Borgia G (2004) Variable female preferences drive complex male displays. Nature 428:742–745. https://doi.org/10.1038/nature02419
Cuthill I, Witter M, Clarke L (1992) The function of bill-wiping. Anim Behav 43:103–115. https://doi.org/10.1016/S0003-3472(05)80076-4
DuVal EH (2007a) Cooperative display and lekking behavior of the lance-tailed manakin (Chiroxiphia lanceolata). Auk 124:1168–1185. https://doi.org/10.1642/0004-8038(2007)124[1168:CDALBO]2.0.CO;2
DuVal EH (2007b) Social organization and variation in cooperative alliances among male lance-tailed manakins. Anim Behav 73:391–401. https://doi.org/10.1016/j.anbehav.2006.05.017
DuVal EH, Kapoor JA (2015) Causes and consequences of variation in female mate search investment in a lekking bird. Behav Ecol 26:1537–1547. https://doi.org/10.1093/beheco/arv110
DuVal EH, Kempenaers B (2008) Sexual selection in a lekking bird: the relative opportunity for selection by female choice and male competition. Proc R Soc Lond B 275:1995–2003. https://doi.org/10.1098/rspb.2008.0151
Endler JA, Endler LC, Doerr NR (2010) Great bowerbirds create theaters with forced perspective when seen by their audience. Curr Biol 20:1679–1684. https://doi.org/10.1016/j.cub.2010.08.033
Fox J, Weisberg S (2019) An R companion to Applied Regression, 3rd edn. Sage, Thousand Oaks, CA
Grieves LA, Bernards MA, MacDougall-Shackleton EA (2019a) Behavioural responses of songbirds to preen oil odour cues of sex and species. Anim Behav 156:57–65. https://doi.org/10.1016/j.anbehav.2019.06.035
Grieves LA, Gloor GB, Bernards MA, MacDougall-Shackleton EA (2019b) Songbirds show odour-based discrimination of similarity and diversity at the major histocompatibility complex. Anim Behav 158:131–138. https://doi.org/10.1016/j.anbehav.2019.10.005
Hicks RE, Larned A, Borgia G (2013) Bower paint removal leads to reduced female visits, suggesting bower paint functions as a chemical signal. Anim Behav 85:1209–1215. https://doi.org/10.1016/j.anbehav.2013.03.007
Janetos AC (1980) Strategies of female mate choice: a theoretical analysis. Behav Ecol Sociobiol 7:107–112
Jennings SL, Ebeler SE (2020) Individual chemical profiles in the Leach’s storm-petrel. J Chem Ecol 46:845–864. https://doi.org/10.1007/s10886-020-01207-0
Jennings SL, Hoover BA, Sin SYW, Ebeler SE (2022) Feather chemicals contain information about the major histocompatibility complex in a highly scented seabird. Proc R Soc B 289:20220567. https://doi.org/10.1098/rspb.2022.0567
Johansson BG, Jones TM (2007) The role of chemical communication in mate choice. Biol Rev 82:265–289. https://doi.org/10.1111/j.1469-185X.2007.00009.x
Keagy J, Hosler LC, Borgia G (2016) Female active sampling of male paint on bowers predicts female uncertainty in mate choice. Anim Behav 116:131–137
Krause ET, Krüger O, Kohlmeier P, Caspers BA (2012) Olfactory kin recognition in a songbird. Anim Behav 327–329. https://doi.org/10.1098/rsbl.2011.1093
Krause ET, Brummel C, Kohlwey S, Baier MC, Müller C, Bonadonna F, Caspers BA (2014) Differences in olfactory species recognition in the females of two Australian songbird species. Behav Ecol Sociobiol 68:1819–1827. https://doi.org/10.1007/s00265-014-1791-y
Kunert J, Stufken J (2002) Optimal crossover designs in a model with self and mixed carryover effects. J Am Stat Assoc 97:898–906. https://doi.org/10.1198/016214502388618681
Leclaire S, Strandh M, Mardon J, Westerdahl H, Bonadonna F (2017) Odour-based discrimination of similarity at the major histocompatibility complex in birds. Proc R Soc B 284:20162466. https://doi.org/10.1098/rspb.2016.2466
Lenth RV (2021) emmeans: Estimated Marginal Means, aka Least-Squares Means, https://CRAN.R-project.org/package=emmeans
Magdaleno FR, O’Loghlen AL, Rothstein SI (2022) An agonistic visual signal during birdsong: Bill wiping in multimodal song displays by the male Brown-headed Cowbird (Molothrus ater). Wilson J Ornithol 134:215–226. https://doi.org/10.1676/21-00004
Norton JE, DuVal EH (2023) Causes and consequences of nest-site fidelity in a tropical lekking bird: Win-stay-lose-shift tactics are unrelated to subsequent success, but site-faithful females nest earlier. Ornithol 140:ukad016. https://doi.org/10.1093/ornithology/ukad016
Penn DJ (2002) The scent of genetic compatibility: sexual selection and the major histocompatibility complex. Ethology 108:1–21
Potier S, Duriez O, Célérier A, Liegeois J-L, Bonadonna F (2019) Sight or smell: which senses do scavenging raptors use to find food? Anim Cogn 22:49–59. https://doi.org/10.1007/s10071-018-1220-0
R Core Team (2023) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Real L (1990) Search theory and mate choice. I. models of single-sex discrimination. Am Nat 136:376–405
Robson TE, Goldizen AW, Green DJ (2005) The multiple signals assessed by female satin bowerbirds: could they be used to narrow down females’ choices of mates? Biol Lett 1:264–267. https://doi.org/10.1098/rsbl.2005.0325
Rowe M, Veerus L, Trosvik P, Buckling A, Pizzari T (2020) The reproductive microbiome: an emerging driver of sexual selection, sexual conflict, mating systems, and reproductive isolation. Trends Ecol Evol 35:P220–234. https://doi.org/10.1016/j.tree.2019.11.004
RStudio Team (2023) RStudio: integrated development environment for R. http://www.rstudio.com
Sardell RJ, Kempenaers B, DuVal EH (2014) Female mating preferences and offspring survival: testing hypotheses on the genetic basis of mate choice in a wild lekking bird. Mol Ecol 23:933–946. https://doi.org/10.1111/mec.12652
Schaedelin FC, Taborsky M (2006) Mating craters of Cyathopharynx furcifer (Cichlidae) are individually specific, extended phenotypes. Anim Behav 72:753–761. https://doi.org/10.1016/j.anbehav.2005.11.028
Searcy WA, Nowicki S (2005) The evolution of animal communication: reliability and deception in signaling systems. Princeton University Press, Princeton
Slade JWG, Watson MJ, Kelly TR, Gloor GB, Bernards MA, MacDougall-Shackleton EA (2016) Chemical composition of preen wax reflects major histocompatibility complex similarity in songbirds. Proc R Soc B 283:20161966. https://doi.org/10.1098/rspb.2016.1966
Sony Creative Software Inc (2013) Movie Studio Platinum, http://www.sonycreativesoftware.com
Trail PW, Adams ES (1989) Active mate choice at cock-of-the-rock leks: tactics of sampling and comparison. Behav Ecol Sociobiol 25:283–292
Uy JAC, Patricelli GL, Borgia G (2001) Complex mate searching in the satin bowerbird Ptilonorhynchus violaceus. Am Nat 158:530–542. https://doi.org/10.1086/323118
Van Huynh A, Rice AM (2019) Conspecific olfactory preferences and interspecific divergence in odor cues in a chickadee hybrid zone. Ecol Evol 9:9671–9683. https://doi.org/10.1002/ece3.5497
Vanderbilt CC (2019) Understanding variation in complex displays and mate choice in a lekking species with cooperative dual-male courtship. Dissertation, Florida State University
Vanderbilt CC, Kelley JP, DuVal EH (2015) Variation in the performance of cross-contextual displays suggests selection on dual-male phenotypes in a lekking bird. Anim Behav 107:213–219. https://doi.org/10.1016/j.anbehav.2015.06.023
Whittaker DJ, Hagelin JC (2021) Female-based patterns and social function in avian chemical communication. J Chem Ecol 47:43–62. https://doi.org/10.1007/s10886-020-01230-1
Whittaker DJ, Richmond KM, Miller AK, Kiley R, Bergeon Burns C, Atwell JW, Ketterson ED (2011) Intraspecific preen oil odor preferences in dark-eyed juncos (Junco hyemalis). Behav Ecol 22:1256–1263. https://doi.org/10.1093/beheco/arr122
Whittaker DJ, Reichard DG, Drouilly M, Battle K, Ziegenfus C (2015) Avian olfactory displays: a hypothesis for the function of bill-wiping in a social context. Behav Ecol Sociobiol 69:159–167. https://doi.org/10.1007/s00265-014-1829-1
Whittaker DJ, Gerlach NM, Slowinski SP, Corcoran KP, Winters AD, Soini HA, Novotny MV, Ketterson ED, Theis KR (2016) Social environment has a primary influence on the microbial and odor profiles of a chemically signaling songbird. Front Ecol Evol 4:90. https://doi.org/10.3389/fevo.2016.00090
Whittaker DJ, Kuzel M, Burrell M, Soini HA, Novotny MV, DuVal EH (2019a) Chemical profiles reflect heterozygosity and seasonality in a tropical lekking passerine bird. Anim Behav 151:67–75
Whittaker DJ, Slowinski SP, Greenberg JM et al (2019b) Experimental evidence that symbiotic bacteria produce chemical cues in a songbird. J Exp Biol 222:jeb202978. https://doi.org/10.1242/jeb.202978
Wiegmann DD, Seubert SM, Wade GA (2010) Mate choice and optimal search behavior: fitness returns under the fixed sample and sequential search strategies. J Theor Biol 262:596–600. https://doi.org/10.1016/j.jtbi.2009.08.033
Acknowledgements
We thank M. Kuzel, E. Filiberti, M. Marsh, K. Hobbs, L. Campbell, M. Bleitz, and E. Resendiz for their assistance with data collection in the field, and J. Norton, D. Brown, J. Albrighton, C. O’Donnell, E. Abel, K. Simmons, and (A) Degenhart for assistance with video processing. We also thank E. Schunke for assistance with Y-maze construction, and (B) Jones for methodology discussions. We thank Dustin Rubenstein and two anonymous reviewers for their insightful comments that improved this manuscript. Access to the field site was granted by F. Koehler and the residents of Isla Boca Brava. Research permits were attained with the assistance of the staff and scientists of Ministerio de Ambiente, Republica de Panamá and the Museo de Vertebrados de la Universidad de Panama.
Funding
This work was supported by grants and awards from the Philanthropic Educational Organization, Sigma Xi: The Scientific Research Society, Florida State University, and the Society for Integrative and Comparative Biology (to PRR) and by NSF grants 1453408 and 2243423 (to EHD) and NSF grant 1457541.
Author information
Authors and Affiliations
Contributions
PRR: Conceptualization, Methodology, Formal analysis, Investigation, Data Curation, Writing – Original Draft, Writing – Review and Editing, Visualization, Supervision, Funding acquisition, Project administration. EHD: Methodology, Resources, Writing – Review and Editing, Supervision, Project administration, Funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
All research methods were approved by the Institutional Animal Care and Use Committee at Florida State University (Protocol #1706). All applicable international, national, and institutional guidelines for the use of animals were followed. Local research permits were granted by the Ministerio de Ambiente, Republica de Panama.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Communicated by D. Rubenstein.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rivers, P.R., DuVal, E.H. Chemical cues in the mating behavior of a highly polygynous bird. Behav Ecol Sociobiol 78, 69 (2024). https://doi.org/10.1007/s00265-024-03477-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-024-03477-0