Abstract
In birds, accumulating energy is far slower than spending energy during flight. During migration, birds spend, therefore, most of the time at stopover refueling energy used during the previous flight. This elucidates why current energy stores and actual rate of accumulating energy are likely crucial factors influencing bird’s decision when to resume migration in addition to other intrinsic (sex, age) and extrinsic (predation, weather) factors modulating the decision within the innate migration program. After first summarizing how energy stores and stopover durations are generally determined, we critically review that high-energy stores and low rates of accumulating energy were significantly related to high departure probabilities in several bird groups. There are, however, also many studies showing no effect at all. Recent radio-tracking studies highlighted that migrants leave a site either to resume migration or to search for a better stopover location, so-called “landscape movements”. Erroneously treating such movements as departures increases the likelihood of type II errors which might mistakenly suggest no effect of either trait on departure. Furthermore, we propose that energy loss during the previous migratory flight in relation to bird’s current energy stores and migration strategy significantly affects its urge to refuel and hence its departure decision.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In bird migrants, variation in the arrival timing at the migratory destination is proximately caused by onset of the seasonal migration (Jahn et al. 2013; Stanley et al. 2012; Tøttrup et al. 2012; Schmaljohann et al. 2016; Yamaura et al. 2016; Ouwehand and Both 2017), total migration distance (Visser et al. 2009), and total speed of migration (Nilsson et al. 2013). The latter is a function of travel speed (Liechti and Schaller 1999; Liechti 2006), i.e., the migratory distance covered between daily locations on travel days, i.e., excluding stopovers, (Nilsson et al. 2013), and total stopover duration (Knudsen et al. 2011; Nilsson et al. 2013). Stopovers are resting and refueling phases that most migrants make in between their migratory flights (Alerstam et al. 2003; Delingat et al. 2006; Åkesson and Hedenström 2007). Because the rate of accumulating energy (refueling) during stopover is slower than the rate of energy expenditure during migratory flights (Alerstam and Lindström 1990; Hedenström and Alerstam 1997; Alerstam 2011), only a minor proportion of time and energy is allocated to the migratory flights (Hedenström and Alerstam 1997; Green et al. 2002; Wikelski et al. 2003; Schmaljohann et al. 2012). Hence, variation in total stopover duration of a migratory journey affects total speed of migration to a larger extent than variation in travel speed. Although the latter can be significantly increased by wind assistance (Liechti and Bruderer 1998; Liechti and Schaller 1999; Weber and Hedenström 2000; Chevallier et al. 2010; Shamoun-Baranes et al. 2010), stopovers remain the most crucial periods of the migratory journey affecting most strongly arrival timing at the migratory destination (Nilsson et al. 2013). Quantifying the relative contributions of different intrinsic (e.g., body condition) and extrinsic factors (e.g., temperature) to variation of stopover duration is therefore important, and will directly indicate the mechanisms how total speed of migration is affected by environmental conditions encountered en route. Many studies have done so and have shown that there is a complex interplay of intrinsic and extrinsic factors jointly affecting departure probability from a stopover site (Moore and Kerlinger 1987a; Richardson 1990; Woodrey and Moore 1997; Yong and Moore 1997; Liechti and Bruderer 1998; Yong et al. 1998; Weber and Hedenström 2000; Erni et al. 2002a; Bolshakov et al. 2003; Jenni and Schaub 2003; Liechti 2006; Tsvey et al. 2007; Bauer et al. 2008; Duriez et al. 2009; Goymann et al. 2010; Chernetsov 2012; McLaren et al. 2013; Smith and McWilliams 2014; Deppe et al. 2015; Dossmann et al. 2015; Henkel and Taylor 2015; Mitchell et al. 2015; Schmaljohann et al. 2017) within the endogenously controlled migration program (Berthold 1996; Gwinner 1996, 2009; Ramenofsky and Wingfield 2006; Bairlein et al. 2015a).
In this review, we take a simplified approach and discuss only the effect that body condition may have on the stopover departure decision. We not only focus on songbirds, but also consider studies on waders, swans, geese, ducks, and soaring birds. We excluded seabirds and swifts from our review, as relating individual body condition to stopover behavior of these groups is extremely difficult due to their offshore (Jouventin and Weimerskirch 1990; Weimerskirch and Wilson 2000; Shaffer et al. 2006) and/or airborne lifestyle (Åkesson et al. 2012, 2016; Liechti et al. 2013).
Songbirds usually have a stop-and-go strategy (Delingat et al. 2006; Åkesson and Hedenström 2007) and perform broad-front migration (Newton 2008), but they may concentrate in coastal regions (Alerstam 1990; Bruderer and Liechti 1998; Fortin et al. 1999; Archibald et al. 2017). There is evidence from single site studies that songbirds show no to little stopover site fidelity (Drost 1941; Dierschke 2002; Catry et al. 2004), though site fidelity might increase when birds require specific habitats for refueling (Cantos and Tellerìa 1994). Songbirds can be grouped into daytime and nighttime migrants. The former migrates either solitarily or in loose flocks, but not in family units. They usually depart from stopover sites to resume migration shortly after sunrise and continue flying until midday; afterwards migration intensity often considerably drops (Dorka 1966; Alerstam 1990; Newton 2008). Nocturnal songbird migrants usually migrate on their own and are not guarded by parents or conspecifics. Radar observations on some nights demonstrated that birds with similar wing beat frequency, air speed, and flight altitude migrated in loose flocks (Larkin and Szafoni 2008), though it remains unknown whether birds aggregated because they selected the same conditions for migration or whether they migrated as a unit. Nevertheless, it is generally assumed that nocturnal songbird migrants ‘entirely’ rely on an innate migration program to find their wintering grounds on their first autumn migration (Bairlein et al. 2015a). Nocturnal migrants usually set off within the first 1–3 h after sunset and land before sunrise (Dorka 1966; Gauthreaux 1971; Bruderer 1994; Bruderer and Liechti 1995, 1999; Schmaljohann et al. 2007b). There are, however, deviations from this pattern; some individuals set off much later in the night (Müller et al. 2016), some prolong their migratory flights into the day when experiencing favorable wind conditions (Schmaljohann et al. 2007a), and some depart earlier or land later to be able to cross ecological barriers non-stop (Bairlein et al. 2012; DeLuca et al. 2015; Adamík et al. 2016; Ouwehand and Both 2016). Waders usually seek specific areas to accumulate large energy stores for the upcoming migratory flights (Piersma et al. 2005; Lisovski et al. 2016), depart in flocks around sunset (Piersma et al. 1990a, b), time their departures in relation to wind (Leyrer et al. 2009; Gill et al. 2009; Conklin and Battley 2011), and fly long stretches of their migration route non-stop (Gill et al. 2009; Klaassen et al. 2011; Battley et al. 2012). The latter might be terminated early when encountering unfavorable wind conditions (Shamoun-Baranes et al. 2010). As in waders, swans, geese and ducks have specific areas for refueling (Green et al. 2002; Arzel et al. 2006). Although some species cover long distances non-stop, they usually do not migrate more than one day in row (Pennycuick et al. 1996; Green et al. 2002; Clausen et al. 2003; Miller et al. 2005; van Wijk et al. 2012; Shariatinajafabadi et al. 2013). In swans and geese, families usually migrate together. Most soaring birds migrate in loose flocks only during daytime periods when thermals are available (Kerlinger 1989; Liechti et al. 1996; Spaar and Bruderer 1996, 1997b; Bohrer et al. 2011), though some raptors switch to flapping flight before or after this period, during headwind conditions and more often when being young (Spaar and Bruderer 1997a; Bohrer et al. 2011; Sapir et al. 2011; Rotics et al. 2016).
The first objective of our review is to give a brief overview on how body condition, in our case the current energy stores of a migratory bird, can be evaluated at a stopover site and how stopover duration is estimated. This is important because we should be aware of the advantages and disadvantages of different methods used to estimate these traits. Our other objective is to review the current knowledge of how energy stores and changes in these affect the probability of a bird departing from a stopover site to resume migration. Recently, radio-tracking studies have shown that sometimes migrants leave a stopover site not to continue migration, but to either search for another nearby stopover site (Mills et al. 2011; Taylor et al. 2011; Stach et al. 2015) or perform exploratory flights, likely for assessing wind conditions aloft (Schmaljohann et al. 2011). Based on these findings we scrutinize as our next objective whether leaving a stopover site indicates that the bird has actually resumed migration. Finally, we discuss why birds terminate their migratory flight, as this provides some insights about the energy loss of the previous migratory flight. This trait has been so far mostly neglected, but together with bird’s arrival condition it might be significant for explaining in more detail why energy stores and/or changes in these are relevant for the departure decision in some but not all birds (Morris 1996; Eikenaar and Bairlein 2014; Eikenaar et al. 2014a). With these critical views we do not intend to criticize, let alone reject the findings of former studies by others or ourselves, but we would like to highlight some future perspectives of stopover ecology research.
How to measure energy stores in bird migrants
The term “body condition” describes the physiological state of an individual. It is assumed that variation in this state will affect to a certain extent the behavior of a bird, in our case the departure probability from a stopover site, cf. Brown (1996). The physiological properties most commonly considered in migration studies are a bird’s fat content (Kaiser 1993) and muscle size (Bairlein 1994), i.e., protein in skeletal muscle (Jenni and Jenni-Eiermann 1998). The pectoral muscle comprises about 50% of total skeletal muscle of a bird (Ward 1969); in migrants the size of the pectoral muscle changes in course of migration (Dietz et al. 1999a, b; Battley et al. 2000; Lindström et al. 2000). Glycogen is the third type of energy store, but its contribution to the overall energy stores is low (Jenni and Jenni-Eiermann 1998) and in comparison to the other two types rarely estimated on the individual level, but see Marsh (1983). Other measurements of body condition include muscle damage (Guglielmo et al. 2001), metabolically available water (Klaassen 2004), and oxidative state (Jenni-Eiermann et al. 2014; Skrip et al. 2015; Eikenaar et al. 2017). Here we will focus on the amount of fat and protein, because these together describe well the energy stores (~95% fat and ~5% protein) available to the individual bird to use for migratory flights and maintenance (Jenni and Jenni-Eiermann 1998; McWilliams et al. 2004).
The available energy stores of a bird can be estimated internally or externally. By extracting fat and protein from a bird’s body, one obtains precise estimates for both, but dead individuals must be used (Johnson et al. 1985; Brown 1996; Piersma et al. 1999; Battley et al. 2000; Bauchinger and Biebach 2001; Bauchinger and McWilliams 2009). A non-invasive method to estimate bird’s energy stores internally is quantitative magnetic resonance, which only requires a few minutes of scanning a live bird and provides information on dry fat, wet lean mass, and total water (Guglielmo et al. 2011). Another non-invasive method is ultrasound (examination duration about 5–15 min), employed to, for example, monitor changes in organ size, e.g., pectoral muscle as the main reserve of protein (Newton 1993; Dietz et al. 1999a; Lindström et al. 2000). A bird’s fat content can be roughly estimated with this method but is subjective (Sears 1988).
There are several approaches how to estimate the energy stores externally. As different morphometric indices of body condition were recently critically reviewed by Labocha and Hayes (2012), we briefly summarize the different approaches mentioned by them and add some more. Weighing body mass is a simple method with a high repeatability and generally explains about 50% of the variation in fat (Labocha and Hayes 2012). To correct for the effect of between-individual variation in body size, body mass is divided by a structural body size measurement. For this wing chord, tail length, bill length or tarsus length are often used. However, body mass and structural body size are commonly correlated with each other (Hayes and Shonkwiler 2001), so that, e.g., comparisons of differently sized birds may not capture the real difference in their energy stores (Labocha and Hayes 2012). Some studies used the residuals of the model explaining variation in body mass by variation in structural body size (Labocha and Hayes 2012), but there are several statistical and biological concerns about this approach (Green 2001; Schulte-Hostedde et al. 2005). To account for these, the scaled mass index was recently introduced (Peig and Green 2009). Fat score (Helms and Drury 1960; Kaiser 1993) and muscle sore (Gosler 1991; Bairlein 1994) qualify the relative amount of visible subcutaneous fat and the relative size of the pectoral muscle. From these scores, the energy stores can be modeled (Salewski et al. 2009). Based on information of dissected birds or birds with a fat and muscle score indicating hardly any visible energy stores, the lean body mass of a bird can be estimated. The relative difference of bird’s actual body mass to its lean body mass is simplified as the energy stores of that bird (Alerstam and Lindström 1990; Lindström and Alerstam 1992; Dänhardt and Lindström 2001; Bayly 2006, 2007; Tsvey et al. 2007; Schaub et al. 2008; Schmaljohann and Naef-Daenzer 2011; Bulyuk 2012). If a bird’s body mass was estimated at the end of a stopover day, this energy store was often termed “evening fuel load” and when the estimate was derived from shortly before the actual departure event “departure fuel load”. Difference in the evening to evening estimates of energy stores relative to bird’s lean body mass describes the rate of accumulating energy for that period (Alerstam and Lindström 1990). Thus, it integrates what the bird experienced over the considered time period expressed as the amount of energy stores temporally gained or lost. In contrast to all other methods, abdominal profiles estimate the energy stores based on body’s shape between legs and the tail as observed in the field so that the observer does not have direct contact with the birds (Owen 1981; Bowler 1994; Boyd and Fox 1995; Wiersma and Piersma 1995; Clausen et al. 2003; Duriez et al. 2009). Although some studies have verified that this approach seems to be valid (Féret et al. 2005; Madsen and Klaassen 2006; Moriguchi et al. 2006), production of eggs (Brown 1996), bird’s posture (Owen 1981), and an observer effect (Féret et al. 2005) have to be considered. This index is mostly used for swans, geese, and waders.
How to identify when birds arrive at and depart from their stopover sites
When studying how energy stores at arrival and/or on evenings are related to the departure probability of a bird, we should be aware that each method to estimate arrival and departure events provides a certain temporal uncertainty.
Many studies on stopover ecology were carried out in coastal areas, peninsulas, or islands (Moore and Kerlinger 1987b; Moore et al. 1990; Kuenzi et al. 1991; Moore and Yong 1991; Yong et al. 1998; Dierschke and Delingat 2001, 2003; Bolshakov et al. 2003; Schmaljohann and Dierschke 2005; Bayly 2006, 2007; Bulyuk and Tsvey 2013; Schmaljohann et al. 2013; Smolinsky et al. 2013; Deppe et al. 2015; Dossmann et al. 2015; Mitchell et al. 2015; Sjöberg et al. 2015; Woodworth et al. 2015) or at oases in deserts which are similarly isolated as islands in the sea (Bairlein et al. 1983; Biebach 1985; Bairlein 1985a, b; Biebach et al. 1986; Bächler and Schaub 2007; Salewski and Schaub 2007; Salewski et al. 2007, 2010) but only few at inland sites surrounded by benign landscape (Cohen et al. 2012, 2014; Woodworth et al. 2014). This spatial bias exists because isolated areas at or in the sea/deserts usually support few breeding individuals and are species poor, meaning that most birds occurring at these areas during migration are “real” migrants and not local breeders. Furthermore, due to the transition from land to sea high numbers of birds arrive at these areas at certain weather conditions (Newton 2008) and there commonly is a high turn-over rate (Schmaljohann et al. 2013). In stopover studies, researchers often simply treat trapped birds as having arrived on the same day owing, e.g., to the apparent large variation in daily numbers of observed migrants present at the study site (Dierschke et al. 2005; Schmaljohann and Dierschke 2005; Schmaljohann et al. 2013). If formerly marked birds are not re-sighted in the research area during daily routine surveys, researchers commonly assume that these birds have departed between the last and the current routine survey (Dierschke et al. 2005; Schmaljohann and Dierschke 2005; Schmaljohann et al. 2013). Stopover duration is, therefore, often simplified as the difference between the estimated “arrival day” and the day of last re-sighting (Dierschke et al. 2005; Schmaljohann and Dierschke 2005; Schmaljohann et al. 2013). This estimate is usually called “minimum stopover duration” (Schaub et al. 2001).
This approach might be applicable for species which are easily observed, e.g., geese, swans, waders, and that rest in open and isolated habitats (Madsen 2001; Gillings et al. 2009; Lourenço et al. 2010; Navedo et al. 2010; Lok et al. 2015). In others, e.g., small land birds stopping over in more complexly structured habitats, pelagic seabirds or aerial birds feeding on the wing (swifts, swallows, some raptors), it is rather difficult to identify the space covered and time spent at stopover, because detection probability can be rather low (Bächler and Liechti 2007) and birds can leave the trapping site but stay in the stopover area (Bächler and Schaub 2007). As birds could have arrived in the study area before first sighting/capture and stayed longer after last re-sighting, the uncertainty in estimated arrival and departure date leads to an underestimation of the actual stopover duration (Schaub et al. 2001). This uncertainty can be reduced by modeling stopover durations with individual re-sightings using Cormack–Jolly–Seber (CJS) capture–recapture models (Cormack 1964; Jolly 1965; Seber 1965; Schaub et al. 2001; Arizaga et al. 2011; Schmaljohann et al. 2013). How much these estimates of stopover duration still vary from the true stopover durations depends among others on the frequency of the re-sightings (Bächler and Schaub 2007; Salewski and Schaub 2007) and local movements (Bächler and Schaub 2007).
Another approach to estimate stopover duration is based on site-specific recaptures in high mist-nets. Here nocturnally migrating birds are initially captured and ringed at a stopover site during daytime. This date is treated as the arrival date of the bird. The date of their recapture in high mist-nets during the night then indicates their departure date (Bolshakov et al. 2000, 2003; Bulyuk 2012; Bulyuk and Tsvey 2013). As recapture probability in such high mist-nets is rather low, a high number of daytime captures is required to achieve a reasonable number of nighttime recaptures (Bolshakov et al. 2003). As in the other methods, it remains unknown when birds had actually arrived at the stopover site. An additional issue is whether nocturnal flights resulting in captures in the high mist-nets served to explore wind conditions aloft (Liechti 2006; Schmaljohann et al. 2011) or to leave the stopover site.
By marking birds with radio tags within a large-scale array of radio-tracking stations covering the specific stopover landscape for a certain species (Taylor et al. 2011) and/or on isolated islands (Goymann et al. 2010; Schmaljohann et al. 2011; Crysler et al. 2016) one can discriminate between these two motivations to perform nocturnal flights.
True stopover duration of individual birds can, however, only be determined when birds are marked before reaching the stopover site. Some studies have started to provide such precise data by individually following marked birds throughout single to several migratory flights (Wikelski et al. 2003; Bairlein et al. 2015b), by tracking migratory movements in a high spatiotemporal resolution (Mandel et al. 2008; Bouten et al. 2013) or by setting up a large-scale array of digital automated radio-tracking stations so that birds tagged at one site can be continuously tracked during consecutive migratory flights and/or picked up at another radio-tracking station (Brown and Taylor 2015; Deppe et al. 2015; Dossmann et al. 2015; Mitchell et al. 2015; Woodworth et al. 2015; Crysler et al. 2016). Tracking devices transmitting bird’s location via satellite connection in real time to the researcher can so far only be used with relatively large species (Fiedler 2009; Bridge et al. 2011). For such species it is, however, generally difficult to estimate their energy stores at arrival and departure because of the difficulty of catching the individual birds. Abdominal profiles estimates might be an option for swans, geese, and waders (Wiersma and Piersma 1995; Clausen et al. 2003; Prop et al. 2003; Duriez et al. 2009).
The above-listed disadvantages pertaining to the different methods lead to uncertain estimates of stopover duration. This increases the variation in estimated energy stores at arrival and departure which in turn reduces the power to find potential effects of energy stores on departure probability. Although we cannot easily solve the problem of identifying when a bird arrived at certain stopover sites for most species, we can at least increase the accuracy of when a stopover is terminated by tracking individual birds.
How do energy stores and rate of accumulating energy affect departure decisions
Fat and protein are the main energy stores required for locomotion. As the size of energy stores limits the duration of any locomotion, birds with no or small energy stores are unlikely to resume migration before having accumulated a sufficient amount of energy to fly at least some hours. In contrast, birds with high-energy stores might depart at any time. Thus, it seems obvious that energy stores will have a paramount effect on the departure decision.
This effect was indeed observed in many studies on songbirds, waders, geese, ducks, and other species irrespective of how energy stores were estimated: body mass relative to body size (Safriel and Lavee 1988), fat class (Meissner 1998; Dierschke and Delingat 2001; Goymann et al. 2010; Cohen et al. 2014; Smith and McWilliams 2014; Deppe et al. 2015; Dossmann et al. 2015; Woodworth et al. 2015), fuel load (Rabøl and Peterson 1973; Cherry 1982; Moore and Kerlinger 1987b; Loria and Moore 1990; Bairlein 1985b; Biebach et al. 1986; Morris 1996; Schmaljohann et al. 2013; Cohen et al. 2014; Stach et al. 2015) or abdominal profile (Prop et al. 2003). However, there are also many studies on the same bird groups which failed to show this effect in relation to body mass (Holmgren et al. 1993), body mass relative to body size (Safriel and Lavee 1988), fat class (Kuenzi et al. 1991; Skagen and Knopf 1994; Lyons and Haig 1995; Morris et al. 1996; Dierschke and Delingat 2001; Salewski and Schaub 2007; Schaub et al. 2008), fuel load (Ellegren 1991; Dierschke and Delingat 2001; Tsvey et al. 2007), and abdominal profile (Duriez et al. 2009). The biological significance of actual energy store on the departure decision from a stopover site, therefore, appears to remain controversial (Jenni and Schaub 2003).
In the studies where energy stores were not positively related to departure probability, intrinsic factors (sex, age, molt, endogenous time program) and extrinsic factors (competition, predation, temperature, habitat quality, wind, season) likely play an important role for the departure decision (Morris 1996; Erni et al. 2002a; Jenni and Schaub 2003; Wikelski et al. 2003; Liechti 2006; Duriez et al. 2009; Chernetsov 2012; Cohen et al. 2012, 2014; Schmaljohann et al. 2013; Smith and McWilliams 2014; Eikenaar et al. 2016). These factors might have either a direct effect on a bird’s departure decision, e.g., rain (Erni et al. 2002b; Schaub et al. 2004) or wind (Liechti 2006), or influence the rate at which energy is accumulated during stopover (see below). As the rate of accumulating energy stores determines the time until a certain energy level is reached at stopover, this rate itself was suggested to affect the departure probability in bird migrants (Alerstam and Lindström 1990; Jenni and Schaub 2003; Hedenström 2008; Alerstam 2011). Estimating the true rate of energy accumulation in free-flying birds is rather difficult (Delingat et al. 2009), because ideally body mass of a given individual is recorded every evening after its food intake during its entire stopover. This type of data was collected for several songbird and one hummingbird species by attracting wild birds to artificial feeding stations with balances for self-weighting (Fig. 1a). Although supplementary feeding might influence the feeding behavior of birds, rates of accumulating energy and amount of energy stored were generally not higher than those observed under natural feeding conditions, reviewed by Schmaljohann et al. (2013). Further, Fig. 1b indicates that field estimates were generally within the same order of magnitude as the theoretically predicted values based on an upper limit to daily metabolisable energy intake (Lindström 1991). The high rates were likely an overestimation though, because of undigested food items ingested shortly before reading the evening body mass. Between individual variation in the rate of accumulating energy has further been explained with individual-specific refueling efficiency (Corman et al. 2014; Eikenaar et al. 2016), time within season (Bauchinger and Klaassen 2005; Eikenaar et al. 2016), food quality and gut length (Van Gils et al. 2008), nocturnal energy loss associated with ambient temperature (Wikelski et al. 2003; Maggini and Bairlein 2013), predation risk (Schmaljohann and Dierschke 2005), competition (Lindström et al. 1990; Moore and Yong 1991; Moore et al. 2003), but see Carpenter et al. (1983) and Dierschke et al. (2005), time (days) before departure (Fransson 1998b; Bayly 2007), and the position along the migration route (Fransson et al. 2001). It should be noted that the high refueling rates in the sedge warbler (Acrocephalus schoenobaenus) data (Fig. 1b) are accounted for by their specific migration strategy (Bayly 2007). They are thought to migrate without additional feeding from northwest Europe to their sub-Sahelian wintering grounds. Hence, when encountering superabundant food supply in form of reed aphids, they intensively exploit this food source by showing high rates of accumulation and resuming migration with large energy stores in preparation for the long stretch with few feeding opportunities (Bibby and Green 1981). The high values in the study of Dierschke et al. (2005) are explained by a different way of calculating the rate of accumulating energy in comparison to the others, see Fig. 1b.
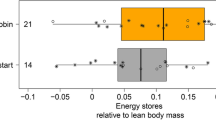
a An individually colour-ringed northern wheatear (Oenanthe oenanthe) perched at a bowl with mealworms (Tenebrio molitor). Balance’s display was read with a telescope the moment before the bird approached the bowl and when the bird was feeding mealworms. By subtracting the former reading from the latter the current body mass of the bird was estimated, for further information see Schmaljohann and Dierschke (2005). Photo: Heiko Schmaljohann. b Rates of accumulating energy (presented as boxplots) during entire stopover of free-flying birds being attracted to balances for reading body mass by ad libitum supplied food. Rates of accumulating energy were calculated based on bird’s body mass on the evening of departure subtracted from its body mass at arrival (“date of capture”, i.e., time of capture or first evening body mass) relative to its lean body mass and the number of stopover days. In Dierschke et al. (2005) the rate was calculated as the difference between evening body mass on departure night and morning body mass of first stopover divided by bird’s lean body mass and number of stopover days (§). Sample size is given above the corresponding boxes. Boxes present 5, 25, 50, 75, and 95% percentiles and outliners (open circles). The grey area indicates the theoretically predicted maximum rate of accumulating energy for a songbird of 10–30 g after Lindström (1991). c Departure fuel load over rates of accumulating energy. Departure fuel load is the difference between bird’s body mass on the evening of departure (“last resighting”) and its lean body mass relative to its lean body mass. Study species in which departure fuel load and rate of accumulating energy significantly correlated with each other: □ rufous hummingbird (Selasphorus rufus), birds stayed 1–2 weeks at the stopover site (Carpenter et al. 1983); ○ reed warbler (Acrocephalus scirpaceus), birds stayed at least 3 days (Bayly 2006); + sedge warbler (A. schoenobaenus), birds stayed at least 1 day (Bayly 2007); Χ bluethroat (Luscinia svecica), birds stayed at least 4 days (Lindström and Alerstam 1992); ◇ northern wheatear (Oenanthe oenanthe) on Helgoland in autumn, birds stayed at least 4 days (Schmaljohann and Dierschke 2005) and △ in spring (males only), birds stayed at least 3 days (Dierschke et al. 2005), *on Iceland in autumn, birds stayed at least 1 day (Delingat et al. 2008). Study species/sexes in which both traits did not correlate: ■ whitethroat, birds stayed at least 3 days (Sylvia communis) (Fransson 1998a); ● European robin (Erithacus rubecula), birds stayed at least 3 days (Dänhardt and Lindström 2001); ▲ female northern wheatears on Helgoland in spring, birds stayed at least 3 days (Dierschke et al. 2005), ♦ northern wheatears in Alaska, birds stayed at least 1 day (Schmaljohann et al. 2013)
Within optimal migration theory, it is predicted for birds minimizing the overall time spent on migration that the rate of accumulating energy is the crucial determinant of departure decision from a stopover site (Alerstam and Lindström 1990). The so-called time minimizers are assumed to exploit the stopover sites where experiencing a high rate of accumulating energy stores and finally depart with high fuel loads. If the currently experienced rate of accumulation drops below a certain level, indicating a slow overall migration speed, they are thought to leave the stopover site. Consequently, energy stores at departure are expected to correlate positively with the rate of accumulating energy stores in these time minimizers (Alerstam and Lindström 1990; Lindström and Alerstam 1992; Hedenström and Alerstam 1997). In the studies providing individual estimates on both traits, this pattern was found in most (Carpenter et al. 1983; Lindström and Alerstam 1992; Fransson 1998a; Dierschke et al. 2005; Schmaljohann and Dierschke 2005; Bayly 2006, 2007; Delingat et al. 2006, 2008; Schmaljohann et al. 2013) but not all species (Dänhardt and Lindström 2001) and not in both sexes (Dierschke et al. 2005; Delingat et al. 2006) (Fig. 1c). Although this highlights the potential importance of the rate of accumulating energy stores for the departure decision (Jenni and Schaub 2003; Hedenström 2008; Schaub et al. 2008; Alerstam 2011), little is known how daily rates of accumulating energy affect the actual departure probability.
To get to grips with this we re-analysed existing data on migratory northern wheatears (Oenanthe oenanthe) (Schmaljohann and Dierschke 2005). These birds are typical nocturnal songbird migrants that spent their non-breeding period in sub-Sahelian Africa (Bairlein et al. 2012; Schmaljohann et al. 2016). For this study, data were collected on Helgoland, a small offshore island in the German Bight, in autumn 2001. Stopover duration of color-ringed northern wheatears was considered as the difference between “arrival day” and the day of last sighting. The resighting probability the day following ringing was 83% (for all birds pooled the number of days with observations divided by the number of days between ringing and last observation). Remote weighing of color-marked birds supplied with ad libitum food (mealworms, Tenebrio molitor) provided individual data on rate of accumulating energy (Fig. 2b) and evening energy stores (Fig. 2c). The rate was only calculated when evening (max. 2 h before sunset) body mass estimates were recorded on two consecutive evenings. This difference was then divided by bird’s lean body mass. Evening energy stores were expressed as the difference between bird’s evening body mass and its lean body mass relative to its lean body mass; for further details see Schmaljohann and Dierschke (2005). The day of last sighting at a feeding location indicated departure from Helgoland on that night. Based on these data Schmaljohann and Dierschke (2005) demonstrated that fuel load at departure was positively correlated with the average rate of accumulating energy during the stopover duration suggesting that northern wheatears behaved in accordance with the time minimization hypothesis (Alerstam and Lindström 1990; Lindström and Alerstam 1992). Here we analysed the data in a different and novel way. Instead of considering values averaged over birds’ stopover, we related the daily rates of accumulating energy, evening energy stores, and bird’s stopover day (going from 1 to max. 10 days in this study, Fig. 2a) to the probability to resume migration in a generalized linear mixed effect model with bird as a random factor to account for multiple readings of the same bird during its stay, see Fig. 2 for more information. In this study, the rate of accumulating energy significantly negatively affected the departure probability (Fig. 2c). Birds experiencing a loss of energy stores or a low rate of accumulating energy had a high probability of leaving Helgoland, whereas high rates were observed in birds staying on the island. In accordance with former studies, the departure probability seemed to increase with energy stores (Goymann et al. 2010; Schmaljohann et al. 2013), although the effect was not significant in the current re-analysis (Fig. 2c).
a Minimum stopover duration as estimated by colour-ring observations on Helgoland for the 40 northern wheatears (Oenanthe oenanthe) that stayed at least one day on the island after the day of catching; data from Schmaljohann and Dierschke (2005). Median stopover duration was 2.5 days. b, c Departure probability of these 40 northern wheatears was modeled for each day of stopover with a generalized linear mixed effect model, bird as a random factor to account for multiple readings of the same bird during its stay, with functions of the R package “lme4” (Bates et al. 2014). If available, individually specific daily values of rate of accumulating energy (n = 96), evening fuel load (n = 106), and day of stopover (n = 126) were z transformed, all tested against each other for collinearity [which was not the case, vif < 1.29 (Babak 2013; Zuur et al. 2010)], and considered as explanatory variables. The 95% CrI of the possible two-way interactions all included zero and were, therefore, removed from the model. b Black dots jittered in the vertical direction demonstrate stay (0) or departure (1). Rate of accumulating energy had a significant negative effect on departure probability with low values being associated with high departure probabilities; 95% credible interval (CrI): −1.29 to −0.22. Given are the fitted values (solid line), with the 95% CrI (dashed lines). To show the effect of rate of accumulating energy on departure probability, evening fuel load and day of stopover were set to their corresponding mean values. c Evening fuel load had a near significant positive effect on departure probability with high values tending to be associated with high departure probabilities; 95% CrI: −0.02–1.06; fitted values (solid line), 95% CrI (dashed lines). To show the effect of evening fuel load on departure probability, the other two variables were set to their corresponding mean values
Ours and previous data thus suggest that there might be a simple departure rule: birds having arrived at a new stopover site may have low energy stores and may initially experience low rate of accumulating energy because of search and settling costs (Alerstam and Lindström 1990; Hedenström and Alerstam 1997). If abiotic (weather) and biotic (food supply, competition) conditions are favourable, an individual will accumulate fuel at a high rate which will, initially, decrease its probability to depart. Once sufficient energy stores are accumulated to at least support the next migratory flight bout, the rate of accumulating further energy is reduced (Fransson 1998b; Delingat et al. 2006). Then, if environmental conditions are additionally favourable, the individual bird is likely to depart. Under the circumstances that an individual is unable to accumulate fuel, it will, given favourable weather conditions, have a high probability to leave the stopover site. Some support for these departure rules comes from cage experiments on wild northern wheatears in which Eikenaar and Schläfke (2013) showed that birds having high energy stores did not refuel, but showed high amounts of nocturnal migratory restlessness. Migratory restlessness in this species is a good approximation for departure probability (Eikenaar et al. 2014b). In contrast, birds with little energy stores showed high rates of accumulating energy, but showed little nocturnal migratory restlessness (Eikenaar and Schläfke 2013). A similar behavioural rule was suggested by Schaub et al. (2008) using body mass change between first and last capture relative to the time elapsed for describing the rate of accumulating energy. Their data suggested that birds experiencing a medium rate of accumulating energy had the highest departure probability (Schaub et al. 2008). Regardless of the differences between our model and that of Schaub et al. (2008), which could be related to the way how the rate of accumulating energy was estimated (Delingat et al. 2009), Schaub et al. (2008) and our analysis (Fig. 2b) provide strong correlative evidence that this rate is indeed a significant factor influencing in a non-linear relationship the probability of an individual to depart.
In addition to other intrinsic and extrinsic factors modulating the decision when to resume migration within the endogenously controlled migratory program (Jenni and Schaub 2003; Bairlein et al. 2015a), there are two more important aspects likely affecting departure decisions that have been largely neglected in stopover ecology studies: first, leaving a stopover site does not always involve truly resuming migration. Second, do the reasons why birds terminate their migratory flight affect their refueling behavior and departure decisions?
Leaving a stopover site does not always involve truly resuming migration
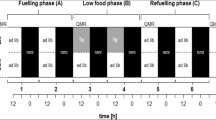
We define here stopover as any time period spent on the ground between migratory flights, i.e., from the point of time when a migratory flight was terminated until the point of time when the next migratory flight begins. During this time a bird, e.g., sleeps, rests, preens itself, seeks shelter, feeds and/or searches for a site within the current stopover landscape with higher temperatures (Wikelski et al. 2003) or better refueling conditions (Mills et al. 2011; Taylor et al. 2011; Stach et al. 2015). Birds might do so by moving from bush to bush (Baird and Nisbet 1960) or by ascending and flying up to some tens (or even more) of kilometers (Mills et al. 2011; Taylor et al. 2011; Brown and Taylor 2015; Stach et al. 2015). These are the two extreme behaviors set at opposing ends of a continuum and any behavior between these extremes could be regarded as local movements towards more favorable stopover sites and thus be disregarded as migration. We adopt here the term first mentioned by Taylor et al. (2011) and summarize all these movements as “landscape movements”, in contrast to migratory flights (Fig. 3). Flight movements contrasting to the seasonally appropriate migratory direction, commonly termed reverse migration (Alerstam 1978; Richardson 1978, 1982; Liechti 1993), in our opinion also belong to “landscape movements”. They were explained by inaccuracy in the orientation skills of birds (Komenda-Zehnder et al. 2002; Zehnder et al. 2002), as an energy-saving strategy to reduce costs for thermoregulation (Wikelski et al. 2003), as a reaction when facing an ecological barrier (Bruderer and Liechti 1998) with low energy stores (Åkesson et al. 1996; Sandberg and Moore 1996; Sandberg et al. 2002; Deutschlander and Muheim 2009; Schmaljohann and Naef-Daenzer 2011; Smolinsky et al. 2013; Deppe et al. 2015; Nilsson and Sjöberg 2016), as a reaction to strong competition and high predation risk (Nilsson and Sjöberg 2016) and they appear more often in young than in adults birds (Nilsson and Sjöberg 2016).
A graphical description of landscape movements and migratory flights. With a migratory flight a bird arrives at the initial landing site within a certain stopover landscape. If abiotic (temperature, precipitation) and biotic (food availability, predation, competition) factors do not provide favourable conditions for resting and refuelling, the bird moves on in search for better conditions. Such a search could include several short-term stays at different locations within the same stopover landscape. Moving away from the initial landing site and these shortly visited locations to others is not a resumption of migration. After Taylor et al. (2011) these movements are called “landscape movements”. Eventually, the bird encounters favourable stopover conditions somewhere within the stopover landscape from where it later resumes migration and starts its next migratory flight. Landscape movements are not necessarily oriented towards a certain direction and can head in any direction from the initial landing site. Long movements towards the seasonally appropriate migratory direction might be indicative for resuming migration
Clearly thus, if a bird leaves the current stopover site, it does not necessarily mean that it has actually resumed migration. Likewise, new arrivals could have terminated a migratory flight or a landscape movement. Although distinguishing between landscape movements and migratory flights is difficult on the level of the individual, the flight direction from a stopover being directed towards the seasonally appropriate migratory direction might be a good first indication that migration was resumed (Fig. 3). To estimate the seasonally appropriate migratory direction, we can use ring recoveries (Bairlein et al. 2014), stable isotopes (Rubenstein and Hobson 2004), a combination of both (Van Wilgenburg and Hobson 2011), genetic differences between populations (Bensch et al. 2002) and various tracking devices (Bridge et al. 2011; Fiedler 2009).
It seems that we, stopover ecologists, have not properly distinguished between landscape movements and migratory flights in the past. Landscape movements may serve either to search for a more appropriate stopover site (in terms of food, shelter, predation or navigation), or to assess weather conditions aloft, which may unintendedly get the birds to land at a different site. Hence, leaving a stopover site is not necessarily related to bird’s current energy stores. Thus, variation in the estimated energy stores of the “departing” birds was overestimated resulting in a lower power to find a significant effect and therefore yielding more type II errors in former studies than statistically expected. This may also have an effect on the predictions of the optimal migration theory which are based on the assumption that all birds that leave a stopover site, truly resume migration, and not relocate within the stopover landscape. Separating the departures from a stopover site resulting in landscape movements from those serving the bird to actually resume migration is crucial, because this will increase the power to identify the importance of energy stores or other factors on departure decisions.
Why do birds terminate their migratory flight to arrive at a stopover site during migration?
The urge of a migrant to refuel depends on the current energy stores and the upcoming migratory flight, e.g., long non-stop flight vs. single daytime flight. Recent fasting-refueling experiments with caged northern wheatears in migratory disposition additionally showed that the loss rate of energy stores had a significant positive effect on bird’s urge to refuel (Eikenaar and Bairlein 2014; Eikenaar et al. 2014a). The authors, therefore, suggested that the energy loss during the previous migratory flight is also predictive of a bird’s urge to refuel. Here we hypothesize that energy stores at arrival together with the amount of previous energy loss will describe well birds’ urge to accumulate energy in relation to its migration strategy (Eikenaar et al. 2014a). If we had information about all three traits for an individual migrant, i.e., arrival energy stores, energy loss during the previous flight and the bird’s migration strategy, we would better understand how the rate of accumulating energy regulates departure probabilities in migratory birds (Schaub et al. 2008) (Fig. 2). Energy loss is, however, difficult to measure in free-flying birds because the energetic cost of a flight bout is a function of, among other things, airspeed (Pennycuick 1975; Hedenström 2002), actual body mass (Norberg 1995, 1996), flight style (Rayner 1999; Rayner et al. 2001), air density (Schmaljohann and Liechti 2009), and the time of flying. To our knowledge, a study on New World Catharus thrushes (Wikelski et al. 2003) and a study on northern bald ibis (Geronticus eremite) (Bairlein et al. 2015b) are the only ones quantifying migrants’ energy expenditure during free-flight. In addition to the doubly labelled water approach used in these two studies, energy expenditure could be estimated by measuring heart beat rates in free-flying birds (Butler and Woakes 1980; Masman and Klaassen 1987; Butler et al. 2000; Ward et al. 2002; Bowlin and Wikelski 2008; Sapir et al. 2010). Both methods are, however, not feasible when working at a specific stopover site, because individual birds have to be tagged somewhere away from the study site on the evening of their departure and later have to land at the study stopover site.
Below we present several different scenarios in which migrants terminate their migratory flight and for these qualify the extent of energy loss during the preceding flight and the urge to refuel (Table 1). For this we have to assume that diurnal/nocturnal migrants usually set off at the beginning of the day/night and continue migration until midday/close to the next sunrise (Dorka 1966; Bäckman et al. 2017; Müller et al. 2016).
-
1.
When flying over benign habitat and during favorable weather, i.e., no strong headwind and no rain (Erni et al. 2002b; Schaub et al. 2004; Delingat et al. 2008), migrants will generally find suitable areas for refueling (Bairlein 1983, 1985b, 1991; Hutto 1985; Ormerod 1990; Moore and Aborn 2000; Chernetsov 2006; Alerstam 2009; Jenni-Eiermann et al. 2011). Migrants in general carry slightly larger energy stores than would be required for the upcoming migratory flight (Bairlein 1985b; Biebach et al. 1986; Bolshakov et al. 2003; Delingat et al. 2006; Salewski et al. 2010), possibly as a safety margin either for unexpected energetically more demanding migratory flights and/or because it is difficult to foresee future feeding conditions at upcoming stopover sites (Schaub and Jenni 2001). Under such circumstances and the assumption that birds exploit most of the day/night for the migratory flight we hypothesize that migrants show a medium loss of energy stores and that they still have sufficient energy stores to weather unexpected unfavorable feeding conditions at the current site for a few days. Thus, the urge to refuel is moderate and lean birds are only an exception.
-
2.
Migrants approaching crucial stopover landscapes for refueling before crossing large-scale ecological barriers and/or flying long stretches non-stop have to stopover somewhere within this landscape and thus terminate their migratory flight independent from the current energy stores, e.g., the coastal regions of large bodies of water (Smith et al. 2007; Johnson and Winker 2008; Bayly and Gómez 2011; Bayly et al. 2013), the border area of large deserts (Smith 1966; Fry et al. 1970; Dowsett and Fry 1971; Moreau 1972; Finlayson 1981; Bairlein 1991; Jones 1995; Ottosson et al. 2001, 2005; Maggini and Bairlein 2010a; Bayly et al. 2012; Trierweiler et al. 2014), and tidal mud flats (Battley et al. 2005; Piersma et al. 2005; Zwarts et al. 1990). How to reach these crucial stopover landscapes might be endogenously controlled (Gwinner and Wiltschko 1978; Berthold and Querner 1981; Fransson et al. 2001; Maggini and Bairlein 2010b; Bulte et al. 2017) or learnt (Harrington et al. 1988; Reed et al. 1998; Fox et al. 2002; Kruckenberg and Borbach-Jaene 2004). Hence, birds are unlikely to arrive at these stopover landscapes with fully depleted energy stores. For this scenario we hypothesize a medium loss of energy stores during the flight to the stopover landscape and sufficient energy stores to search within this landscape for a favorable stopover site. In contrast to scenario 1, birds have a strong urge to accumulate large amounts of energy in preparation for the upcoming high-energy demanding flight period.
-
3.
Upon arrival at the “first” stopover landscapes after an energetically highly demanding flight migrants have lost a large fraction of their energy stores (Loria and Moore 1990; Moore and Yong 1991; Pilastro and Spina 1997; Yong and Moore 1997; Spina and Pilastro 1999; Battley et al. 2000; Ottosson et al. 2002; Yohannes et al. 2008; Maggini and Bairlein 2010a), might be physically exhausted (Schwilch et al. 2002), and may experience water stress when migrated at high temperatures (Biesel and Nachtigall 1987; Carmi et al. 1992; Giladi and Pinshow 1999; Ward et al. 1999; Michaeli and Pinshow 2001), but see Schmaljohann et al. (2008). Birds with fully depleted of energy stores stay and refuel at the surroundings of the first site (Maggini and Bairlein 2010a), while others with some remaining energy stores might search for a specific stopover site. In both cases the urge to accumulate energy and recover is strong and bird’s energy stores are more depleted than in the other scenarios.
-
4.
If departure of a migratory flight was not well timed with respect to the bird’s energy stores and wind conditions, a bird may deplete its energy stores and/or could get physically exhausted, so that it has to land earlier than intended. Such emergency landings could happen anywhere and anytime during migration, which increases the variation in the energy stores we find in arriving migrants and which increases birds’ urge to refuel at the stopover site.
-
5.
Birds usually time their departure from a stopover site to avoid unfavorable weather conditions for the next migratory flight bout (Erni et al. 2002b; Schaub et al. 2004; Delingat et al. 2008; Deppe et al. 2015). If the weather conditions deteriorate during the night because of heavy rainfall and/or increasing headwind, birds likely terminate their flight early. If, in this scenario, birds are able to land, less energy has been used than during a normal migratory flight, and the urge to refuel will be small. However, if such a change in the weather condition occurs over an ecological barrier, birds either reverse to a former site or continue until landing is possible. Under these circumstances birds often have to invest much more energy for flying than usual and arrive in a depleted state so that they have a high urge to refuel and only little energy stores left.
Depending on the scenarios why birds terminate their migratory flight and in relation to their migration strategy, energy stores and the urge to refuel can differ substantial between species and individuals. These differences significantly affect the importance of energy stores and the rate of accumulating energy for the departure decision within an individual. Regarding the distribution of migrating birds on land, the majority of birds land most of the times at conditions described under scenario (1), less often as described under scenario (4), and even less frequently as described under the other scenarios. Nevertheless, scenarios (2), (3), and (5) are biologically important, because most stopover ecology studies are performed at coastal areas or islands, where birds land under such scenarios on a regular basis. Although in many instances it may not be possible to determine why a bird has landed at a stopover site, this knowledge would very much facilitate formulating clear hypotheses of how energy stores and rates of accumulating energy influence bird’s departure probability, given the species-/bird-group-specific migration strategy.
Conclusion
Our review shows that to better understand the relationship between energy stores and departure from stopover, several issues need to be tackled in future stopover ecology studies. The large-scale radio-tracking systems in North and South America (Brown and Taylor 2015; Deppe et al. 2015; Dossmann et al. 2015; Mitchell et al. 2015; Woodworth et al. 2015; Crysler et al. 2016) or satellite transmitters revealing bird’s whereabouts at a high spatiotemporal resolution in real time provide an excellent basis for this scientific task. With such systems we can identify when a bird arrives at a stopover site and whether leaving the site results in a migratory flight. Furthermore, flight duration, wind conditions experienced en route, and/or heart rates (Bowlin and Wikelski 2008; Sapir et al. 2010) allow estimating energy loss during a given migratory flight. Investigating a bird’s energy loss during the previous flight bout, its arrival condition, and combining this knowledge with its migration strategy will significantly increase our understanding on the factors controlling the departure probability at a stopover site. Still, whether the phenotypic reaction norm of a migrant in response to energy stores or the rate of accumulating energy studied at one stopover site reflects the behavioral reaction pattern along its entire migration route is questionable. This seems unlikely, because birds encountering an ecological barrier adjust their departure decision to the specific ahead conditions (Schmaljohann and Naef-Daenzer 2011; Smolinsky et al. 2013; Deppe et al. 2015; Schmaljohann et al. 2017) and because the remaining migration distance to the migratory destination influences bird’s movement ecology (Alerstam 2001; Karlsson et al. 2012; Schmaljohann et al. 2017) and with that also its departure decision (Schmaljohann et al. 2017). Ideally, in addition to the traits mentioned above, individual migrants’ breeding areas and the wintering grounds are known so that we can pinpoint each individual’s location within its migration route.
References
Adamík P, Emmenegger T, Briedis M, Gustafsson L, Henshaw I, Krist M, Laaksonen T, Liechti F, Procházka P, Salewski V, Hahn S (2016) Barrier crossing in small avian migrants: individual tracking reveals prolonged nocturnal flights into the day as a common migratory strategy. Sci Rep 6:21560
Åkesson S, Hedenström A (2007) How migrants get there: migratory performance and orientation. Bioscience 57:123–133
Åkesson S, Karlsson L, Walinder G, Alerstam T (1996) Bimodal orientation and the occurrence of temporary reverse bird migration during autumn in south Scandinavia. Behav Ecol Sociobiol 38:293–302
Åkesson S, Klaassen RHG, Holmgren J, Fox JW, Hedenström A (2012) Migration routes and strategies in a highly aerial migrant, the Common Swift Apus apus, revealed by light-level geolocators. PLoSONE 7:e41195
Åkesson S, Bianco G, Hedenström A (2016) Negotiating an ecological barrier: crossing the Sahara in relation to winds by common swifts. Phil Trans R Soc B 371:20150393
Alerstam T (1978) Reoriented bird migration in coastal areas: dispersal to suitable resting grounds? Oikos 30:405–408
Alerstam T (1990) Bird Migration. Cambridge University Press, Cambridge
Alerstam T (2001) Detours in Bird Migration. J Theor Biol 209:319–331
Alerstam T (2009) Flight by night or day? Optimal daily timing of bird migration. J Theor Biol 258:530–536
Alerstam T (2011) Optimal bird migration revisited. J Ornithol 152:5–23
Alerstam T, Lindström à (1990) Optimal bird migration: the relative importance of time, energy, and safety. In: Gwinner E (ed) Bird migration: physiology and ecophysiology. Springer, Berlin Heidelberg, pp 331–351
Alerstam T, Hedenström A, Åkesson S (2003) Long-distance migration: evolution and determinants. Oikos 103:247–260
Archibald KM, Buler JJ, Smolinsky JA, Smith RJ (2017) Migrating birds reorient toward land at dawn over the Great Lakes. Auk 134:193–201
Arizaga J, Belda EJ, Barba E (2011) Effect of fuel load, date, rain and wind on departure decisions of a migratory passerine. J Ornithol 152:991–999
Arzel C, Elmberg J, Guillemain M (2006) Ecology of spring-migrating Anatidae: a review. J Ornithol 147:167–184
Babak N (2013) usdm: Uncertainty analysis for species distribution models. R package version 11–12. http://CRAN.R-project.org/package=usdm. Accessed 08 Mar 2017
Bächler E, Liechti F (2007) On the importance of g(0) for estimating bird population densities with standard distance-sampling: implications from a telemetry study and a literature review. Ibis 149:693–700
Bächler E, Schaub M (2007) The effects of permanent local emigration and encounter technique on stopover duration estimates as revealed by telemetry and mark-recapture. Condor 109:142–154
Bäckman J, Andersson A, Alerstam T, Pedersen L, Sjöberg S, Thorup K, Tøttrup AP (2017) Activity and migratory flights of individual free-flying songbirds throughout the annual cycle: method and first case study. J Avian Biol 48:309–319
Baird J, Nisbet ICT (1960) Northward fall migration on the Atlantic coast and its relation to offshore drift. Auk 77:119–149
Bairlein F (1983) Habitat selection and associations of species in European passerine birds during southward, post-breeding migrations. Ornis Scand 14:239–245
Bairlein F (1985a) Autumn migration of palaearctic waterbirds across the Algerian Sahara. Avocetta 9:63–72
Bairlein F (1985b) Body weights and fat deposition of Palaearctic passerine migrants in the central Sahara. Oecologia 66:141–146
Bairlein F (1991) Body mass of Garden Warblers (Sylvia borin) on migration: a review of field data. Vogelwarte 36:48–61
Bairlein F (1994) Manual of field methods. European-African Songbird Migration. Institut für Vogelforschung, Wilhelmshaven
Bairlein F, Beck P, Feiler W, Querner U (1983) Autumn weights of some Palaearctic passerine migrants in the Sahara. Ibis 125:404–407
Bairlein F, Norris DR, Nagel R, Bulte M, Voigt CC, Fox JW, Hussell DJT, Schmaljohann H (2012) Cross-hemisphere migration of a 25-gram songbird. Biol Lett 8:505–507
Bairlein F, Dierschke J, Dierschke V, Salewski V, Geiter O, Hüppop K, Köppen U, Fiedler W (2014) Atlas des Vogelzugs. Ringfunde deutscher Brut- und Gastvögel. AULA-Verlag, Wiebelsheim
Bairlein F, Eikenaar C, Schmaljohann H (2015a) Routes to genes: unravelling the control of avian migration—an integrated approach using Northern Wheatear Oenanthe oenanthe as model organism. J Ornithol 156:S3–S14
Bairlein F, Fritz J, Scope A, Schwendenwein I, Stanclova G, van Dijk G, Meijer HAJ, Verhulst S, Dittami J (2015b) Energy expenditure and metabolic changes of free-flying migrating Northern Bald Ibis. PLoSONE 10:e0134433
Bates D, Mächler M, Bolker B, Walker S (2014) lme4: Linear mixed-effects models using Eigen and S4. R package version 11–7. http://CRAN.R-project.org/packages=lme4. Accessed 08 Mar 2017
Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K (2000) Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc R Soc Lond B 267:191–195
Battley PF, van Gils JA, Piersma T, Hassell CJ, Boyle A, Hong-Yan Y (2005) How do red knots Calidris canutus leave Northwest Australia in May and reach the breeding grounds in June? Predictions of stopover times, fuelling rates and prey quality in the Yellow Sea. J Avian Biol 36:494–500
Battley PF, Warnock N, Tibbitts TL, Gill RE, Piersma T, Hassell CJ, Douglas DC, Mulcahy DM, Gartell BD, Schuckard R, Melville DS, Riegen A (2012) Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica. J Avian Biol 43:21–32
Bauchinger U, Biebach H (2001) Differential catabolism of muscle protein in Garden Warblers (Sylvia borin): flight and leg muscle act as a protein source during long-distance migration. J Comp Physiol B 171:293–301
Bauchinger U, Klaassen M (2005) Longer days in spring than in autumn accelerate migration speed of passerine birds. J Avian Biol 36:3–5
Bauchinger U, McWilliams S (2009) Carbon turnover in tissues of a passerine bird: Allometry, isotopic clocks, and phenotypic flexibility in organ size. Physiol Biochem Zool 82:787–797
Bauer S, Gienapp P, Madsen J (2008) The relevance of environmental conditions for departure decision changes en route in migrating geese. Ecology 89:1953–1960
Bayly NJ (2006) Optimality in avian migratory fuelling behaviour: a study of a trans-Saharan migrant. Anim Behav 71:173–182
Bayly NJ (2007) Extreme fattening by sedge warblers, Acrocephalus schoenobaenus, is not triggered by food availability alone. Anim Behav 74:471–479
Bayly NJ, Gómez C (2011) Comparison of autumn and spring migration strategies of Neotropical migratory landbirds in northeast Belize. J Field Ornithol 82:117–131
Bayly NJ, Atkinson PW, Rumsey SJR (2012) Fuelling for the Sahara crossing: variation in site use and the onset and rate of spring mass gain by 38 Palearctic migrants in the western Sahel. J Ornithol 153:931–945
Bayly NJ, Gómez C, Hobson KA (2013) Energy reserves stored by migrating Gray-cheeked Thrushes Catharus minimus at a spring stopover site in northern Colombia are sufficient for a long-distance flight to North America. Ibis 155:271–283
Bensch S, Akesson S, Irwin DE (2002) The use of AFLP to find an informative SNP: genetic differences across a migratory divide in willow warblers. Mol Ecol 11:2359–2366
Berthold P (1996) Control of bird migration. 1 edn. Chapman & Hall, London
Berthold P, Querner U (1981) Genetic basis of migratory behavior in European warblers. Science 212:77–79
Bibby CJ, Green RE (1981) Autumn migration strategies of reed and sedge warblers. Ornis Scand 12:1–12
Biebach H (1985) Sahara stopover in migratory flycatchers: fat and food affect the time progam. Experientia 41:695–697
Biebach H, Friedrich W, Heine G (1986) Interaction of bodymass, fat, foraging and stopover period in trans-sahara migrating passerine birds. Oecologia 69:370–379
Biesel W, Nachtigall W (1987) Pigeon flight in a wind tunnel. IV. Thermoregulation and water homeostasis. J Comp Physiol B 157:117–128
Bohrer G, Brandes D, Mandel JT, Bildstein KL, Miller TA, Lanzone M, Katzner T, Maisonneuve C, Tremblay JA (2011) Estimating updraft velocity components over large spatial scales: contrasting migration strategies of golden eagles and turkey vultures. Ecol Lett 15:96–103
Bolshakov CV, Bulyuk VN, Sinelschikova A (2000) Study of nocturnal departures in small passerine migrants: retrapping of ringed birds in high mist-nets. Vogelwarte 40:250–257
Bolshakov CV, Bulyuk VN, Chernetsov N (2003) Spring nocturnal migration of Reed Warblers Acrocephalus scirpaceus: departure, landing and body condition. Ibis 145:106–112
Bouten W, Baaij EW, Shamoun-Baranes J, Camphuysen KCJ (2013) A flexible GPS tracking system for studying bird behaviour at multiple scales. J Ornithol 154:571–580
Bowler JM (1994) The condition of Bewick’s swans Cygnus columbianus bewickii in winter as assessed by their abdominal profile. Ardea 82:241–248
Bowlin MS, Wikelski M (2008) Pointed wings, low wingloading and calm air reduce migratory flight costs in songbirds. PLoSONE 3:e2154
Boyd H, Fox AD (1995) Abdominal profiles of Icelandic pink-footed geese Anser brachyrhynchus in spring. Wildfowl 46:161–175
Bridge ES, Thorup K, Bowlin MS, Chilson PB, Diehl RH, Fléron RW, Hartl P, Kays R, Kelly JF, Robinson WD, Wikelski M (2011) Technology on the move: Recent and forthcoming innovations for tracking migratory birds. Bioscience 61:689–698
Brown ME (1996) Assessing body condition in birds. Curr Ornithol 13:67–135
Brown JM, Taylor PD (2015) Adult and hatch-year blackpoll warblers exhibit radically different regional-scale movements during post-fledging dispersa. Biol Lett 11:20150593
Bruderer B (1994) Radar studies on nocturnal bird migration in the Negev. Ostrich 65:204–212
Bruderer B, Liechti F (1995) Variation in density and height distribution of nocturnal migration in the south of Israel. Isr. J Zool 41:477–487
Bruderer B, Liechti F (1998) Flight behaviour of nocturnally migrating birds in coastal areas—crossing or coasting. J Avian Biol 29:499–507
Bruderer B, Liechti F (1999) Bird migration across the Mediterranean. In: Adam N, Slotow R (eds) Proc. Int. Ornithol. Congr. Birdlife South Africa, Durban, pp 1983–1999
Bulte M, Heyers D, Mouritsen H, Bairlein F (2017) Geomagnetic information modulates nocturnal migratory restlessness but not fueling in a long distance migratory songbird. J Avian Biol 48:75–82
Bulyuk VN (2012) Influence of fuel load and weather on timing of nocturnal spring migratory departures in European robins, Erithacus rubecula. Behav Ecol Sociobiol 66:385–395
Bulyuk VN, Tsvey A (2013) Regulation of stopover duration in the European Robin Erithacus rubecula. J Ornithol 154:1115–1126
Butler PJ, Woakes AJ (1980) Heart rate, respiratory frequency and wing beat frequency of free flying barnacle geese Branta leucopsis. J Exp Biol 85:213–226
Butler PJ, Woakes AJ, Bevan RM, Stephenson R (2000) Heart rate and rate of oxygen consumption during flight of the barnacle goose, Branta leucopsis. Comp Biochem Phys A 126:379–385
Cantos FJ, Tellerìa JL (1994) Stopover site fidelity of four migrant warblers in the Iberian Peninsula. J Avian Biol 25:131–134
Carmi N, Pinshow B, Porter WP, Jaeger J (1992) Water and energy limitations on flight duration in small migrating birds. Auk 109:268–276
Carpenter FL, Paton DC, Hixon MA (1983) Weight gain and adjustment of feeding territory size in migrant hummingbirds. PNAS 80:7259–7263
Catry P, Encarnacão V, Araújo A, Fearon P, Fearon A, Armelin M, Delaloye P (2004) Are long-distance migrant passerines faithful to their stopover sites? J Avian Biol 35:170–181
Chernetsov N (2006) Habitat selection by nocturnal passerine migrants en route: mechanisms and results. J Ornithol 47:185–191
Chernetsov N (2012) Passerine migration—stopovers and flight. Springer-Verlag, Berlin
Cherry JD (1982) Fat deposition and length of stopover of migrant white-crowned sparrows. Auk 99:725–732
Chevallier D, Handrich Y, Georges J-Y, Baillon F, Brossault P, Aurouet A, Le Maho Y, Massemin S (2010) Influence of weather conditions on the flight of migrating black storks. Proc R Soc Lond B 277:2755–2764
Clausen P, Green M, Alerstam T (2003) Energy limitations for spring migration and breeding: the case of brent geese Branta bernicla tracked by satellite telemetry to Svalbard and Greenland. Oikos 103:426–445
Cohen EB, Moore FR, Fischer RA (2012) Experimental evidence for the interplay of exogenous and endogenous factors on the movement ecolgy of a migrating songbird. PLoSONE 7:e41818
Cohen EB, Moore FR, Fischer RA (2014) Fuel stores, time of spring, and movement behvior influence stopover duration of Red-eyed Vireo Vireo olivaceus. J Ornithol 155:785–792
Conklin J, Battley PF (2011) Impacts of wind on individual migration schedules of New Zealand bar-tailed godwits. Behav Ecol 22:854–861
Cormack RM (1964) Estimates of survival from the sighting of marked animals. Biometrika 51:429–438
Corman AM, Bairlein F, Schmaljohann H (2014) The nature of the migration route shapes physiological traits and aerodynamic properties in a migratory songbird. Behav Ecol Sociobiol 68:391–402
Crysler ZJ, Ronconi RA, Taylor PD (2016) Differential fall migratory routes of adult and juvenile Ipswich Sparrows (Passerculus sandwichensis princeps). Mov Ecol 4:3
Dänhardt J, Lindström à (2001) Optimal departure decisions of songbirds from an experimental stopover site and the significance of weather. Anim Behav 62:235–243
Delingat J, Dierschke V, Schmaljohann H, Mendel B, Bairlein F (2006) Daily stopovers as optimal migration strategy in a long-distance migrating passerine: the Northern Wheatear. Ardea 94:593–605
Delingat J, Bairlein F, Hedenström A (2008) Obligatory barrier crossing and adaptive fuel management in migratory birds: the case of the Atlantic crossing in Northern Wheatears (Oenanthe oenanthe). Behav Ecol Sociobiol 62:1069–1078
Delingat J, Dierschke V, Schmaljohann H, Bairlein F (2009) Diurnal patterns in body mass change during stopover in a migrating songbird. J Avian Biol 40:625–634
DeLuca WV, Woodworth BK, Rimmer CC, Marra PP, Taylor PD, McFarland KP, Mackenzie SA, Norris DR (2015) Transoceanic migration by a 12 g songbird. Biol Lett 11:20141045
Deppe JL, Ward MP, Bolus RT, Diehl RH, Celis-Murillo A, Zenzal TJ, Moore FR, Benson TJ, Smolinsky JA, Schofield LN, Enstrom DA, Paxton EH, Bohrer G, Beveroth TA, Raim A, Obringer RL, Delaney D, Cochran WW (2015) Fat, weather, and date affect migratory songbirds’ departure decisions, routes, and time it takes to cross the Gulf of Mexico. PNAS 112:E6331–E6338
Deutschlander ME, Muheim R (2009) Fuel reserves affect migratory orientation of thrushes and sparrows both before and after crossing an ecological barrier near their breeding grounds. J Avian Biol 40:1–5
Dierschke V (2002) Kaum ein Vogel kehrt zurück: Geringe Rastplatztreue von ziehenden Landvögeln zur Nordseeinsel Helgoland. Vogelwarte 41:190–195
Dierschke V, Delingat J (2001) Stopover behaviour and departure decision of northern wheatears, Oenanthe oenanthe, facing different onward non-stop flight distances. Behav Ecol Sociobiol 50:535–545
Dierschke V, Delingat J (2003) Stopover of Northern Wheatears Oenanthe oenanthe at Helgoland: where do the migratory routes of Scandinavian and Nearctic birds join and split? Ornis Svecica 13:53–61
Dierschke V, Mendel B, Schmaljohann H (2005) Differential timing of spring migration in northern wheatears Oenanthe oenanthe: hurried males or weak females? Behav Ecol Sociobiol 57:470–480
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999a) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72:28–37
Dietz MW, Piersma T, Dekinga A (1999b) Body-building without power training: endogenously regulated pectoral muscle hypertrophy in confined shorebirds. J Exp Biol 202:2831–2837
Dorka V (1966) Das jahres- und tageszeitliche Zugmuster von Kurz- und Langstreckenziehern nach Beobachtungen auf den Alpenpässen Cou/Bretolet (Wallis). Ornithol Beob 63:165–223
Dossmann BC, Mitchell GW, Norris DR, Taylor PD, Guglielmo CC, Matthews SN, Rodewald PG (2015) The effects of wind and fuel stores on stopover departure behavior across a migratory barrier. Behav Ecol 27:567–574
Dowsett JR, Fry CH (1971) Weight losses of trans-saharan migrants. Ibis 113:531–533
Drost R (1941) Zieht der einzelne Vogel stets auf demselben Weg? Ardea 30:215–223
Duriez O, Bauer S, Destin A, Madsen J, Nolet BA, Stillman RA, Klaassen M (2009) What decision rules might pink-footed geese use to depart on migration? An individual-based model. Behav Ecol 20:560–569
Eikenaar C, Bairlein F (2014) Food availability and fuel loss predict Zugunruhe. J Ornithol 155:65–70
Eikenaar C, Schläfke L (2013) Size and accumulation of fuel reserves at stopover predict nocturnal restlessness in a migratory bird. Biol Lett 9:0130712
Eikenaar C, Klinner T, de Lille T, Bairlein F, Schmaljohann H (2014a) Fuel loss and flexible fuel deposition rates in a long-distance migrant. Behav Ecol Sociobiol 68:1465–1471
Eikenaar C, Klinner T, Szostek KL, Bairlein F (2014b) Migratory restlessness in captive individuals predicts actual departure in the wild. Biol Lett 10:20140154
Eikenaar C, Fritzsch A, Kämpfer S, Schmaljohann H (2016) Migratory restlessness increases and refuelling rate decreases over the spring migration season in northern wheatears. Anim Behav 112:75–81
Eikenaar C, Källstig E, Andersson MN, Herrera-Dueñas A, Isaksson C (2017) Oxidative challenges of avian migration: a comparative field study on a partial migrant. Physiol Biochem Zool 90:223–229
Ellegren H (1991) Stopover ecology of autumn migrating Bluethroats Luscinia s. svecica in relation to age and sex. Ornis Scand 22:340–348
Erni B, Liechti F, Bruderer B (2002a) Stopover strategies in passerine bird migration: a simulation study. J Theor Biol 219:479–493
Erni B, Liechti F, Underhill LG, Bruderer B (2002b) Wind and rain govern the intensity of nocturnal bird migration in central Europe—a log-linear regression analysis. Ardea 90:155–166
Féret M, Bêty J, Gauthier G, Giroux J-F, Picard G (2005) Are abdominal profiles useful to assess body condition of spring staging Greater Snow Geese? Condor 107:694–702
Fiedler W (2009) New technologies for monitoring bird migration and behaviour. Ringing Migration 24:175–179
Finlayson JC (1981) Seasonal distribution, weights and fat of passerine migratns at Gibraltar. Ibis 123:88–95
Fortin D, Liechti F, Bruderer B (1999) Variation in the nocturnal flight behaviour of migratory birds along the northwest coast of the Mediterranean Sea. Ibis 141:480–488
Fox AD, Hilmarsson JO, Einarsson O, Walsh AJ, Boyd H, Kristiansen JN (2002) Staging site fidelity of Greenland white-fronted geese (Anser albifrons flavirostris) in Iceland. Bird Study 49:42–49
Fransson T (1998a) A feeding experiment on migratory fuelling in whitethroats, Sylvia communis. Anim Behav 55:153–162
Fransson T (1998b) Patterns of migratory fuelling in Whitethroats Sylvia communis in relation to departure. J Avian Biol 29:569–573
Fransson T, Jakobsson S, Johansson P, Kullberg C, Lind J, Vallin A (2001) Magnetic cues trigger extensive refuelling. Nature 414:35–36
Fry CH, Ash JS, Ferguson-Lees IJ (1970) Spring weights of some Palaearctic migrants at lake Chad. Ibis 112:58–82
Gauthreaux SA Jr (1971) A radar and direct visual study of Passerine spring migration in Southern Louisiana. Auk 88:343–365
Giladi I, Pinshow B (1999) Evaporative and excretory water loss during free flight in pigeons. J Comp Physiol B 169:311–318
Gill RE, Tibbitts TL, Douglas DC, Handel CM, Mulcahy DM, Gottschalck JC, Warnock N, McCaffery BJ, Battley PF, Piersma T (2009) Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc R Soc Lond B 276:447–457
Gillings S, Atkinson PW, Baker AJ, Bennett KA, Clark NA, Cole KB, González PM, Kalasz KS, Minton CDT, Niles LJ, Porter RC, Serrano IDL, Sitters HP, Woods JL (2009) Staging Behavior in Red Knot (Calidris Canutus) in Delaware BAY: Implications for Monitoring Mass and Population Size. Auk 126:54–63
Gosler AG (1991) On the use of greater covert moult and pectoral muscle as measures of condition in passerines with data for the Great Tit Parus major. Bird Study 38:1–9
Goymann W, Spina F, Ferri A, Fusani L (2010) Body fat influences departure from stopover sites in migratory birds: evidence from whole island telemetry. Biol Lett 6:478–481
Green AJ (2001) Mass/length residuals: Measures of body cndition or generators of spurious results? Ecology 82:1473–1483
Green M, Alerstam T, Clausen P, Drent R, Ebbinge BS (2002) Dark-bellied Brent Geese Branta bernicla bernicla, as recorded by satellite telemetry, do not minimze flight distance during spring migration. Ibis 144:106–121
Guglielmo CC, Piersma T, Williams TD (2001) A sport-physiological perspective on bird migration: evidence for flight-induced muscle damage. J Exp Biol 204:2683–2690
Guglielmo CC, McGuire LP, Gerson AR, Seewagen CL (2011) Simple, rapid, and non-invasive measurement of fat, lean, and total water masses of live birds using quantitative magnetic resonance. J Ornithol 152:S75–S85
Gwinner E (1996) Circadian and circannual programmes in avian migration. J Exp Biol 199:39–48
Gwinner E (2009) Circannual rhythms. Endogenous annual clocks in the organizations of seasonal processes. Springer, Berlin Heidelberg New York
Gwinner E, Wiltschko W (1978) Endogenously controlled changes in migratory direction of the garden warbler, Sylvia borin. J Comp Physiol A 125:267–273
Harrington BA, Hagan JH, Leddy LE (1988) Site fidelity and survival differences between two groups of New World red knots (Calidris canutus). Auk 105:439–445
Hayes JP, Shonkwiler JS (2001) Morphometric indicators of body condition: worthwhile or wishful thinking? In: Spearman JR (ed) Body composition analysis of animals: a handbook of non-destructive methods. Cambridge University Press, Cambridge, pp 8–38
Hedenström A (2002) Aerodynamics, evolution and ecology of avian flight. TREE 17:415–422
Hedenström A (2008) Adaptations to migration in birds: behavioural strategies, morphology and scaling effects. Phil Trans R Soc B 363:287–299
Hedenström A, Alerstam T (1997) Optimum fuel loads in migratory birds: Distinguishing between time and energy minimization. J Theor Biol 189:227–234
Helms CW, Drury WHJ (1960) Winter and migratory weight and fat field studies on some north American buntings. Bird Banding 31:1–36
Henkel JR, Taylor CM (2015) Migration strategy predicts stopover ecology in shorebirds on the northern Gulf of Mexico. Anim Mig 2:63–75
Holmgren N, Ellegren H, Pettersson J (1993) Stopover length, body mass and fuel deposition rate in autumn migrating adult Dunlins Calidris alpina: evaluating the effects of moulting status and age. Ardea 81:9–20
Hutto RL (1985) Habitat selection by nonbreeding, migratory land birds. In: Cody ML (ed) Habitat selection in birds. Acedemic Press, London, pp 455–476
Jahn AE, Cuteo VR, Fox JW, Husak MS, Kim DH, Landoll DV, Ledezma JP, LePage HK, Levey DJ, Murphy MT, Renfrew RB (2013) Migration timing and wintering areas of three species of flycatchers (Tyrannus) breeding in the Great Plains of North America. Auk 130:247–257
Jenni L, Jenni-Eiermann S (1998) Fuel supply and metabolic constraints in migrating birds. J Avian Biol 29:521–528
Jenni L, Schaub M (2003) Behavioural and Physiological Reactions to Environmental Variation in Bird Migration: a review. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian Migration. Springer, Berlin Heidelberg, pp 155–171
Jenni-Eiermann S, Almasi B, Maggini I, Salewski V, Bruderer B, Liechti F, Jenni L (2011) Numbers, foraging and refuelling of passerine migrants at a stopover site in the western Sahara: diverse strategies to cross a desert. J Ornithol 152:S113–S128
Jenni-Eiermann S, Jenni L, Smith S, Costantini D (2014) Oxidative stress in endurance flight: An unconsiderd factor in bird migration. PLoSONE 9:e97650
Johnson AB, Winker K (2008) Autumn stopover near the Gulf of Honduras by Nearctic-Neotropic migrants. Wilson. J Ornithol 120:277–285
Johnson DH, Krapu GL, Reinecke KJ, Jorde DG (1985) An evaluation of condition indices for birds. J Wildlife Manage 49:569–575
Jolly G (1965) Explicit estimates from capture-recapture data with both death and immigration-stochastic model. Biometrika 52:225–247
Jones PJ (1995) Migration strategies of palearctic passerines in Africa. Isr. J Zool 41:393–406
Jouventin P, Weimerskirch H (1990) Satellite tracking of Wandering albatrosses. Nature 343:746–748
Kaiser A (1993) A new multi-category classification of subcutaneous fat deposits of songbirds. J Field Ornithol 64:246–255
Karlsson H, Nilsson C, Bäckman J, Alerstam T (2012) Nocturnal passerine migrants fly faster in spring than in autumn: a test of the time minimization hypothesis. Anim Behav 83:87–93
Kerlinger P (1989) Flight strategies of migrating hawks. University of Chicago Press, Chicago
Klaassen M (2004) May dehydration risk govern long-distance migratory behaviour? J Avian Biol 35:4–6
Klaassen RHG, Alerstam T, Carlsson P, Fox JW, Lindström à (2011) Great flights by great snipes: long and fast non-stop migration over benign habitats. Biol Lett 7:833–835
Knudsen E, Lindén A, Both C, Jonzén N, Pulido F, Saino N, Sutherland WJ, Bach LA, Coppack T, Ergon T, Gienapp P, Gill JA, Gordo O, Hedenström A, Lehikoinen E, Marra PP, Møller AP, Nilsson ALK, Péron G, Ranta E, Rubolini D, Sparks TH, Spina F, Studds CE, Sæther SA, Tryjanowski P, Stenseth NC (2011) Challenging claims in the study of migratory birds and climate change. Biol Rev 86:928–946
Komenda-Zehnder S, Liechti F, Bruderer B (2002) Is reverse migration a common feature of nocturnal bird migration?—an analysis of radar data from Israel. Ardea 90:325–334
Kruckenberg H, Borbach-Jaene J (2004) Do greylag geese (Anser anser) use traditional roosts? Site fidelity of colour-marked nordic greylag geese during spring migration. J Ornithol 145:117–122
Kuenzi AJ, Moore FR, Simons TR (1991) Stopover of Neotropical landbird migrants on East Ship Island following trans-gulf migration. Condor 93:869–883
Labocha MK, Hayes JP (2012) Morphometric indices of body condition in birds: a review. J Ornithol 153:1–22
Larkin RP, Szafoni RE (2008) Evidence for widely dispersed birds migrating together at night. Integrative Comp Biol 48:40–49
Leyrer J, Pruiksma S, Piersma T (2009) On 4 June 2008 Siberian Red Knots at Elbe mouth kissed the canonical evening migration departure rule goodbye. Ardea 97:71–79
Liechti F (1993) Nächtlicher Vogelzug im Herbst über Süddeutschland: Winddrift und Kompensation. J Ornithol 134:373–404
Liechti F (2006) Birds: blowin’ by the wind? J Ornithol 147:202–211
Liechti F, Bruderer B (1998) The relevance of wind for optimal migration theory. J Avian Biol 29:561–568
Liechti F, Schaller E (1999) The use of low-level jets by migrating birds. Naturwissenschaften 86:549–551
Liechti F, Erich D, Bruderer B (1996) Flight behaviour of White Storks Ciconia ciconia on their migration over Southern Israel. Ardea 84:3–13
Liechti F, Witvliet W, Weber R, Bächler E (2013) First evidenve of a 200-day non-stop flight in a bird. Nat Comm 4:2554
Lindström à (1991) Maximum fat deposition rates in migrating birds. Ornis Scand 22:12–19
Lindström A, Alerstam T (1992) Optimal fat loads in migrating birds: a test of the time-minimization hypothesis. Am Nat 140:477–491
Lindström Å, Hasselquist D, Bensch S, Grahn M (1990) Asymmetric contests over resources for survival and migration: a field experiment with bluethroats. Anim Behav 40:453–461
Lindström Å, Kvist A, Piersma T, Dekinga A, Dietz MW (2000) Avian pectoral muscle size rapidly tracks body mass changes during flight, fasting and fuelling. J Exp Biol 203:913–919
Lisovski S, Gosbell K, Christie M, Hoye BJ, Klaassen M, Steward ID, Taysom AJ, Minton C (2016) Movement patterns of Sanderling (Calidris alba) along the East Asian Australasian Flyway and a comparison of methods to identify crucial areas for conservation. Emu 116:168–177
Lok T, Overdijk O, Piersma T (2015) The cost of migration: spoonbills suffer higher mortality during trans-Saharan spring migrations only. Biol Lett 11:20140944
Loria DE, Moore FR (1990) Energy demands of migration on Red-eyed Vireos, Vireo olivaceus. Behav Ecol 1:24–33
Lourenço PM, Kentie R, Schroeder J, Alves JA, Groen NM, Hooijmeijer JCEW, Piersma T (2010) Phenology, stopover dynamics and population size of migrating black-tailed godwits Limosa Limosa Limosa in Portuguese rice plantations. Ardea 98:35–42
Lyons JE, Haig SM (1995) Fat content and stopover ecology of spring migrant Semipalmated Sandpipers in South Carolina. Condor 97:427–437
Madsen J (2001) Spring migration strategies in pink-footed geese Anser brachyrhynchus and consequences for spring fattening and fecundity. Ardea 89:43–55
Madsen J, Klaassen M (2006) Assessing body condition and energy budget components by scoring abdominal profiles in free-ranging pink-footed geese Anser brachyrhynchus. J Avian Biol 37:283–287
Maggini I, Bairlein F (2010a) Body condition and stopover of trans-Saharan spring migrant passerines caught at a site in southern Morocco. Ring Migr 26:31–37
Maggini I, Bairlein F (2010b) Endogenous rhythms of seasonal migratory body mass changes and nocturnal restlessness in different populations of Northern wheatear Oenanthe oenanthe. J Biol Rhythm 25:268–276
Maggini I, Bairlein F (2013) Metabolic response to changes in temperature in northern wheatears from an arctic and a temperate populations. J Avian Biol 44:1–7
Mandel JT, Bildstein KL, Bohrer G, Winkler DW (2008) Movement ecology of migration in turkey vultures. PNAS 105:19102–19107
Marsh RL (1983) Adaptations of the Gray Catbird Dumetella carolinensis to long distance migration: energy stores and substrate concentrations in plasma. Auk 100:170–179
Masman D, Klaassen M (1987) Energy expenditure during free flight in trained and free-living Eurasian Kestrels Falco tinnunculus. Auk 104:603–616
McLaren J, Shamoun-Baranes J, Bouten W (2013) Stop early to travel fast—modelling risk-averse scheduling among nocturnally migrating birds. J Theor Biol 316:90–98
McWilliams SR, Guglielmo CG, P. P, Klaassen M (2004) Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35:377–393
Meissner W (1998) Fat reserves in Dunlins Clidris alpina during autumn migration. Ornis Svecica 8:91–102
Michaeli G, Pinshow B (2001) Respiratory water loss in free-flying pigeons. J Exp Biol 204:3803–3814
Miller MR, Takekawa JY, Fleskes JP, Orthmeyer DL, Casazza ML, Perry WM (2005) Spring migration of Northern Pintails from California’s Central Valley wintering area tracked with satellite telemetry: routes, timing, and destinations. Can J Zool 83:1314–1332
Mills AM, Thurber BG, Mackenzie SA, Taylor PD (2011) Passerines use nocturnal flights for landscape-scale movements during migration stopover. Condor 113:597–607
Mitchell GW, Woodworth BK, Taylor PD, Norris DR (2015) Automated telemetry reveals age specific differences in flight duration and speed are driven by wind conditions in a migratory songbird. Mov Ecol 3:19
Moore FR, Aborn DA (2000) Mechanisms of en route habitat selection: how do migrants make habitat decisions during stopover. Stud Avian Biol 20:34–42
Moore F, Kerlinger P (1987a) Stopover and fat deposition by North American wood-warblers (Parulinae) following spring migration over the Gulf of Mexico. Oecologia 74:47–54
Moore FR, Kerlinger P (1987b) Stopover and fat deposition by North American wood-warblers (Parulidae) following spring migration over the Gulf of Mexico. Oecologia 74:47–54
Moore FR, Yong W (1991) Evidence of food-based competition among passerine migrants during stopover. Behav Ecol Sociobiol 28:83–90
Moore FR, Kerlinger P, Simons TR (1990) Stopover on a gulf coast barrier island by spring trans-gulf migrants. Wilson. J Ornithol 102:487–500
Moore FR, Mabey S, Woodrey M (2003) Priority access to food in migratory birds: age, sex and motivational asymmetries. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian Migration. Springer, Berlin Heidelberg New York, pp 281–292
Moreau RE (1972) The Palaearctic-African Bird Migration Systems. Academic Press, London and New York
Moriguchi S, Amano T, Ushiyama K, Fujita G, Higuchi H (2006) The relationship between abdominal profile index and body condition of greater white-fronted geese Anser albifrons. Ornithol Sci 5:193–198
Morris SR (1996) Mass loss and probability of stopover by migrant warblers during spring and fall migration. J Field Ornithol 67:456–462
Morris SR, Holmes DW, Richmond ME (1996) A ten-year study of the stopover patterns of migratory passerines during fall migration on Appledore island, Maine. Auk 113:395–409
Müller F, Taylor PD, Sjöberg S, Muheim R, Tsvey A, Schmaljohann H (2016) Towards a conceptual framework for explaining variation in the nocturnal departure time of songbird migrants. Mov Ecol 4:24
Navedo JG, Masero JA, Overdijk O, Orizaola G, Sánchez-Guzmán JM (2010) Assessing the role of multiple environmental factors on Eurasian Spoonbill departure decisions from stopover sites. Ardea 98:3–12
Newton SF (1993) Body condition of a small passerine bird: ultrasonic assessment and significance in overwinter survival. J Zool 229:561–580
Newton I (2008) The migration ecology of birds. Academic Press, London
Nilsson C, Sjöberg S (2016) Causes and characteristics of reverse bird migration: an analysis based on radar, radio tracking and ringing at Falsterbo, Sweden. J Avian Biol 47:354–362
Nilsson C, Klaassen RHG, Alerstam T (2013) Differences in speed and duration of bird migration between spring and autumn. Am Nat 181:837–845
Norberg UM (1995) How a long tail and changes in mass and wing shape affect the cost for flight in animals. Func Ecol 9:48–54
Norberg U (1996) Energetics of flight. Avian energetics and nutritional ecology. Chapman and Hall, New York
Ormerod SJ (1990) Time of passage, habitat use and mass change of Acrocephalus warblers in a South Wales reedswamp. Ring Migr 11:1–11
Ottosson U, Bairlein F, Hall P, Hjort C, Rumsey SJR, Spina F, Waldenström J (2001) Timing of migration and spring mass of some Palaearctic migrants at Lake Chad. Ostrich S15:60
Ottosson U, Bairlein F, Hjort C (2002) Migration patterns of Palaearctic Acrocephalus and Sylvia warblers in north-eastern Nigeria. Vogelwarte 41:249–262
Ottosson U, Waldenström J, Hjort C, McGregor R (2005) Garden Warbler Sylvia borin migration in sub-Saharan West Africa: phenology an body mass changes. Ibis 147:750–757
Ouwehand J, Both C (2016) Alternate non-stop migration strategies of pied flycatchers to cross the Sahara desert. Biol Lett 12:20151060
Ouwehand J, Both C (2017) African departure rather than migration speed determines variation in spring arrival in pied flycatchers. J Anim Ecol 86:88–97
Owen M (1981) Abdominal profile—a condition index for wild geese in the field. J Wildlife Manage 45:227–230
Peig J, Green AJ (2009) New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos 118:1883–1891
Pennycuick CJ (1975) Mechanics of flight. In: Farner DS (ed) Avian Biology. 5 edn. Academic Press, New York, pp 1–75
Pennycuick CJ, Einarsson O, Bradbury TAM, Owen M (1996) Migrating Whooper Swans Cygnus cygnus: satellite tracks and fligth performance calculations. J Avian Biol 27:118–134
Piersma T, Klaasen K, Bruggemann HJ, Blomert A, Gueye A, Ntiamoa-Baidu Y, Van Brederode NE (1990a) Seasonal timing of the spring departure of waders from the Banc d’Arguin, Mauritania. Ardea 78:123–133
Piersma T, Zwarts L, Bruggemann JH (1990b) Behavioural aspects of the departure of waders before long-distance flights: flocking, vocalizations, flight paths and diurnal timing. Ardea 78:157–184
Piersma T, Gudmundsson GA, Lilliendahl K (1999) Rapid changes in the size of different functional organ and muscle groups during refueling in a long-distance migrating shorebird. Physiol Biochem Zool 71:405–415
Piersma T, Rogers DI, Gonzáles PM, Zwarts L, Niles LJ, de Lima I, do Nascimento S, Minton CDT, Baker AJ (2005) Fuel storage rates before northward flights in red knots worldwide. Facing the severest ecological constraints in tropical intertidal environments. In: Greenberg R, Marra PP (eds) Birds of two Worlds. Johns Hopkins University Press, Baltimore, pp 262–273
Pilastro A, Spina F (1997) Ecological and morphological correlates of residuel fat reserves in passerine migrants at their spring arrival in southern Europe. J Avian Biol 28:309–318
Prop J, Black JM, Shimmings P (2003) Travel schedules to the high arctic: barnacle geese trade-off the timing of migration with accumulation of fat deposits. Oikos 103:403–414
Rabøl J, Peterson FD (1973) Lengths of resting time in various night-migrating passerines at Hesselo, Southern Kattegat, Denmark. Ornis Scand 22:33–46
Ramenofsky M, Wingfield J (2006) Behavioral and physiological conflicts in migrants: the transition between migration and breeding. J Ornithol 147:135–145
Rayner JMV (1999) Estimating power curves of flying vertebrates. J Exp Biol 202:3449–3461
Rayner JMV, Viscardi PW, Ward S, Speakman JR (2001) Aerodynamics and Energetics of Intermittent Flight in Birds. Am Zool 41:188–204
Reed ET, Cooch EG, Goudie RI, Cooke F (1998) Site fidelity of Black Brant wintering and spring staging in the Strait of Georgia, British Columbia. Condor 100:426–437
Richardson WJ (1978) Timing and amount of bird migration in relation to weather: a review. Oikos 30:224–272
Richardson WJ (1982) Northeastward reverse migration of birds over Nova Scotia, Canada, in Autumn. Behav Ecol Sociobiol 10:193–206
Richardson WJ (1990) Timing of bird migration in relation to weather: updated review. In: Gwinner E (ed) Bird migration. Springer, Berlin Heidelberg, pp 78–101
Rotics S, Kaatz M, Resheff YS, Turjeman SF, Zurell D, Sapir N, Eggers U, Flack A, Fiedler W, Jeltsch F, Wikelski M, Nathan R (2016) The challenges of the first migration: movement and behaviour of juvenile vs. adult white storks with insights regarding juvenile mortality. J Anim Ecol 85:938–947
Rubenstein DR, Hobson KA (2004) From birds to butterflies: animal movement patterns and stable isotopes. TREE 19:257–263
Safriel UN, Lavee D (1988) Weight changes of cross-desert migrants at an oasis—do energetic considerations alone determine the length of stopover? Oecologia 76:611–619
Salewski V, Schaub M (2007) Stopover duration of Palearctic passerine migrants in the western Sahara—independent of fat sores? Ibis:223–236
Salewski V, Thoma M, Schaub M (2007) Stopover of migrating birds: simultaneous analysis of different marking methods enhances the power of capture-recapture analyses. J Ornithol 148:29–37
Salewski V, Kéry M, Herremans M, Liechti F, Jenni L (2009) Estimating fat and protein fuel from fat and muscle scores in passerines. Ibis 151:640–653
Salewski V, Schmaljohann H, Liechti F (2010) Spring passerine migrants stopping over in the Sahara are not fall-outs. J Ornithol 151:371–378
Sandberg R, Moore FR (1996) Migratory orientation of red-eyed vireos, Vireo olivaceus, in relation to energetic condition and ecological context. Behav Ecol Sociobiol 39:1–10
Sandberg R, Moore FR, Bäckman J, Lohmus M (2002) Orientation of nocturnally migrating Swainson’s Trush at dawn and dusk: Importance of energetic conditon and geomagnetic cues. Auk 119:201–219
Sapir N, Wikelski M, McCue MD, Pinshow B, Nathan R (2010) Flight modes in migrating European bee-eaters: heart rate may indicate low metabolic rate during soaring and gliding. PLoSONE 5:e13956
Sapir N, Horvitz N, Wikelski M, Avissar R, Mahrer Y, Nathan R (2011) Migration by soaring or flapping: numerical atmospheric simulations reveal that turbulence kinetic energy dictates bee-eater flight mode. Proc R Soc Lond B 278:3380–3386
Schaub M, Jenni L (2001) Variation of fuelling rates among sites, days and individuals in migrating passerine birds. Func Ecol 15:584–594
Schaub M, Pradel R, Jenni L, Lebreton J-D (2001) Migrating birds stop over longer than usually thought: an improved capture-recapture analysis. Ecology 82:852–859
Schaub M, Liechti F, Jenni L (2004) Departure of migrating European robins, Erithacus rubecula, from a stopover site in relation to wind and rain. Anim Behav 67:229–237
Schaub M, Jenni L, Bairlein F (2008) Fuel stores, fuel accumulation, and the decision to depart from a migration stopover site. Behav Ecol 19:657–666
Schmaljohann H, Dierschke V (2005) Optimal bird migration and predation risk: a field experiment with northern wheatears Oenanthe oenanthe. J Anim Ecol 74:131–138
Schmaljohann H, Liechti F (2009) Adjustments of wingbeat frequency and airspeed to air density in free flying migratory birds. J Exp Biol 212:3633–3642
Schmaljohann H, Naef-Daenzer B (2011) Body condition and wind support initiate shift in migratory direction and timing of nocturnal departure in a free flying songbird. J Anim Ecol 80:1115–1122
Schmaljohann H, Liechti F, Bruderer B (2007a) Daytime passerine migrants over the Sahara—are these diurnal migrants or prolonged flights of nocturnal migrants? Paper presented at the Ostrich, Proc. Pan African Ornithol. Congress, Djerba, 2007
Schmaljohann H, Liechti F, Bruderer B (2007b) Songbird migration across the Sahara—the non-stop hypothesis rejected! Proc R Soc Lond B 274:735–739
Schmaljohann H, Bruderer B, Liechti F (2008) Sustained bird flights occur at temperatures beyond expected limits of water loss rates. Anim Behav 76:1133–1138
Schmaljohann H, Becker PJJ, Karaardic H, Liechti F, Naef-Daenzer B, Grande C (2011) Nocturnal exploratory flights, departure time, and direction in a migratory songbird. J Ornithol 152:439–452
Schmaljohann H, Fox JW, Bairlein F (2012) Phenotypic response to environmental cues, orientation and migration costs in songbirds flying halfway around the world. Anim Behav 84:623–640
Schmaljohann H, Korner-Nievergelt F, Naef-Daenzer B, Nagel R, Maggini I, Bulte M, Bairlein F (2013) Stopover optimization in a long-distance migrant: the role of fuel load and nocturnal take-off time in Alaskan northern wheatears (Oenanthe oenanthe). Front Zool 10:26
Schmaljohann H, Meier C, Arlt D, Bairlein F, van Oosten H, Morbey YE, Åkesson S, Buchmann M, Chernetsov N, Desaever R, Elliott J, Hellström M, Liechti F, López A, Middleton J, Ottosson U, Pärt T, Spina F, Eikenaar C (2016) Proximate causes of avian protandry differ between subspecies with contrasting migration challenges. Behav Ecol 27:321–331
Schmaljohann H, Lisovski S, Bairlein F (2017) Flexible reaction norms to environmental variables along the migration route and the significance of stopover duration for total speed of migration in a songbird migrant. Front Zool 14:17
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: valildating body condition indices. Ecology 86:155–163
Schwilch R, Piersma T, Holmgren NMA, Jenni L (2002) Do migratory birds need a nap after a long non-stop flight? Ardea 90:149–154
Sears J (1988) Assessment of body condition in live birds; measurements of protein and fat reserves in the mute swan, Cygnus olor. J Zool 216:295–308
Seber GAF (1965) A note on the multiple-recapture census. Biometrika 52:249–259
Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR, Sagar PM, Moller H, Taylor GA, Foley DG, Block BA, Costa DP (2006) Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. PNAS 103:12799–12802
Shamoun-Baranes J, Leyrer J, van Loon E, Bocher P, Robin F, Meunier F, Piersma T (2010) Stochastic atmospheric assistance and the use of emergency staging sites by migrants. Proc R Soc Lond B 277:1505–1511
Shariatinajafabadi M, Wang T, Skidmore AK, Toxopeus AG, Kölzsch A, Nolet BA, Exo K-M, Griffin L, Stahl J, Cabot D (2013) Migratory herbivorous waterfowl track satellite-derived green wave index. PLoSONE 9:e108331
Sjöberg S, Alerstam T, Åkesson S, Schulz A, Weidauer A, Coppack T, Muheim R (2015) Weather and fuel reserves determine departure and flight decisions in passerines migrating across the Baltic Sea. Anim Behav 104:59–68
Skagen SK, Knopf FL (1994) Residency pattern of migrating Sandpipers at a midcontinental stopover. Condor 96:949–958
Skrip MM, Bauchinger U, Goymann W, Fusani L, Cardinale M, Alan RR, McWilliams SR (2015) Migrating songbirds on stopover prepare for, and recover from, oxidative challenges posed by long-distance flight. Ecol Evol 5:3198–3209
Smith VW (1966) Autumn and spring weights of some palearctic migrants in Central Nigeria. Ibis 108:492–512
Smith AD, McWilliams SR (2014) What to do when stopping over: behavioral decisions of a migrating songbird during stopover are dictated by initial change in their body condition and mediated by key environmental conditions. Behav Ecol 25:1423–1435
Smith RJ, Moore FR, May CA, Lank DB (2007) Stopover habitat along the shoreline of northern Lake Huron, Michigan: emergent aquatic insects as a food resource for spring migrating landbirds. Auk 124:107–121
Smolinsky JA, Diehl RH, Radzio TA, Delaney DK, Moore FR (2013) Factors influencing the movement biology of migrant songbirds confronted with an ecological barrier. Behav Ecol Sociobiol 67:2041–2051
Spaar R, Bruderer B (1996) Soaring migration of Steppe Eagles Aquila nipalensis in southern Israel: Flight behaviour under various wind and thermal conditions. J Avian Biol 27:289–301
Spaar R, Bruderer B (1997a) Migration by flapping or soaring: Flight strategies of Marsh, Montagu’s and Pallid Harriers in southern Israel. Condor 99:458–469
Spaar R, Bruderer B (1997b) Optimal flight behaviour of soaring migrants? A case study of migrating Steppe Buzzards Buteo buteo vulpinus. Behav Ecol 8:288–297
Spina F, Pilastro A (1999) Strategy of sea and desert crossing in spring passerine migrants as suggested by the analysis of intra- and inter-specific variation of residual fat levels. Proceedings of the 22 International Ornithological Congress, Durban. BirdLife South Africa, Johannesburg
Stach R, Fransson T, Jakobsson S, Kullberg C (2015) Wide ranging stopover movements and substantial fuelling in first year garden warblers at a northern stopover site. J Avian Biol 46:315–322
Stanley CQ, MacPherson M, Fraser KC, McKinnon EA, Stutchbury BJM (2012) Repeat tracking of individual songbirds reveals consistent migration timing but flexibility in route. PLoSONE 7:e40688
Taylor PD, Mackenzie SA, Thurber BG, Calvert AM, Mills AM, McGuire LP, Guglielmo CG (2011) Landscape movements of migratory birds and bats reveal an expanded scale of stopover. PLoSONE 6:e27054
Tøttrup AP, Klaassen RHG, Strandberg R, Thorup K, Willemoes Kristensen M, Søgaard Jørgensen P, Fox JW, Afanasyev V, Rahbek C, Alerstam T (2012) The annual cycle of a trans-equatorial Eurasian-African passerine migrant: different spatio-temporal strategies for autumn and spring migration. Proc R Soc Lond B 279:1008–1016
Trierweiler C, Klaassen RHG, Drent R, Exo K-M, Komdeur J, Bairlein F, Koks BJ (2014) Migratory connectivity and population-specific migration routes in a long-distance migratory bird. Proc R Soc Lond B 281:20132897
Tsvey A, Bulyuk VN, Kosarev V (2007) Influence of body condition and weather on departures of first-year European robins, Erithacus rubecula, from an autumn migratory stopover site. Behav Ecol Sociobiol 61:1665–1674
Van Wilgenburg SL, Hobson KA (2011) Combining stable-isotope (δD) and band recovery data to improve probabilistic assignment of migratory birds to origin. Ecol Appl 21:1340–1351
van Gils JA, Beekman JH, Coehoorn P, Corporaal E, Dekkers T, Klaassen M, Van Kraaij R, De Leeuw R, De Vries PP (2008) Longer guts and higher food quality increases energy intake in migratory swans. J Anim Ecol 77:1234–1241
van Wijk RE, Kölzsch A, Kruckenberg H, Ebbinge BS, Müskens GJDM, Nolet BA (2012) Individually tracked geese follow peaks of temperature acceleration during spring migration. Oikos 121:655–664
Visser ME, Perdeck AC, van Balen JH, Both C (2009) Climate change leads to decreasing bird migration distances. Glob Change Biol 15:1859–1865
Ward P (1969) The annual cycle of the Yellow-vented bulbul Pycnonotus goiavier in a humid equatorial environment. J Zool 157:25–45
Ward S, Rayner JMV, Möller U, Jackson DM, Nachtigall W, Speakman JR (1999) Heat transfer from starlings Sturnus vulgaris during flight. J Exp Biol 202:1589–1602
Ward S, Bishop CM, Woakes AJ, Butler PJ (2002) Heart rate and the rate of oxygen consumption of flying and walking barnacle geese (Branta leucopsis) and bar-headed geese (Anser indicus). J Exp Biol 205:3347–3356
Weber TP, Hedenström A (2000) Optimal stopover decisions under wind influence: the effects of correlated winds. J Theor Biol 205:95–104
Weimerskirch H, Wilson RP (2000) Oceanic respite for wandering albatrosses. Nature 406:955–956
Wiersma P, Piersma T (1995) Scoring abdominal profiles to characterize migratory cohorts of shorebirds—an example with red knots. J Field Ornithol 66:88–98
Wikelski M, Tarlow EM, Raim A, Diehl RH, Larkin RP, Visser GH (2003) Costs of migration in free-flying songbirds. Nature 423:704
Woodrey MS, Moore FR (1997) Age-related differences in the stopover of fall landbird migrants on the coast of Alabama. Auk 114:695–707
Woodworth BK, Francis CM, Taylor PD (2014) Inland flights of young red-eyed vireos Vireo olivaceus in relation to survival and habitat in a coastal stopover landscape. J Avian Biol 45:387–395
Woodworth BK, Mitchell GW, Norris DR, Francis CM, Taylor PD (2015) Patterns and correlates of songbird movements at an ecological barrier during autumn migration assessed using landscap- and regional-scale automated radiotelemetry. Ibis 157:326–339
Yamaura Y, Schmaljohann H, Lisovski S, Senzaki M, Kawamura K, Fujimaki Y, Nakamura F (2016) Tracking the Stejneger’s stonechat Saxicola stejnegeri along the East Asian–Australian Flyway from Japan via China to Southeast Asia. J Avian Biol 48:197–202
Yohannes E, Biebach H, Nikolaus G, Pearson DJ (2008) Passerine migration strategies and body mass variation along geographic sectors across East Africa, the Middle East and the Arabian Peninsula. J Ornithol 150:369–381
Yong W, Moore FR (1997) Spring stopover of intercontinental migratory thrushes along the northern coast of the Gulf of Mexico. Auk 114:263–278
Yong W, Finch DM, Moore FR, Kelly JF (1998) Stopover ecology and habitat use of migratory Wilson’s warblers. Auk 115:829–842
Zehnder S, Åkesson S, Liechti F, Bruderer B (2002) Observation of free-flying nocturnal migrants at Falsterbo: occurrence of reverse flight directions in autumn. Avian Sci 2:103–113
Zuur AE, Irwin DE, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Meth Ecol Evol 1:3–14
Zwarts L, Ens BJ, Kersten M, Piersma T (1990) Moult, mass and flight range of waders ready to take off for long-distance migrations. Ardea 78:339–364
Acknowledgements
This work was supported by a Deutsche Forschungsgemeinschaft (DFG) Grant awarded to HS (SCHM 2647/2-1). We thank one reviewer for very helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmaljohann, H., Eikenaar, C. How do energy stores and changes in these affect departure decisions by migratory birds? A critical view on stopover ecology studies and some future perspectives. J Comp Physiol A 203, 411–429 (2017). https://doi.org/10.1007/s00359-017-1166-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-017-1166-8