Abstract
To African–Palearctic migratory birds that spend the non-breeding season in sub-Saharan Africa, the Sahara desert is an ecological barrier that must be crossed using large energy reserves stored at stopover sites either side. Given the coincidence of spring migration with the height of the dry season in the west African Sahel, spring stopover conditions may be a limiting factor that regulates populations. By determining where, when and how reserves are accumulated prior to crossing the desert, we can identify possible population regulation mechanisms in Palearctic migrants and effectively target stopover sites for protection. We present data on the non-breeding season use of Djoudj National Park in the western Sahel by 38 Palearctic migrants based on constant effort mist-netting data. Twenty-four species showed evidence of using Djoudj either throughout the non-breeding period or during spring migration. The remaining 14 species were largely absent from Djoudj and presumably accumulated energy reserves for spring migration south of the Sahel. Species preparing for spring migration at Djoudj began increasing their energy reserves in January or February. Energy reserves were generally accumulated at slow rates during long stopovers, and in all species examined, predicted take-off fuel loads were sufficient to cross the Sahara desert under certain conditions. Three species, Common Redstart Phoenicurus phoenicurus, Common Whitethroat Sylvia communis and Common Blackcap Sylvia atricapilla, achieved faster rates and had concurrently lower stopover durations. We predict that vulnerability to fluctuating environmental conditions in the Sahel region is greater in species that initiate fuelling later in the spring and in species with short stopover durations.
Zusammenfassung
Auftanken für die Sahara-Überquerung—Variation bei der Gebietsnutzung und in Beginn und Ausmaß der Gewichtszunahme im Frühling bei achtunddreißig paläarktischen Zugvogelarten im westlichen Sahel.
Für Zugvögel, die in der Paläarktis brüten und im subsaharischen Afrika überwintern, stellt die Sahara eine ökologische Barriere dar, für deren Überwindung sie große Energiereserven benötigen, die sie bei Zwischenstopps auf beiden Seiten einlagern. Da der Frühjahrszug mit dem Höhepunkt der Trockenzeit in der westafrikanischen Sahelzone zusammentrifft, könnten die dortigen Rastbedingungen im Frühjahr einen limitierenden, die Populationen regulierenden Faktor darstellen. Kann man ermitteln, wann, wo und wie die Energiereserven vor der Überquerung der Wüste angelegt werden, lassen sich mögliche Mechanismen der Populationsregulation bei paläarktischen Zugvögeln ausmachen und Rastplätze gezielt schützen. Hier präsentieren wir Daten über die Nutzung des Djoudj Nationalparks als Winterquartier durch 38 paläarktische Zugvogelarten, die auf der Grundlage standardisierter Netzfänge gewonnen wurden. Vierundzwanzig Arten nutzten Djoudj entweder während der ganzen Überwinterungszeit oder auf dem Frühjahrszug. Die restlichen 14 Arten suchten Djoudj kaum auf und legten ihre Energiereserven für den Frühjahrszug vermutlich südlich der Sahelzone an. Arten, die sich in Djoudj für den Frühjahrszug rüsteten, begannen im Januar oder Februar mit der Aufstockung ihrer Energiereserven. Diese wurden in der Regel allmählich während längerer Rastaufenthalte angelegt; bei allen untersuchten Arten waren die geschätzten Energiereserven beim Abflug ausreichend, um die Sahara unter bestimmten Bedingungen zu überqueren. Drei Arten—Gartenrotschwanz Phoenicurus phoenicurus, Dorngrasmücke Sylvia communis und Mönchsgrasmücke Sylvia atricapilla—erreichten schnellere Zunahmeraten und hatten folglich kürzere Verweildauern. Unsere Prognose wäre eine größere Anfälligkeit für veränderliche Umweltbedingungen in der Sahelzone bei Arten, die erst später im Frühling auftanken.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Sahara desert represents a large ecological barrier in the annual journeys of billions of migratory birds that breed in Europe and Asia and have non-breeding grounds in sub-Saharan Africa (Hahn et al. 2009; Moreau 1972). The Sahara is termed a barrier, as it is a large area providing few or no opportunities for a typical migrant to stop and feed, and therefore must be crossed using energy stored beforehand. A typical migratory passerine, such as a warbler, must accumulate energy reserves equivalent to a 40% increase in body mass to fuel the approximately 1,750-km flight over the desert (Biebach 1992). These reserves are largely accumulated at stopover sites either side of the Sahara (e.g. Hernandez 2009; Ottosson et al. 2001; Schaub and Jenni 2000a), and site quality is expected to have a significant impact on the success of migration (Weber et al. 1999). This is especially true in sub-Saharan Africa, where preparations for the northward journey across the desert to Europe coincide with the end of a long dry season when resources are at low levels (Moreau 1972; Morel and Morel 1992). Indeed, given that the desert is the greatest energetic challenge faced by many migrants on their annual journeys, stopover sites south of the desert should be considered a conservation priority (Newton 2006). Information on sub-Saharan stopovers is required to fully understand long-term population declines observed in many Afro-Palearctic migrants (Sanderson et al. 2006).
Currently, there is a paucity of information on the sites or regions used by many species of migrants in sub-Saharan Africa, especially those used to prepare for the desert crossing (Fransson et al. 2006; Ottosson et al. 2001; Hjort et al. 1996; Salewski and Schaub 2007). While some species will stopover in the Sahel before crossing the Sahara (Ottosson et al. 2001), others are thought to fatten further south (Bayly and Rumsey 2010; Ottosson et al. 2005). In the past, species stopping over in the Sahel may have been impacted by the well-documented relationship between drought in the Sahel region and population declines in a range of migratory species (e.g. Baillie and Peach 1992). Currently, the Sahel is experiencing a wetting phase, and concern is now focussed on those species that winter in the humid zone of west Africa, as many of these populations are showing very rapid population declines (Sanderson et al. 2006). Arrival time of these species in Europe has often not advanced (whereas for Sahelian wintering migrants it has), possibly indicating some kind of constraint is operating in west Africa preventing earlier spring arrival in the breeding grounds (Ockendon et al. 2012). Understanding which species stage where and for how long is an important first step in understanding how migrants prepare for the desert crossing.
For species that stopover in the Sahel, it has been suggested that much of the mortality associated with past droughts may have occurred during the migratory phase (Bayly et al. 2011; Wilson and Cresswell 2006). For high levels of mortality to be associated with migration is not unprecedented (Newton 2006), indeed a study of annual survival in Black-throated Blue Warblers Dendroica caerulescens showed that approximately 85% of annual mortality occurred during migration (Sillett and Holmes 2002). Identifying and conserving sub-Saharan stopover sites is therefore expected to be essential if we are to adequately protect Palearctic migrants at all stages of their life cycle.
Our objective was to examine how 37 migratory passerines and 1 non-passerine utilised Djoudj National Park in Senegal during the non-breeding season, with particular emphasis on spring migration. Djoudj National Park is located in the western Sahel region of sub-Saharan Africa and is part of a larger complex of wetlands in the Senegal river valley. The Sahel is a key wintering and staging area for many Palearctic–African migrants (Moreau 1972; Morel and Morel 1992). By providing information on site use in terms of seasonal presence, use at the population level, migratory condition on arrival in spring, fuel deposition rates and stopover durations, we aim to provide a case study of how migrants may be using the wider Sahel region during the non-breeding season and as a stopover site prior to crossing the Sahara desert. By understanding where, when and how Palearctic migrants accumulate the energy required to cross the Sahara, we can make predictions regarding the vulnerability of species to environmental fluctuations, such as drought, that may affect the success of migration.
Methods
Study site
Parc National des Oiseaux du Djoudj (16°10′N, 16°18′W; referred to as Djoudj from here on) is situated within the Senegal River delta in northwest Senegal approximately 30 km from the coast (Fig. 1), and is comprised of seasonally flooded waterways, open water, reed and sedge beds, and areas of inundated and dry scrub dominated by tamarisk Tamarix senegalensis (Sauvage et al. 1998). Water levels in the park peak between September and December and decrease thereafter (unpublished data).
Data collection and species selection
Data collection was part of an international ornithological expedition organised by the Wetland Trust, during which mist-netting took place daily from October to May between 1990 and 1993 (see Ottosson et al. 2001 for full details). Mist-net effort was distributed between eight main sites and was operated on rotation such that equal effort was exerted in any one site every 7 days. The sites contained three identifiable habitat types: reed and sedge beds, seasonally flooded tamarisk and dry scrub containing either acacia Acacia sp. or Salvadora persica.
Birds captured in mist-nets were fitted with a uniquely coded metal ring and the following data were taken prior to release: date; mist-net site; hour of extraction; age; maximum wing chord—measured to nearest 1 mm following Svensson (1992); body mass—measured to nearest 0.1 g using a spring balance; fat score—visible fat was scored on a five point scale following Busse and Kania (1970).
Thirty-eight migratory landbirds were selected for analysis on the basis of having regular wintering populations in west Africa (Borrow and Demey 2004; Cramp et al. 2004), being likely to use the habitats available at Djoudj during migration and being likely to be trapped in mist-nets. These are listed in Table 1 except for the following which were omitted for having <10 captures: Whinchat Saxicola rubetra, Aquatic Warbler Acrocephalus paludicola, Icterine Warbler Hippolais icterina, Wood Warbler Phylloscopus sibilatrix, Golden Oriole Oriolus oriolus, Ortolan Bunting Emberiza hortulana.
Temporal site use at Djoudj and population indices
We calculated the number of individuals (determined by ring number) captured for each species between October and May during two ‘winters’ (1991/1992 and 1992/1993), making no correction for mist-net effort, as an indication of sample sizes and capture rates. To examine temporal site use at Djoudj, we divided the non-breeding period into three ‘seasons’ defined as autumn, winter and spring, each of 2 months duration (15 October–14 December, 15 December–14 February, 15 February–15 April, respectively; note that the definition of ‘spring’ varies between the analyses presented here depending on data needs and due to variability in the onset of migration between species), and selected captures between 15 October 1992 and 15 April 1993 for analysis (in other ‘winters’, not all sites or habitats were sampled in all three periods). For each species, the number of individuals captured by season was then summed and corrected for mist-net effort, before being converted into a percentage of total captures across all seasons. Mist-net effort varied between sites, habitats and seasons. To correct capture totals to reflect equal mist-net effort between habitats and seasons, captures were first assigned to one of three habitats (defined under “Data collection and species selection”) according to site of capture. Three species targeted for capture at communal roosts, Sand Martin Riparia riparia, Barn Swallow Hirundo rustica and Yellow Wagtail Motacilla flava, could not be analysed as above due to uneven distribution of capture sessions in time. Only Sand Martin roosts were targeted in all three seasons, and capture totals were corrected for the number of roosts targeted in each period.
To objectively classify seasonal use of Djoudj by each species into a series of categories, we ran a cluster analysis entering the percentage of captures by season, as defined above, for 30 species. The cluster analysis was carried out using the complete linkage setting, to account for small differences between species, and Euclidean distance in the program Minitab release 13.3.
To determine the importance of Djoudj to the western European population of individual species, and by extrapolation the Sahel, we calculated a crude winter and spring population index using Eq. 1.
‘Capture total’ was calculated by season and corrected for mist-net effort (see above). ‘Population size’ was derived from Birdlife International (2004) by summing all population estimates for countries in ‘western Europe’ (defined as all countries west of and including Sweden, Germany, Switzerland and Italy), based on the assumption that western and eastern European populations migrate differentially to west and east Africa, respectively (Alerstam 1990). The multiplication factor mf was the value required to give a winter index value of 100% for Savi’s Warbler Locustella luscinioides and was applied to all species. Savi’s Warbler was chosen to determine a value for mf, as it winters almost exclusively in the Sahel region, i.e. approximately 100% of the population is expected to winter in the Sahel (Cramp et al. 2004).
The final index is intended to give a crude approximation of the percentage of each species’ population present in the Sahel in either winter or spring—its cautious use must consider the following. Pied Wagtail Motacilla alba, Sardinian Warbler Sylvia melanocephala, Common Blackcap Sylvia atricapilla and Common Chiffchaff Phylloscopus collybita winter both north and south of the Sahara; the index will therefore underestimate the percentage of the sub-Saharan population present. Resident and migratory populations of Olivaceous Warbler Hippolais pallida and Rufous-tailed Scrub Robin Cercotrichas galactotes were present at Djoudj; the index will therefore overestimate presence. The restricted availability of wetland habitats in the Sahel is expected to concentrate wetland specialists at a small number of sites. The scaling of our index was based on a wetland specialist, and therefore the presence of species favouring more broadly distributed habitats is expected to be underestimated. Finally, the index assumes that species are equally catchable, and any interpretation must consider the probability of capture in the habitats sampled.
Stopover behaviour on spring migration
To determine which species prepared for the crossing of the Sahara desert at Djoudj and how they did so, we calculated six measures of stopover and fuelling behaviour. Ideally, we would have fewer measures to ensure comparison between species, but the calculation of each measure has differing data requirements and not all measures could be calculated for all species. Underlying several of the measures below is a value for lean body mass (LBM; the body mass of a bird carrying no visible fat reserves), which was estimated from Eq. 2. The values of a and b in Eq. 2 were estimated for each species by modelling body mass as a function of wing chord for all birds with a fat score of zero in a general linear model with normal errors and an identity link function.
We make the assumption that increases in body mass above LBM reflect increasing fuel stores based on studies relating fat content and body mass, e.g. Redfern et al. (2004). Muscle scores were not recorded in this study, and by not considering them in our estimations of LBM, our LBM values are likely to give rise to small underestimates when used to calculate fuel loads and flight ranges (Salewski et al. 2009). Nevertheless, our flight range estimates may be more realistic given that the optimal strategy is likely to involve arriving at a destination before consuming all possible combustible reserves.
Initiation of fuelling
A turning point analysis was applied to the body mass data for each individual species to identify the week/s after which body mass showed an increasing trend. The week of each capture after 1 July was calculated and the data truncated to include only weeks 20 (11 November) to 42 (20 April). This encompassed the majority of capture dates for all migrants. For each species, a bootstrapping procedure with replacement was used to produce 499 separate datasets that included species identity, the week of capture and the mass at capture. Using a General Additive model (GAM), we fitted a smooth curve such that mass was a smooth function of week. The degrees of freedom in the model were calculated by taking the integer value of the equation (last week − first week) and dividing this number by 3 as recommended by Fewster et al. (2000) and Atkinson et al. (2003). A turning point was defined as a point at which the smoothed values for the previous and following week were greater than the value for the current week (i.e. a U-shaped curve). For species which showed a very rapid increase at a defined point in time, a large number of turning points would be identified for certain weeks, giving a noticeable spike in the plot of week versus number of turning points. For species where the onset of mass gain was more spread out, a shallower curve would be expected in this plot. This analysis indicated a number of different strategies were occurring. We therefore defined the date on which fuelling was initiated as the midpoint of the week after 1 January with the highest number of positive turning points. We verified the selected week by visual inspection of the smoothed curves generated using GAMs. In three cases, visual inspection revealed that the week with the second highest number of turning points was a better approximation to the start of fuelling.
Mean fuel load
Fuel load, defined as any increase in body mass above LBM, is expressed as a percentage of LBM to allow inter-species comparisons (Eq. 3). Fuel load was calculated for all new ‘spring’ captures in 1992 and 1993, for species with a small or no wintering population at Djoudj (selection criteria <15% of captures must have occurred in winter; see Table 1).
For species with no wintering population at Djoudj, the spring period was defined as 1 February–1 May. For species with a small wintering population, the start of the spring period was defined by the turning point analysis described above. Given that constant effort mist-netting was carried out for up to 5 months prior to spring migration, new spring captures were expected to be recently arrived, and therefore their fuel load should reflect the extent to which fuel was accumulated elsewhere. This approach relies on the assumption that birds are caught shortly after arrival and/or they are accumulating reserves at slow rates. We test the latter assumption by estimating fuel deposition rates (see below). New spring captures of species that regularly winter at Djoudj will contain an unknown proportion of wintering individuals and therefore cannot be reliably analysed by this method.
Max fuel deposition rate (max FDR)
We calculated max FDR based on changes in body mass between successive captures of individual birds in spring, on the assumption that increases in body mass relate to the deposition of energy reserves in the form of fat and protein. To minimise the inclusion of birds in a non-fuelling state, we selected captures occurring after each species’ respective turning point. Species with less than five recaptured individuals were not analysed. Body mass was positively correlated with hour of capture in most species, and therefore it was necessary to adjust body mass for hour of capture before estimating daily fuel deposition rates. We modelled a condition index (CI) against hour of capture relative to sunrise for all captures, to estimate size-specific hourly rates of increase in body mass, where CI = body mass/wing chord. If the regression was significant (p < 0.05), body mass was adjusted to the median hour of capture in each species to minimise alteration of the raw data. Following correction for hour of capture, FDR was calculated for all recaptured individuals using Eq. 4.
We express FDR as the percentage of a bird’s size specific LBM accumulated per day. As capture effects are believed to cause mass reductions (Schwilch and Jenni 2001), summary statistics of max FDR do not include negative rates and are based on the maximum rate observed in any given individual.
Model fuel deposition rate (model FDR)
The calculation of model FDR was more data intensive than max FDR, and we limited analyses to species with at least 20 recaptures. As with max FDR, only individuals captured after a species’ respective turning point were considered and all body masses were corrected to the median hour of capture prior to analysis. To estimate model FDR, we modelled body mass change between the first and successive captures of individual birds based on a modification of the method adopted by Atkinson et al. (2007); see Bayly et al. (2011) for further discussion of this method. Multiple captures of the same individual were arranged as 1st–2nd, 1st–3rd, 1st–4th, etc., in order to capture the shape of individual mass change trajectories (see Fig. 2). We modelled the resulting body mass changes using General Linear Models with normal errors in the Program R (preliminary analysis with Generalised Additive Models did not support the use of non-linear models) and the factors, number of days between captures (D), date of first capture (S), body mass at first capture (M), year of capture (Y), Sex (sexually dimorphic species only), and their interactions. The model set included all permutations of the above factors entered sequentially into models, including nesting of continuous factors in the categorical variable year. Subsequent model selection was based on Akaike Information Criteria adjusted for sample size (AICc; Burnham and Anderson 1998) and the model receiving the greatest support (lowest AICc) was used to estimate model FDR, entering mean values for continuous factors, e.g. date, and the mean of coefficients for categorical factors, e.g. year.
Spring captures as defined here are expected to consist primarily of actively fuelling birds, but they will also include individuals that have yet to commence fuelling. As the presence of non-fuelling individuals will result in an underestimation of FDR, we conservatively excluded individuals from model FDR analyses that gained <0.5% of lean body mass per day (mean max FDR in this study = 1.6% LBM per day) after day n from first capture, where n was species-dependent. Day n was the first day after initial capture where mean mass change across all individuals was positive, on the condition that the mean was positive on the following day as well. In all species except Common Redstart Phoenicurus phoenicurus, mean mass gain was negative immediately following capture and n varied between species such that n = 5 days in Sedge Warbler Acrocephalus schoenobaenus and 10 days in Grasshopper Warbler Locustella naevia (Fig. 2).
To examine the relationship between resulting model FDRs and max FDRs, we carried out a paired t test.
Minimum stopover duration
Minimum stopover duration was defined as the number days elapsed between the first and last capture of individual birds, inclusive of the first and last day. To calculate minimum stopover duration, we only considered individuals recaptured on at least one occasion, individuals captured after the respective turning point of each species and species with at least five recaptures.
Stopover after and total stopover durations
We estimated ‘stopover after’ and ‘total stopover’ durations by analysing mark–recapture data in the Program MARK 5.1 (White and Burnham 1999), following the methods outlined in Schaub et al. (2001) and Efford (2005). We calculate both stopover estimates, as the most applicable method with standard mist-netting data remains to be resolved (Schaub et al. 2001; Efford 2005). These methods predict longer durations than the minimum stopover method, as they use recapture probabilities to estimate how long a bird is likely to have remained at a site after last capture (stopover after) or, in the case of ‘total stopover’, the time present at a site before first capture in combination with ‘stopover after’.
The reliability of ‘stopover after’ and ‘total stopover’ estimates decreases with decreasing sample sizes and recapture rates. We set minimum sample size at 60 individuals. Stopover duration was estimated separately by year and by site for each species (if sites were over 1 km apart) and considering only individuals captured after a species’ respective turning point. Lumping distant sites would break the assumption of equal probability of capture between individuals. The number of capture occasions (c) varied between sites. Where c > 30, capture occasions were pooled over 2 days to reduce the number of parameters to be estimated, otherwise capture occasions were not pooled and the number of days between occasions was defined in MARK.
To estimate ‘stopover after’ and ‘total stopover’, we estimated survival probability (Φ) and seniority probability (γ). Starting models included time-dependence in Φ, γ and the recapture probability p. We also included an age-dependent structure following Pradel et al. (1997) to account for the presence of transients in the population. For each combination of site, year and species, five models were run [Phi (t) p (t); Phi (t) p (.); Phi (.) phi (t); Phi (.) p (.); Phi (transient) p (.)], where t indicate time-dependence. Estimates for Φ and γ were obtained from models with the lowest AICc value except where the ΔAIC (difference in AICc value between two models) value of the nearest model was <2 (Burnham and Anderson 1998)—an indication that models are equally plausible. In this study, where ΔAIC was <2, the two models involved were always Phi (.) p (.) and Phi (trans) p (.)—we selected the latter model to estimate Φ and γ on the basis that transients or birds that enter an uncatchable state are always expected to be present in a population at a stopover site. A goodness of fit test based on the median ĉ method implemented in MARK (White and Burnham 1999) was run for all selected models, and models with ĉ > 1.5 are not presented to minimise uncertainty in stopover estimates. Finally, ‘stopover after’ durations were calculated from estimates of Φ without time-dependence using the life expectancy formula, LE = −1/lnΦ. Where a transient model was supported, we estimate stopover duration in non-transients only (Φ2). ‘Total stopover’ was estimated as ‘total stopover’ = (−1/lnΦ) + (−1/lnγ). In all models selected on the basis of AICc values, Φ and γ were not time-dependent except one. For this exception, we adopted the non-time-dependent model for uniformity.
As ‘stopover after’ or ‘total stopover’ estimates are considered more precise than minimum stopover durations, but unlike the latter they could not be estimated for all species, we examined the relationship between minimum stopover duration and ‘stopover after’ in order to determine whether minimum stopover duration can act as surrogate for ‘stopover after’ in order to examine between species patterns. We did this by regressing the difference in estimates for each species against its respective ‘stopover after’ estimate, to see whether the degree of underestimation by the minimum stopover method varied markedly with changing stopover duration.
Variation between species in stopover duration may be caused by differences in when a species begins preparing for migration or by variation in the rate of fuel deposition. We therefore tested the null hypothesis that stopover duration is not related to species’ turning points or to their mean FDR using linear regression.
Multiplying estimated FDRs by stopover durations produces an estimate of take-off fuel load. If migrants fly directly across the Sahara desert on leaving Djoudj, we estimate that take-off fuel loads should be >32.5% LBM based on flight range estimates for Sedge Warbler and Grasshopper Warbler. Flight range estimates were calculated using the program Flight 1.15 (Pennycuick 2008), assuming that a bird required sufficient energy to cover 1,750 km to cross the Sahara. The following values required by Flight were entered for each species: Grasshopper Warbler wingspan = 0.184 m (mean of 14 UK birds), aspect ratio = 4.99 (mean of 7 UK birds); Sedge Warbler wingspan = 0.183 m (mean of 12 UK birds), aspect ratio = 5.27 (mean of 5 UK birds). Flight altitude was set at 2,250 m (Liechti and Schmaljohann 2007), otherwise all other settings were set to default. During spring migration, tailwinds are common over the Sahara and final energy needs were calculated assuming a tailwind of 2 m/s (Schmaljohann et al. 2009). There is increasing evidence that birds make daytime stops while crossing the desert (Schmaljohann et al. 2007). Fuel needs may therefore be up to 11% greater if birds consume energy during these stops (rates of loss in the desert are unknown), e.g. 43.5% LBM, based on estimated hourly rates of mass loss of 0.5% LBM (Salewski et al. 2010) and 2 daytime stops.
Results
Temporal site use at Djoudj and population indices
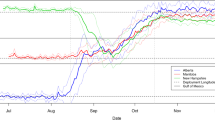
Raw mist-net capture totals over two winters ranged from 0 in Wood Warbler to 9,298 in Reed Warbler Acrocephalus scirpaceus. Capture totals for 32 species with >5 captures are provided in Table 1. Less than 5 captures were registered in the following species (capture total): Whinchat (3), Aquatic Warbler (1), Icterine Warbler (0), Wood Warbler (0), Golden Oriole (0), Ortolan Bunting (1). A cluster analysis of seasonal presence identified three main clusters with less than 10% similarity (Table 1), which can be broadly defined as species present in all seasons (16 species), autumn migrants (4 species) and spring migrants (10 species). Within these clusters we have identified two further distinct clusters, primarily wintering species (2 species) and spring migrants that also occur in autumn (3 species). Four of these patterns of seasonal presence are presented in Fig. 3. Further clusters are identifiable within species present in all seasons but they are not differentiated in Table 1.
Seasonal occurrence of four Palearctic migrants at Djoudj. Each species illustrates one of the main strategies of site use identified in Table 1 (one strategy is not illustrated). Strategy (left to right, top to bottom): present in all seasons; autumn passage migrant; spring passage migrant; autumn and spring passage migrant
Our population indices are expected to provide a preliminary indication of the degree to which Djoudj and, by extrapolation, the western Sahel region are used by species at the population level. We consider a population index of >10% to determine a subset of species which rely on the western Sahel; however, we do not consider an index of <10% as evidence for non-use, given the shortfalls of the index outlined in the methods. Species with an index >10% included Sand Martin, Common Redstart (mainly spring), Savi’s Warbler, Grasshopper Warbler, Sedge Warbler, Reed Warbler (mainly spring), Orphean Warbler Sylvia hortensis and Subalpine Warbler Sylvia cantillans. We exclude Rufous-tailed Scrub Robin and Olivaceous Warbler as resident populations of both species may have been present.
Stopover behaviour on spring migration
The initiation of fuel deposition for spring migration, as defined by turning points, began in the week with midpoint of 9 January in the earliest species and in the week with midpoint of 20 March in the latest species (Table 2; Fig. 4). The majority of species began fuelling in January (9 species) or February (7 species), and only two species began in March. Species with no or a small wintering population at Djoudj had mean fuel loads varying between 8 and 60% LBM on first capture (Table 2). In two species, Tree Pipit Anthus trivialis and Garden Warbler Sylvia borin, mean fuel load at first capture was greater than that required to cross the Sahara (32.5–43.5% LBM).
Max FDR varied between 0.9 and 1.7% LBM per day in most species and exceeded 2% in just three species: Common Redstart, Common Whitethroat Sylvia communis and Common Blackcap (Table 2). Model FDRs were slower than Max FDRs in eight of the ten species examined here, but a paired t test revealed no overall significant difference (p = 0.108). Models of body mass change containing a year effect received the greatest support from AIC values in 9 of the 10 species examined (Table 2), providing evidence that fuel deposition rates varied significantly between years in these species. Maximum differences in rates between years within species varied from 12.3 to 50.9%, with a mean difference of 39.8%.
Stopover duration varied according to the method used for its calculation and across species, sites and years (Tables 2 and 3). There are strong arguments for the suitability of ‘stopover after’ estimates (Efford 2005) and ‘total stopover’ estimates (Schaub et al. 2001); however, we present minimum stopover durations, as ‘stopover after’ or ‘total stopover’ was not estimable in all species. Variation in minimum stopover duration between species closely reflected differences in ‘stopover after’ estimates, with a mean difference between estimates of 24%. However, the degree of difference increased significantly at a rate of 1.3% per day as ‘stopover after’ durations increased (F 1,26 = 16.1, p < 0.001), indicating that comparisons of minimum stopover durations will underestimate the degree of difference between species with short durations and those with long durations. Nevertheless, we conclude that minimum durations can be used to examine patterns between species in relation to factors such as the initiation of fuelling.
‘Stopover after’ durations averaged across sites and years ranged from 6.7 to 32.3 days in the eight species considered. Stopover durations decreased the later the turning point of each species (Fig. 5), significantly so with minimum stopover durations (F 1,14 = 4.91, p = 0.045) but not with ‘stopover after’ duration (F 1,7 = 3.43, p = 0.113). ‘Stopover after’ durations decreased significantly with increasing model FDR (F 1,7 = 6.3, p = 0.047, Fig. 6) and max FDR (F 1,7 = 7.9, p = 0.031).
Stopover duration against fuel deposition rate (model FDR) for eight migrant passerines at Djoudj. Both mean ‘stopover after’ (filled square) and mean ‘total stopover’ (open square) are presented for the eight species in which they were estimable, and model FDR is used to represent fuel deposition rate except in one case where max FDR is used (Blackcap). The continuous line represents the required stopover duration for a given FDR in order for birds to have sufficient energy reserves to make a non-stop flights across the Sahara (minimum fuel load of 32.5% LBM), when they are conservatively assumed to be carry a fuel load of 5% LBM on arrival. The dashed line represents the same situation but under the assumption that birds make daytime stops in the desert and therefore require a fuel load of 43.5% LBM. Points above these lines represent species that exceeded the estimated minimum take-off fuel load required to cross the Sahara under a non-stop or intermittent flight scenario
In the eight species for which ‘stopover after’ durations were calculated, estimated take-off fuel loads were not sufficient to cross the Sahara (i.e.<43.5% LBM) in five species, when take-off fuel load = mean ‘stopover after’ × model FDR (Fig. 6). If take-off fuel load was calculated alternatively as: mean ‘stopover after’ × mean max FDR, five of the eight species attained our minimum fuel load requirement (32.5% LBM), but only two species could cross the Sahara if fuel is consumed during daytime stops. If take-off fuel load = mean total stopover × mean model FDR, all species theoretically attained the minimum fuel load requirement regardless of whether they adopted a strategy of a non-stop flight or intermittent flights with daytime stops (Fig. 6).
Discussion
The 38 trans-Saharan migratory landbirds considered here showed considerable variation in their use of Djoudj National Park (Table 1). Approximately 42% of species were present at Djoudj throughout the non-breeding season, while 11% used Djoudj primarily as an autumn migration stopover site and 25% as a spring stopover site. Another group of species were essentially absent from Djoudj (conservatively estimated as 16% of species), apparently using other habitats, routes or regions during both migration and winter, e.g. Wood Warbler. Our population indices suggest that Djoudj and by extrapolation the western Sahel are used by a notable proportion of the non-breeding population of at least eight species, based on having a population index >10%. Species present at Djoudj during spring migration showed strong evidence for replenishing their energy reserves in the park (Table 2; Figs. 2, 4 and 6). Based on estimated FDRs and ‘total stopover’ durations, accumulated energy reserves were sufficient to cross the Sahara desert without refuelling (Fig. 6). Stopover strategies at Djoudj generally involved long stopover durations, as rates of fuel deposition were low; however, a small number of species achieved faster rates and had concurrently lower stopover durations. Spring stopover strategies and the timing of fuelling, which varied widely between species (Table 1), may have important implications for a species’ vulnerability to fluctuating environmental conditions in the Sahel region.
Temporal site use at Djoudj and population indices
Of the 38 Palearctic migrants considered here, 8 species occurred at Djoudj in significant numbers based on our population index (Table 1). Six of these species wintered at Djoudj, while Common Redstart and Reed Warbler were primarily spring migrants. Rufous-tailed Scrub Robin and Olivaceous Warbler also had high index values, but it was not possible to differentiate between migrant populations from Europe and non-migratory African populations. Our population index is expected to underestimate the presence of species whose western European population does not winter exclusively south of the Sahara. Six species in Table 1 have wintering populations north of the Sahara, including Pied Wagtail, Bluethroat Luscinia svecica, Sardinian Warbler, Spectacled Warbler Sylvia conspicillata, Common Blackcap and Common Chiffchaff. If we had considered only sub-Saharan wintering populations, the populations of species such as Common Chiffchaff and Bluethroat recorded at Djoudj would no doubt have assumed greater importance. Our index is also expected to underestimate the presence of species occupying dry habitats. For example, the spring index of 6.5% for Common Whitethroat may underestimate the occurrence of spring stopovers in this species, which has been recorded in high numbers elsewhere in the Sahel (Ottosson et al. 2002).
Six of the 38 species considered here were trapped on fewer than ten occasions. The absence or near absence of these species can be explained by wintering habitat preferences (e.g. Whinchat) and migration route or strategy. Wood Warbler and Icterine Warbler, for example, follow a more easterly route from Africa to Europe (Borrow and Demey 2004; Cramp et al. 2004). Several more species also appear to have a non-breeding season strategy that largely excludes the use of Djoudj and by extrapolation the western Sahel. Among these are species which winter primarily in the Guinea savanna or in the Guinea and Congolian forests to the south of the Sahel zone (Borrow and Demey 2004), and which include widespread European-breeding species such as Tree Pipit, Common Nightingale Luscinia megarhynchos, Garden Warbler, Willow Warbler Phylloscopus trochilus, Pied Flycatcher Ficedula hypoleuca and Spotted Flycatcher Muscicapa striata. These species are currently undergoing rapid declines across much of Europe and understanding their spring migration strategies must be a high priority, especially as there is evidence for constraints acting on their ability to advance their spring arrival time in Europe in order to take advantage of earlier springs (Ockendon et al. 2012).
Stopover behaviour on spring migration
The Sahel is the last vegetated zone encountered by spring migrants before the Sahara desert, and Djoudj forms part of the most northerly wetland complex in the western Sahel. Djoudj and the wider Senegal River valley are therefore expected to provide fuelling opportunities for a wide variety of migrants preparing to cross the desert. Despite the strategic position of Djoudj, a range of species, as discussed above, did not appear to stopover at Djoudj in spring and are expected to be fuelling in regions further south (Ottosson et al. 2005; Yohannes et al. 2008).
Species which did fuel at Djoudj began increasing their energy reserves as early as the first week of January and as late as the middle of March (Fig. 5). The rate of fuel deposition was low in the majority of species in comparison to rates recorded in Europe (Schaub and Jenni 2000b), and may reflect a paucity of resources associated with the west African dry season. Rates were not constant between years, and modelling of body mass changes found a significant year effect in nine out of ten species examined (Table 2). Rates were found to be up to 50% different between years and therefore, under certain conditions, rates will be still lower than the mean rates reported here. Based on our estimate of minimum fuel requirements to cross the Sahara desert (>32.5% or >43.5% LBM), birds achieving rates of between 0.9 and 1.7% LBM per day would be expected to fuel for between 20 and 40 days, if Djoudj was their last stopover before crossing the Sahara. Our stopover estimates agree with this prediction, and ‘total stopover’ durations were sufficiently long to reach the target fuel load in all species for which they were estimated (Fig. 6). The optimal stopover duration for each species appears to lie between our estimates for ‘stopover after’ and ‘total stopover’ duration (Fig. 6). Taking into account that minimum stopover durations underestimate actual stopover durations, the FDRs and stopover durations presented in Table 2 provide evidence of at least 15 species accumulating sufficient energy at Djoudj to cross the Sahara desert. We can conclude that Djoudj and by extrapolation the Senegal River valley represent a key stopover site for a range of Palearctic migrant landbirds.
A minority of species including Common Redstart, Common Whitethroat and Common Blackcap achieved FDRs above 2% LBM/day and had shorter stopover durations presumably as a consequence (Table 2; Fig. 6). These rates may have been achieved by consuming fruit, indeed both Common Whitethroat and Common Redstart consume Salvadora berries during spring migration (Stoate and Moreby 1995), and Blackcaps are highly frugivorous during migration (Jordano 1988).
The majority of species preparing for the Sahara crossing at Djoudj also wintered there (Table 2). In contrast, Common Redstart, Common Whitethroat and Reed Warbler had no or a limited winter presence at Djoudj, but made a spring stopover at Djoudj during which they accumulated energy reserves for the Sahara crossing. Other species that occurred only during migration generally carried large fuel loads in spring (e.g. Tree Pipit and Common Nightingale). These birds presumably consisted mostly of individuals that had no need for further energy reserves and had paused their migration to avoid adverse flight conditions such as high daytime temperatures (Bayly and Rumsey 2010; Salewski et al. 2010; Schmaljohann et al. 2007). A smaller proportion presumably had failed to accumulate sufficient reserves at more southerly stopover sites. Despite carrying relatively small reserves at Djoudj, Melodious Warbler Hippolais polyglotta and Bonelli’s Warbler Phylloscopus bonelli were uncommon and showed no evidence of fuelling at Djoudj (Table 2).
Vulnerability to environmental fluctuations
Spring migration coincides with the Sahelian dry season and consequently conditions and resource levels are expected to deteriorate as migration progresses (Moreau 1972). The severity of the Sahelian dry season has been linked to population regulation in several Palearctic migrants, and droughts have caused dramatic population declines in the past (Baillie and Peach 1992; Newton 2004). Given the high energetic demands associated with crossing the Sahara desert, where, when and how migrants prepare for the crossing should determine their vulnerability to climatic variability in the Sahel region.
Of the 38 species considered here, nearly 40% were either largely absent from Djoudj during spring migration or had large reserves on arrival. This group of migrants, including species such as Pied Flycatcher and Common Nightingale, are expected to be the least vulnerable to climatic conditions and habitat quality in the Sahel in spring—although they may, of course, use the Sahel in early autumn on their way to wintering areas further south. Species that did fuel at Djoudj had a general strategy of initiating fuelling in January or February, long before their arrival in Europe in March or April (Cramp et al. 2004), and accumulated energy reserves at slow rates during long stopovers. This strategy is likely to be relatively robust to variations in resource levels between years, as the range of FDRs (resource levels) across which a target take-off mass can be reached increases with increasing stopover duration. Further, by initiating fuelling ‘early’, migrants may take advantage of higher resource levels earlier in the dry season. The disadvantages of this strategy might include both increased metabolic costs and a heightened predation risk resulting from a prolonged period of intense foraging (Dierschke 2003) and high body mass maintenance (Klaassen and Lindström 1996).
A minority of species adopted an alternative strategy, initiating fuelling or arriving at Djoudj ‘late’ and accumulating energy reserves at rapid rates during short stopovers (Figs. 5 and 6). This strategy may greatly reduce time and energy costs associated with migration (Hedenström and Alerstam 1997), but it is vulnerable to large fluctuations in resources. Indeed, minimum FDR requirements are much higher, and resource failures could result in large fuelling shortfalls if stopover durations are inflexible (Erni et al. 2002). Based on the timing of fuelling, FDRs and stopover durations at Djoudj, we predict that Common Redstart, Common Whitethroat and Common Blackcap should be vulnerable to drought conditions in the Sahel. Following the Sahelian drought of 1968, Common Redstart and Common Whitethroat registered the largest declines observed in Palearctic migrants (Newton 2004; Winstanley et al. 1974). Further, the UK population levels of Common Whitethroat were strongly correlated with Sahelian rainfall in the past (Baillie and Peach 1992), although the same is not true of Common Blackcap, presumably because a large percentage of the UK population winter in Europe. The Sahel is currently in a wetting phase and, in the years 2008–2010, rainfall was at or above the long-term average. It is likely that factors other than rainfall are currently limiting Sahelian migrants. While most population declines linked to factors acting in sub-Saharan Africa have been attributed to climatic conditions (Newton 2004), continued degradation of Sahelian habitats could give rise to long-term declines (Wilson and Cresswell 2006), particularly in species that are most vulnerable to changes in spring fuelling conditions.
References
Alerstam T (1990) Bird Migration. Cambridge University Press, Cambridge
Atkinson PW, Clark NA, Bell MC, Dare PJ, Clark JA, Ireland PL (2003) Changes in commercially fished shellfish stocks and shorebird populations in the Wash, England. Biol Conserv 114:127–141
Atkinson PW, Baker AJ, Bennett KA, Clark NA, Clark JA, Cole KB, Dekinga A, Dey A, Gillings S, González PM, Kalasz K, Minton CDT, Newton J, Niles LJ, Piersma T, Robinson RA, Sitters HP (2007) Rates of mass gain and energy deposition in red knot on their final spring staging site is both time- and condition-dependent. J Appl Ecol 44:885–895
Baillie SR, Peach WJ (1992) Population limitation in Palearctic–African migrant passerines. Ibis 134:120–132
Bayly NJ, Rumsey SJR (2010) Garden Warbler Sylvia borin migration in sub-Saharan west Africa. Ringing Migr 25:59–61
Bayly NJ, Clark JA, Rumsey SJR (2011) Crossing the Sahara Desert – migratory strategies of the grasshopper warbler Locustella naevia. J Ornithol 152:933–946
Biebach H (1992) Flight-range estimates for small trans-Saharan migrants. Ibis 134(suppl):47–54
BirdLife International (2004) Birds in Europe: population estimates, trends and conservation status. BirdLife international, Cambridge
Borrow N, Demey R (2004) Birds of Western Africa. Christopher Helm, London
Burnham KP, Anderson DR (1998) Model selection and inference: A practical information theoretic approach. Springer, New York
Busse P, Kania W (1970) Operation Baltic 1961–1967 working methods. Acta Ornithol 12:231–267
Cramp et al. (ed) (2004) Birds of the Western Palearctic interactive. Oxford University Press, Oxford
Dierschke V (2003) Predation hazard during migratory stopover: are light or heavy birds under risk? J Avian Biol 34:24–29
Efford MG (2005) Migrating birds stop over longer than usually thought: comment. Ecology 86:3415–3418
Erni B, Liechti F, Bruderer B (2002) Stopover strategies in passerine bird migration: a simulation study. J Theor Biol 219:479–493
Fewster RM, Buckland ST, Siriwardena GM, Baillie SR, Wilson JD (2000) Analysis of population trends for farmland birds using generalized additive models. Ecology 81:1970–1984
Fransson T, Jakobsson S, Kullberg C, Mellroth R, Petrersson T (2006) Fuelling in front of the Sahara desert in autumn—an overview of Swedish field studies of migratory birds in the eastern Mediterranean. Ornis Svec 16:74–83
Hahn S, Bauer S, Liechti F (2009) The natural link between Europe and Africa—2.1 billion birds on migration. Oikos 118:624–625
Hedenström A, Alerstam T (1997) Optimum fuel loads in migratory birds: distinguishing between time and energy minimization. J Theor Biol 189:227–234
Hernandez A (2009) Summer-autumn feeding ecology of pied flycatchers Ficedula hypolueca and spotted flycatchers Muscicapa striata: the importance of frugivory in a stopover area in north-west Iberia. Bird Conserv Int 19:224–238
Hjort C, Pettersson J, Lindström Å, King JMB (1996) Fuel deposition and potential flight ranges of blackcaps Sylvia atricapilla and whitethroats Sylvia communis on spring migration in The Gambia. Ornis Svecica 6:137–144
Jordano P (1988) Diet, fruit choice and variation in body condition of frugivorous warblers in Mediterranean scrubland. Ardea 76:193–209
Klaassen M, Lindström A (1996) Departure fuel loads in time-minimising migrating birds can be explained by the energy costs of being heavy. J Theor Biol 183:29–34
Liechti F, Schmaljohann H (2007) Wind-governed flight altitudes of nocturnal spring migrants over the Sahara. Ostrich 78:337–341
Moreau RE (1972) The Palearctic-African bird migration systems. Academic, London
Morel GJ, Morel M (1992) Habitat use by Palearctic migrant passerine birds in West Africa. Ibis 134(suppl):83–88
Newton I (2004) Population limitation in migrants. Ibis 146:197–226
Newton I (2006) Can conditions experienced during migration limit the population levels of birds? J Ornithol 147:146–166
Ockendon N, Hewson CM, Johnston A, Atkinson PW (2012) Declines in British breeding populations of Afro-Palaearctic migrant birds are linked to bioclimatic wintering zone in Africa, possibly via constraints on arrival time advancement. Bird Study. doi:https://doi.org/10.1080/00063657.2011.645798
Ottosson U, Rumsey R, Hjort C (2001) Migration of four Sylvia warblers through northern Senegal. Ringing Migr 20:344–351
Ottosson U, Bairlein F, Hjort C (2002) Migration patterns of Palaearctic Acrocephalus and Sylvia warblers in north-eastern Nigeria. Vogelwarte 41:249–262
Ottosson U, Waldenstrom J, Hjort C, McGregor R (2005) Garden warbler Sylvia borin migration in sub-Saharan West Africa: phenology and body mass changes. Ibis 147:750–757
Pennycuick CJ (2008) Modelling the flying bird. Elsevier, Amsterdam
Pradel R, Hines JE, Lebreton JD, Nichols JD (1997) Capture–recapture survival models taking account of transients. Biometrics 53:60–72
Redfern C, Topp V, Jones P (2004) Fat and pectoral muscle in migrating sedge warblers Acrocephalus schoenobaenus. Ringing Migr 22:24–34
Salewski V, Schaub M (2007) Stopover duration of Palearctic passerine migrants in the western Sahara—independent of fat stores? Ibis 149:223–236
Salewski V, Kéry M, Herremans M, Liechti F, Jenni L (2009) Estimating fat and protein fuel from fat and muscle scores in passerines. Ibis 151:640–653
Salewski V, Schmaljohann H, Liechti F (2010) Spring passerine migrants stopping over in the Sahara are not fall-outs. J Ornithol 151:371–378
Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Brommel FPJ (2006) Long-term population declines in Afro-Palearctic migrant birds. Biol Conserv 131:93–105
Sauvage A, Rumsey SJR, Rodwell SP (1998) Recurrence of Palearctic birds in the lower Senegal river valley. Malimbus 20:33–53
Schaub M, Jenni L (2000a) Body mass of six long-distance migrant passerine species along the autumn migration route. J Ornithol 141:441–460
Schaub M, Jenni L (2000b) Fuel deposition of three passerines along the migration route. Oecologia 122:306–317
Schaub M, Pradel R, Jenni L, Lebreton JD (2001) Migrating birds stop over longer than usually thought: an improved capture–recapture analysis. Ecology 82:852–859
Schmaljohann H, Liechti F, Bruderer B (2007) Songbird migration across the Sahara: the non-stop hypothesis rejected! Proc R Soc Lond B 274:735–739
Schmaljohann H, Liechti F, Bruderer B (2009) Trans-Sahara migrants select flight altitudes to minimize energy costs rather than water loss. Behav Ecol Sociobiol 63:1609–1619
Schwilch R, Jenni L (2001) Low initial refuelling rate at stopover sites: a methodological effect? Auk 118:698–708
Sillett TS, Holmes RT (2002) Variation in survivorship of a migratory songbird throughout its annual cycle. J Anim Ecol 71:296–308
Stoate C, Moreby SJ (1995) Premigratory diet of trans-Saharan migrant passerines in the western Sahel. Bird Study 42:101–106
Svensson L (1992) Identification guide to European Passerines, 4th edn. Naturhistoriska, Stockholm
Weber T, Houston AI, Ens B (1999) Consequences of habitat loss at migratory stopover sites: a theoretical investigation. J Avian Biol 30:416–426
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46(suppl):120–139
Wilson JM, Cresswell W (2006) How robust are Palearctic migrants to habitat loss and degradation in the Sahel. Ibis 148:789–800
Winstanley D, Spencer R, Williamson K (1974) Where have all the whitethroats gone? Bird Study 21:1–14
Yohannes E, Biebach H, Nikolaus G, Pearson DJ (2008) Passerine migration strategies and body mass variation along geographic sectors across East Africa, the Middle East and the Arabian Peninsula. J Ornithol 150:369–381
Acknowledgments
We thank all the ringers who took part in the Wetland Trust expeditions to Djoudj National Park between 1990 and 1993. We are indebted to Phil Jones for digitising large volumes of ringing data and to Jacquie Clark for commenting on the manuscript. H. Schmaljohann and an anonymous reviewer provided invaluable comments that helped improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bairlein.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayly, N.J., Atkinson, P.W. & Rumsey, S.J.R. Fuelling for the Sahara crossing: variation in site use and the onset and rate of spring mass gain by 38 Palearctic migrants in the western Sahel. J Ornithol 153, 931–945 (2012). https://doi.org/10.1007/s10336-012-0823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10336-012-0823-5