Abstract
Purpose
To evaluate the thermal effect of Ho:YAG laser lithotripsy in a standardized in vitro model via real-time temperature measurement.
Methods
Our model comprised a 20 ml test tube simulating the renal pelvis that was immersed in a 37 °C water bath. Two different laser fibers [FlexiFib (15–45 W), RigiFib 1000 (45–100 W), LISA laser products OHG, Katlenburg-Lindau, Germany] were placed in the test tube. An Ho:YAG 100 W laser was used in all experiments (LISA). Each experiment involved 120 s of continuous laser application, and was repeated five times. Different laser settings (high vs. low frequency, high vs. low energy, and long vs. short pulse duration), irrigation rates (0 up to 100 ml/min, realized by several pumps), and human calcium oxalate stone samples were analyzed. Temperature data were acquired by a real-time data logger with thermocouples (PICO Technology, Cambridgeshire, UK). Real-time measurements were assessed using MatLab®.
Results
Laser application with no irrigation results in a rapid increase in temperature up to ∆28 K, rising to 68 °C at 100 W. Low irrigation rates yield significantly higher temperature outcomes. Higher irrigation rates result immediately in a lower temperature rise. High irrigation rates of 100 ml/min result in a temperature rise of 5 K at the highest laser power setting (100 W).
Conclusions
Ho:YAG laser lithotripsy might be safe provided that there is sufficient irrigation. However, high power and low irrigation resulted in potentially tissue-damaging temperatures. Laser devices should, therefore, always be applied in conjunction with continuous, closely monitored irrigation whenever performing Ho:YAG laser lithotripsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endoscopic stone treatment has now replaced shock wave lithotripsy as the first choice of modalities to treat urolithiasis of the upper urinary tract [1]. Urologists performing intracorporeal lithotripsy can choose between ultrasonic, pneumatic, and laser lithotripsy; current guidelines do not recommend electrohydraulic lithotripsy [2]. However, ultrasonic and pneumatic energy sources are limited to rigid scopes. On the contrary, laser devices can be used in both flexible ureteroscopy (RIRS) and percutaneous nephrolithotomy (PNL). Due to laser lithotripsy’s extended range of application, its energy source is being widely utilized to disintegrate urinary stones intracorporeally [3]. According to the latest EAU guidelines, holmium:yttrium–aluminum–garnet (Ho:YAG) laser is the gold standard in laser lithotripsy [4]. Its fragmentation mechanism relies on the photothermal effect, triggering the rapid vapor expansion of the residual water molecules enclosed in the calculi [5, 6]. However, this physical phenomenon is not exclusively limited to the calculus. In fact, the surrounding region could undergo a significant temperature increase in the collecting system, a phenomenon that could lead to the denaturation of urothelium or even parenchymal damage resulting in irreversible kidney failure of the organ being treated. Evidence on the long-term effects of intracorporeal laser lithotripsy is scarce, and there is a little literature on such potentially hazardous side effects of Ho:YAG lasers [7, 8]. High-energy laser systems (> 30 W) have also been continuously exploited in endourology, especially to treat benign prostate enlargement. Their cross-utilization in urolithiasis therapy is becoming an important issue for stone surgeons around the world, since little is known about thermal impacts. It was this study’s objective, therefore, to determine the extent of Ho:YAG laser-induced temperature rise in relation to various irrigation levels and laser settings in a standardized in vitro model.
Materials and methods
Experimental setup
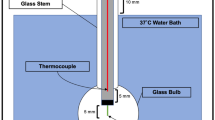
Our experimental setup simulated the renal pelvis with a 20 ml test tube (conventional glass) immersed in a 37 °C water bath that had been heated by an aquarium heater (Thermocontrol 3604, Eheim GmbH & Co. KG, Deizisau, Germany). The role of the water bath was to mimic the surrounding body temperature and thermal convection. Figure 1 illustrates the setup, showing inside the test tube and the irrigation saline used.
To homogenize the water bath, a hose pump (SP 04 L, Otto Huber GmbH, Böttingen, Germany) was used, mimicking heat exchange and blood circulation in the surrounding tissue. Irrigation up to 16.65 ml/min inside the test tube was realized by an infusion pump (Infusomat© fmS, B. Braun Melsungen AG, Melsungen, Germany) and for higher flow rates by the Urology Pump (LUT GmbH, Denzlingen, Germany). Two thermocouples type K, SE002, were placed in the water bath and in the test tube, and temperature data were acquired by a real-time data logger (TC-08 thermocouple data logger and type K thermocouples, PICO Technology, Cambridgeshire, UK). An Ho:YAG laser providing up to 100 W was used with two different laser fibers placed inside the test tube (Sphinx, FlexiFib (for trial runs with 15–45 W, fiber core of 272 µm), RigiFib 1000 (for trial runs with 15–100 W, fiber core of 940 µm), and LISA laser products OHG, Katlenburg-Lindau, Germany). To validate the laser power output before and after each experiment, we used a Fieldmaster power meter (Coherent GmbH, Dieburg, Germany). Data were processed and visualized in real time using MatLab (MatLab® R2016b, The MathWorks, Inc., Natick, US). Each of the following experiments was carried out five times to balance out variations through irrigation and pulse impact. Each experiment involved 120 s of continuous laser application. The standard deviation for each of the five experiments was calculated for all time data points. Figure 2c, d contains this standard deviation as a corridor of ± σ. In Fig. 2a, b, we did not include standard deviation since multiple curves.
Evaluation of different laser settings
Preliminary tests were run to examine all different laser settings:
-
The minimum pulse duration of 150 µs was contrasted to the maximum pulse duration of 800 µs. The power and frequency settings were compared at 15 W (0.5 J/30 Hz) and 100 W (4.5 J/22.3 Hz).
-
The two different laser fibers FlexiFib and RigiFib were compared at 15 W (0.5 J/30 Hz/150 µs) and 45 W (1.5 J/30 Hz/150 µs).
-
Interdependency between energy and frequency at a given power setting was examined at 18 W (1.0 J/18 Hz/400 µs vs. 4.5 J/4 Hz/400 µs) and 54 W (2.0 J/2 Hz/400 µs vs. 4.5 J/12 Hz/400 µs).
-
The difference between a freshly cut and an already used fiber was tested at 18 W (4.5 J/4 Hz/400 µs) and 100 W (4.5 J/22.3 Hz/400 µs).
Impact of human calcium oxalate stone probes
To examine the influence of stone material on the temperature rise, we used 1gr of different stone probes (human calcium oxalate after approval from the local ethics committee, IRB number 79/16) in comparison to pure water at a given power setting (40 W/2.0 J/20 Hz/400 µs).
Evaluation of different irrigation rates and power settings
After the preliminary step, all subsequent test runs were done using a pulse duration of 400 µs and a freshly cut fiber. Irrigation rates were measured in a flexible ureterorenoscope (Cobra Vision®, Richard Wolf, Knittlingen, Germany). Based on these data, we tested irrigation rates between 0 and 100 ml/min according to clinical practice (active and passive irrigation). The irrigation range was swept through (0, 5, 10, 16.65, 30, 40, 50, 75, and 100 ml/min) at a power setting of 15 W (0.5 J/30 Hz/400 µs) and 60 W (2.0 J/30 Hz/400 µs). For a power setting of 100 W (4.5 J/22.3 Hz/400 µs), the irrigation rate was limited to 16.65, 50, and 100 ml/min.
Evaluation of different irrigation rates and power settings in calcium oxalate stones
Final pretests were run with human calcium oxalate stones, different irrigation rates (0, 10, 30, 50, and 100 ml/min), and different power settings (15 W/0.5 J/30 Hz/400 µs and 60 W/2.0 J/30 Hz/400 µs).
Results
The impact of different pulse durations was examined in the first part of the experiments. Shorter laser pulses with a length of 150 µs yielded a significantly greater temperature increase than a pulse duration of 800 µs. At the 15 W laser setting (measured 12.1 W for 150 µs and 12.8 W for 800 µs pulse duration), the temperature increase was 9.5% higher with a short pulse; at a 100 W laser setting (outside the measuring range), the temperature rise was 6.5% higher. Furthermore, shorter pulse durations result in greater turbulence as reflected in curve fluctuations—see Fig. 2a.
When comparing the two laser fibers FlexiFib and RigiFib with diameters of 272 and 940 µm, use of the smaller fiber resulted in a higher temperature increase of 8.8% at a setting of 15 W (measured 12.6 W for 272 µm and 13.1 W for 940 µm fiber diameter) and 10.3% at a setting of 45 W (measured 35.8 W for 272 µm and 39.4 W for 940 µm fiber diameter). Smaller fibers are also associated with higher turbulences, see Fig. 2a.
The next set of experiments contrasted a setting with high single-pulse energy at a low frequency against a setting with low single-pulse energy at a high frequency. At 18 W power (measured 14.8 W for low pulse energy and 14.0 W for high pulse energy), we observed an insignificant difference of 3.5% of higher temperatures in the high-frequency setting. When tested at a higher power setting of 54 W (measured 46.6 W for low pulse energy and 43.7 W for high pulse energy), the higher frequency setting yielded 8.8% higher end temperatures, as shown in Fig. 2b.
In a comparison between a freshly cut laser fiber and a degraded laser fiber, there was no substantial temperature difference at a 15 W power setting (measured 10.8 W for degraded fiber and 13.5 W for freshly cut fiber); see also Fig. 2b. At the 60 W power setting (measured 43.3 W for degraded fiber and 52.0 W for freshly cut fiber), we noted a 10.5% higher temperature difference in conjunction with the freshly cut laser fibers. The differences in capacity which the power meter measured at the two laser settings amounted to 25% (15 W) and 20% (60 W), respectively, but those differences had no effect on the rise in temperature. The freshly cut fibers curves are very smooth, suggesting less turbulence (Fig. 2b).
When adding irrigation, the temperatures dropped at all power settings (all different collected data were combined), as shown in Fig. 2c. At 60 and 100 W settings, even irrigation of 100 ml/min is insufficient and resulted in temperature rises of 5.3 and 8.6 K. Irrigation of 10 ml/min sufficed at the 15 W power setting and resulted in a 4.3 K temperature difference.
Laser application without irrigation results in a quick increase in temperature of up to ∆28 K, rising to 68 °C at the most powerful laser setting (100 W, Fig. 2c). Low irrigation rates yield significant higher temperature outcomes (Fig. 2c). High irrigation rates of 100 ml/min result in a temperature rise of 5 K at the highest laser power setting (100 W). Increased irrigation rates result immediately in a lower temperature rise. Higher power settings at 60 W result in a quicker temperature rise reached after approximately 1 min of laser application.
During experiments with renal calculi consisting of calcium oxalate, the temperature rise was quicker, more turbulent, and 12.9% higher than that reached in the blank test; see Fig. 2d.
Discussion
Ho:YAG laser lithotripsy is widely used in current stone treatment. However, the thermal side effects induced by laser beam radiation still seem to play a minor role among practising urologists, as they are not mentioned in the current guidelines [2]. On the other hand, laser energy does not limit itself to the calculus and the surrounding region—it affects the physical conditions in the entire collecting system on the treated side, as well. This side effect can denaturize urothelial proteins and cause several severe renal parenchymal damage. In the present study, we analyzed the thermal effect of Ho:YAG laser lithotripsy in a standardized in vitro model.
In the preliminary step, we evaluated the impact of different laser settings and implications (pulse duration’s interdependence between energy and the frequency/fiber diameter/freshly cut vs. used fiber) on the temperature increase. Collectively, all the aforementioned factors induced divergent temperature rises around < 10%. To the best of our knowledge, this is the first study to have analyzed the impact of different laser settings like pulse duration on the temperature increase in a standardized in vitro model. However, in daily clinical practice, these analyzed settings and features may have no significant impact, as surgeons can choose whichever they prefer. After finishing the preliminary part of this work, we decided to standardize the subsequent experiments applying a pulse duration of 400 µs and a freshly cut fiber for each trial run.
Our study is the first in urologic research to control the real laser power output using a power meter. It might be surprising that the genuine laser output is significantly lower than that stated on the laser. However, this is a phenomenon that can vary by < 10% between the real and stated laser power, and it might compromise the temperature increase in our study design and clinical practice, because the genuine laser powers were lower than those displayed on the laser. This could enhance intraoperative safety marginally. On the other hand, our findings highlight the need for scientific standards concerning the actual laser capacity in effect (at least in those investigations addressing the effect of different Ho:YAG lasers’ capacities)—and that such capacities should be verified with a power meter to enable the comparability of such studies.
Focus of this investigation was to analyze the impact of different irrigation rates and power settings on the temperature increase. Herein, we analyzed laser powers of 15, 60, and 100 W in relation to irrigation rates ranging from 0 to 100 ml/min. In general, our results show that a steady temperature state is reached following an initial increase (Fig. 2). Without any irrigation, a temperature increase of nearly 8 K (15 W), 20 K (60 W), and 30 K (100 W) was reached. There is evidence that a temperature of only ~ 43 °C is associated with the onset of exponential tissue damage [9, 10]. Exponential tissue damage correlates closely to the duration of exposure [11]. At a starting temperature of 37 °C, even 15 W of power leading to 45 °C can significantly affect the urothelium in the collecting system when there is no irrigation. However, different tissues tolerate different temperature thresholds, calculated to equal the cumulative equivalent at 43 °C (CEM43), at which thermal damage occurs [12]. Regarding the CEM43 values: canine urethras revealed a CEM43 value of ~ 1, the lowest in the urinary tract [11]. We can, therefore, consider 43 °C as the critical threshold at which the initial thermal damage occurs. However, our findings demonstrate that an irrigation rate of 10 ml/min reduced the temperature rise to ~ 4 K (Fig. 2) at 15 W of power, which should cause no harm to the urothelium independent of exposure time. This highlights the importance of sufficient irrigation even in conjunction with weaker laser power of 15 W.
Kallidonis et al. analyzed the thermal effects of thulium:yttrium–aluminum–garnet and Ho:YAG lasers in 2015 [13]. They used a test volume of 40 ml; irrigation rates in their Ho:YAG experiments were 2–25 ml/min, and they applied powers of up to 20 W. However, no water bath simulating body temperature (and thermal convection in particular) was used. Furthermore, they employed no power meter to control the laser power. Kallidonis et al. report a comparable temperature rise at an Ho:YAG laser power of 10 W combined with an irrigation rate of 10 ml/min of 7.9 K compared to a difference of 5.9 K at 15 W with 10 ml/min in the present investigation. This difference might be due to the thermal convection simulated by the water bath in our experiments. On the other hand, our different volumes (20 vs. 40 ml) might have a crucial impact on the temperature rise. Several working groups reported a tremendous temperature rise in different in vitro ureter models in which the volumes were much smaller than in our project [7, 8, 14]. Notably, Wollin et al. showed in their ureter model that temperatures of 100° C were reached after 45 s using only 20 W without irrigation [14].
However, all those studies emphasized the importance of sufficient irrigation during ureteral laser lithotripsy, which was not the focus of the present investigation. In the current study, we focused on intrarenal lithotripsy, which is widely administered and recommended in RIRS [1, 2] but also used frequently in PNL; moreover, we are concentrating on the effects of those high-energy lasers applied in BPS-related procedures but that are also increasingly used in urolithiasis treatment.
In PNL in which larger fiber cores permit a laser power > 30 W, it might be advantageous to administer high power Ho:YAG laser lithotripsy in patients presenting a high stone burden to increase the calculus’ ablation volume [3]. Because of this specific constellation, we analyzed the thermal effect of high power (60) and 100 W in correlation to irrigation rates between 0 and 100 ml/min. Compared to the previous studies, all of which were limited to a laser power ≤ 20 W, the present study is the first to demonstrate the importance of sufficient irrigation in a standardized in vitro model at laser powers of 60 and 100 W [7, 8, 13,14,15,16].
The study by Buttice et al. published 2016 in the Journal of Endourology showed similar results in a similar study design analyzing Ho:YAG laser lithotripsy up to 20 W in an in vitro model [15]. However, direct comparison is hampered due to their differences in study design (e.g., test volume of 10 ml or limited temperature measurements at 45 °C).
Sufficient irrigation is crucial to limiting the temperature increase, as shown in Fig. 2D, in which 10 ml/min seems to be the turnover point for safe temperatures. This confirms the results of earlier studies, e.g., the aforementioned work by Aldoukhi et al., as they also evaluated the temperature decrease [17]. However, Ng et al. showed that the irrigation rate depends on the diameter of the ureteral access sheath, the diameter of the scope’s working channel(s), and baskets/laser fibers inserted [18]. Therefore, it is difficult to offer general advice at which specific constellation irrigation rates < 10 ml/min can be reached.
We forcefully recommend monitored irrigation, as stated in our conclusions. Irrigation can be monitored easily using a syringe between the irrigation bag and the scope and an assistant who actively irrigates using the syringe. If a 10 ml syringe is used, this should be applied at least once a minute to attain a 10 ml/min irrigation rate.
A crucial point regarding the temperature rise is its development over time. In the present study design, we applied laser energy over 120 s to evaluate the theoretically reachable temperature increase; however, one must consider that continuous laser energy over 120 s may not reflect daily clinical practice. In all test runs, we observed a quick temperature increase (approximately during 0–60 s, see Figs. 2a–c) which is followed by a sort of plateau. This has been confirmed in the previous studies, e.g., Aldoukhi et al., which demonstrated a quick temperature increase in a similar study design [17]. They described the temperature decrease with several irrigation rates. In our study, the starting temperature could be reached after approximately 20 s. Therefore, it is extremely important to apply intermittent laser energy in contrast to continuous laser energy as exemplified in the present study design.
Surprisingly, we measured a positive thermal effect in the test runs using human calcium oxalate stone probes (Fig. 2c), though the reasons for that are unclear. Perhaps, the laser energy initiated an exothermal reaction requiring 100–200 °C activation energy at the fiber tip [19]. However, further experimentation is necessary to reveal other effects.
The present experimental setup is limited by several factors. The test tube volume of 20 ml might be excessive compared to the human collecting system. However, we decided to use such a high volume to ensure enough space for the laser fiber, in- and outflow, and thermocouple installation into the test tube. We, therefore, plan to conduct a study using postmortem porcine kidneys immersed in the water bath. In these, we will insert one thermocouple directly into the collecting system and several pairs thereof into the parenchyma. Although we did our experiments in a water bath to simulate the surrounding body and thermal convection, this effect cannot be simulated realistically in the experimental setup which we used. We postulate that thermal convection and blood circulation play major roles in daily clinical practice, and that they prevent thermal tissue damage.
Although the present laboratory study is limited by the aforementioned aspects, we would in conclusion like to mention important issues regarding the clinical use of Holmium laser for intrarenal lithotripsy:
-
Pulse duration, fiber condition, and their interdependence might not have a significant impact on the temperature rise provided that the laser system is set at the same power.
-
Sufficient and monitored irrigation (at least ≥ 10 ml/min) with 37 °C irrigation fluid minimizes the risk of thermal damage to the urinary tract.
-
Intermittent (not continuous) laser energy should be applied.
-
The most important factor besides irrigation is the laser power itself. Surgeons should, therefore, start with low power and increase power slowly under sufficient and well-monitored irrigation until enough lithotripsy effect is reached.
Conclusions
In the present laboratory study, we show that Ho:YAG laser lithotripsy might be safe at all the aforementioned power settings provided that there is sufficient irrigation > 10 ml/min. However, this study has limitations in simulating genuine laser lithotripsy conditions in a patient. High power (> 15 W) and simultaneous low irrigation (< 10 ml/min) may result in potentially tissue-damaging temperatures after a short time (< 30 s.). Therefore, endourologists employing laser to treat kidney stones should be trained in their use, and particular emphasis should be placed on the need for continuous and monitored irrigation during surgery.
Abbreviations
- CEM43 :
-
Cumulative equivalent at 43 °C
- PNL:
-
Percutaneous nephrolithotomy
- RIRS:
-
Retrograde intrarenal surgery
References
Oberlin DT, Flum AS, Bachrach L, Matulewicz RS, Flury SC (2015) Contemporary surgical trends in the management of upper tract calculi. J Urol 193(3):880–884. https://doi.org/10.1016/j.juro.2014.09.006
Turk C, Petrik A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T (2016) EAU guidelines on interventional treatment for urolithiasis. Eur Urol 69(3):475–482. https://doi.org/10.1016/j.eururo.2015.07.041
Kronenberg P, Traxer O (2014) Update on lasers in urology 2014: current assessment on holmium:yttrium-aluminum-garnet (Ho:YAG) laser lithotripter settings and laser fibers. World J Urol. https://doi.org/10.1007/s00345-014-1395-1
Turk C, Knoll T, Petrik A, Sarica K, Skolarikos A, Straub M, Seitz C (2015) EAU guidelines on urolithiasis 2015
Vassar GJ, Chan KF, Teichman JM, Glickman RD, Weintraub ST, Pfefer TJ, Welch AJ (1999) Holmium: YAG lithotripsy: photothermal mechanism. J Endourol Endourol Soc 13(3):181–190. https://doi.org/10.1089/end.1999.13.181
Chan KF, Vassar GJ, Pfefer TJ, Teichman JM, Glickman RD, Weintraub ST, Welch AJ (1999) Holmium:YAG laser lithotripsy: a dominant photothermal ablative mechanism with chemical decomposition of urinary calculi. Lasers Surg Med 25(1):22–37
Molina WR, Silva IN, Donalisio da Silva R, Gustafson D, Sehrt D, Kim FJ (2015) Influence of saline on temperature profile of laser lithotripsy activation. J Endourol Endourol Soc 29(2):235–239. https://doi.org/10.1089/end.2014.0305
Cordes J, Nguyen F, Sievert K-D (2015) First intraluminal temperature measurement during Ho:YAG-laser exposure at an in vitro URS. Open J Urol 05(01):1–5. https://doi.org/10.4236/oju.2015.51001
Thomsen S, Pearce JA (2011) Thermal damage and rate processes in biologic tissues. In: Welch AJ, van Gemert MJC (eds) Optical-thermal response of laser-irradiated tissue. Springer, Dordrecht, pp 487–549. https://doi.org/10.1007/978-90-481-8831-4_13
Bauer KD, Henle KJ (1979) Arrhenius analysis of heat survival curves from normal and thermotolerant CHO cells. Radiat Res 78(2):251–263. https://doi.org/10.2307/3575042
van Rhoon GC, Samaras T, Yarmolenko PS, Dewhirst MW, Neufeld E, Kuster N (2013) CEM43°C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol 23(8):2215–2227. https://doi.org/10.1007/s00330-013-2825-y
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10(6):787–800
Kallidonis P, Amanatides L, Panagopoulos V, Kyriazis I, Vrettos T, Fligou F, Kamal W, Liatsikos EN (2015) Does the heat generation by the Thulium:Yttrium aluminum garnet laser in the irrigation fluid allow its use on the upper urinary tract? An experimental study. J Endourol 30(4):422–427. https://doi.org/10.1089/end.2015.0252
Wollin DA, Carlos EC, Tom WR, Simmons WN, Preminger GM, Lipkin ME (2017) Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J Endourol. https://doi.org/10.1089/end.2017.0658
Buttice S, Sener TE, Proietti S, Dragos L, Tefik T, Doizi S, Traxer O (2016) Temperature changes inside the kidney: what happens during holmium:yttrium-aluminium-garnet laser usage? J Endourol Endourol Soc 30(5):574–579. https://doi.org/10.1089/end.2015.0747
Kallidonis P, Kamal W, Panagopoulos V, Vasilas M, Amanatides L, Kyriazis I, Vrettos T, Fligou F, Liatsikos E (2016) Thulium laser in the upper urinary tract: does the heat generation in the irrigation fluid pose a risk? Evidence from an in vivo experimental study. J Endourol 30(5):555–559. https://doi.org/10.1089/end.2015.0768
Aldoukhi AH, Ghani KR, Hall TL, Roberts WW (2017) Thermal response to high-power holmium laser lithotripsy. J Endourol Endourol Soc 31(12):1308–1312. https://doi.org/10.1089/end.2017.0679
Ng YH, Somani BK, Dennison A, Kata SG, Nabi G, Brown S (2010) Irrigant flow and intrarenal pressure during flexible ureteroscopy: the effect of different access sheaths, working channel instruments, and hydrostatic pressure. J Endourol 24(12):1915–1920. https://doi.org/10.1089/end.2010.0188
Nair CGR, Ninan KN (1978) Thermal decomposition studies: Part X. Thermal decomposition kinetics of calcium oxalate monohydrate—correlations with heating rate and samples mass. Thermochim Acta 23(1):161–169. https://doi.org/10.1016/0040-6031(78)85122-3
Acknowledgements
We would like to thank Michaela von Aichberger for illustration of Fig. 1.
Funding
Institutional funding, Faculty of Medicine, University of Freiburg, Germany.
Author information
Authors and Affiliations
Contributions
SH: protocol/project development, data collection and management, data analysis, and manuscript writing/editing. RP: data collection and management, data analysis, manuscript and figure writing/editing, and performed experiments. MS: manuscript writing/editing and supervision. UW: manuscript writing/editing and supervision. AM: protocol/project development, manuscript writing/editing, and supervision.
Corresponding author
Ethics declarations
Conflict of interest
This work was supported by material support of LISA Laser products (Katlenburg-Lindau, Germany). Ulrich Wetterauer advisory board, DR. KADE Pharmazeutische Fabrik GmbH, Berlin, Germany (unrelated to the presented work). Martin Schoenthaler consultant contract with and NeoTract Inc., Pleasanton, USA (unrelated to the present work). Arkadiusz Miernik consultant contract with KLS Martin GmbH, Tuttlingen, Germany (unrelated to the present work).
Ethical approval
IRB approved protocol number: 79/16 leading ethics committee: Ethik-Kommission der Albert-Ludwigs-Universität Freiburg, Germany.
Research involved in human and animals rights
There are no human participants or animals involved into the study. Human stone probes were analyzed after ethical approval (79/16 leading ethics committee: Ethik-Kommission der Albert-Ludwigs-Universität Freiburg, Germany) and written informed consent.
Additional information
Simon Hein and Ralf Petzold have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hein, S., Petzold, R., Schoenthaler, M. et al. Thermal effects of Ho: YAG laser lithotripsy: real-time evaluation in an in vitro model. World J Urol 36, 1469–1475 (2018). https://doi.org/10.1007/s00345-018-2303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2303-x