Abstract
Purpose
High-power laser lithotripsy can elevate temperature within the urinary collecting system and increase risk of thermal injury. Temperature elevation is dependent on power settings and operator duty cycle (ODC)—the percentage of time the laser pedal is depressed. The objective of this study was to quantify temperature and thermal dose resulting from laser activation at different ODC in an in-vitro model.
Methods
Holmium laser energy (1800 J) was delivered at 30 W (0.5 J × 60 Hz) to a fluid filled glass bulb. Room temperature irrigation was applied at 8 ml/min. ODC was evaluated in 10% increments from 50–100%. Bulb fluid temperature was recorded and thermal dose calculated. Time to reach threshold of thermal injury and maximal allowable energy were also determined at each ODC.
Results
Upon laser activation, there was an immediate rise in fluid temperature with a “saw-tooth” oscillation superimposed on the curves for 50–90% ODC corresponding to periodic activation of the laser. Higher ODC resulted in greater maximum temperature and thermal dose, with ODC ≥ 70% exceeding threshold. Use of 50% compared to 60% ODC resulted in a tenfold increase in time required to reach threshold of thermal injury and an eightfold increase in maximal allowable energy.
Conclusions
Laser activation at higher ODC produced greater fluid temperature and thermal dose. Time to threshold of thermal injury and maximal allowable energy were dramatically higher for 50% compared to 60% ODC at high-power settings. Proper management of laser ODC can enhance patient safety and optimize stone treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With advances in laser technology, the range of lithotripsy techniques has expanded beyond fragmentation. Dusting and popcorn strategies that rely on higher laser pulse frequency and power are now commonly employed for treatment of urinary stones [1]. However, use of high-power settings can produce excessive temperature elevation of fluid within the ureter or collecting system and increase risk of thermal injury to adjacent tissue as seen in prior in-vitro and in-vivo research studies [2,3,4,5,6,7,8,9,10,11]. Recently, several clinical cases of thermal injury of the ureter have been reported to regulatory agencies prompting revised recommendations for 8 W default settings and not to exceed 20 W when using the SOLTIVE laser in the ureter [12].
In addition to laser power, operator duty cycle (ODC) is an important factor when considering thermal safety. ODC is the percentage of time the pedal is depressed. For example, a pattern of 7 s on, followed by 3 s off constitutes a 70% ODC. While it is generally understood that greater ODC produces higher temperature and thermal dose [13], this has not been quantitatively characterized. A proper understanding of this relationship is necessary for the urologist to efficiently perform laser lithotripsy (LL) without inducing thermal injury. The objective of this study was to measure temperature and calculate thermal dose resulting from laser activation at different ODC in an in-vitro model containing a volume of fluid representative of that found in a segment of ureter or within a renal pelvis during ureteroscopy.
Prior work has characterized the temperature profile and thermal dose that result from delivery of 1200 J of laser energy at 50% ODC with a range of pedal activation times from 5 to 30 s [13]. Building on that knowledge, this study examines temperature response from laser activation at ODC from 50–100%. This work is directly relevant to conventional laser treatment strategies; a series of clinical cases revealed that the mean ODC employed during the treatment phase of LL was 63% with a range of 12–100% [13]. Quantitatively measuring fluid temperature from a range of ODC will provide insight to urologists on appropriate LL activation patterns. This knowledge, in conjunction with selection of appropriate power setting and irrigation flow rate, can be used to optimize efficiency of LL while ensuring thermal safety in the ureter and other locations within the urinary tract.
Materials and methods
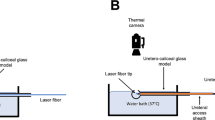
A model consisting of a glass bulb (volume 2.8 ml) and stem (5.7 mm inner diameter, 55.0 mm length) was partially submerged (10 mm of stem above the water surface) in a 17 L water bath maintained at 37.0˚C ± 0.2 °C. A wire thermocouple (Omega, CT) was affixed 5 mm proximal to the tip of a flexible ureteroscope (LithoVue, Boston Scientific). The ureteroscope was positioned in the model and a 200 µm laser fiber (Flexiva, Boston Scientific) inserted through the working channel with fiber tip 5 mm beyond the ureteroscope centered in the fluid filled glass bulb, (Fig. 1).
In-vitro model consisted of a glass bulb (volume 2.8 ml). The top 10 mm of the stem extended above the surface of the water bath. A wire thermocouple was affixed 5 mm proximal to the flexible ureteroscope tip and a laser fiber was secured 5 mm distal. The laser fiber tip was centered in the glass bulb
Irrigation with room temperature (20 ± 1 °C) deionized water was delivered through the ureteroscope working channel from a peristaltic pump (Masterflex; Cole Parmer, IL) at 8 ml/min. Once fluid temperature in the glass bulb reached steady state, the holmium:YAG laser (Pulse120; Lumenis) was activated at 30 W (0.5 J × 60 Hz, short pulse) to deliver 1800 J with 50, 60, 70, 80, 90, or 100% ODC, (Table 1). Four trials at each ODC were performed.
Thermal dose was calculated using the Dewey and Sapareto methodology. The threshold of thermal injury is defined as 43 °C for 120 min or shorter times at higher temperatures [14, 15]. The time to reach threshold of thermal injury was determined from each thermal dose curve. For lower ODC where thermal dose did not reach the threshold of thermal injury, longer trials were performed with extrapolation of the thermal contribution from the last three cycles of laser activation as needed. The maximal energy that could be applied before reaching threshold of thermal injury was also calculated (time to threshold × power setting × ODC).
A second complete set of data was generated by repeating each of the studies described above for delivery of 2400 J of energy at 40 W (0.5 J × 80 Hz, short pulse) and 12 ml/min irrigation rate. These supplementary data and additional notes are presented in the Online Resource.
Mean and standard deviation values were calculated using Microsoft Excel. Statistical significance was determined with two-tailed Student’s t test with probability for type I error set at 0.05 and Bonferroni correction applied for multiple comparisons.
Results
Upon laser activation, an immediate rise in fluid temperature was noted followed by flattening towards a plateau and then decay after deactivation of the laser. A “saw-tooth” oscillation in temperature was superimposed on the curves for 50–90% ODC corresponding to the periodic activation of the laser, (Fig. 2). Maximum temperature for 30 W settings was 58.6, 56.6, 55.2, and 53.0 °C for ODC of 100, 90, 80, and 70%, respectively. Higher ODC yielded greater thermal dose (p < 0.002) which exceeded the threshold of thermal injury for ODC of 70–100%, (Fig. 3, Table 2).
The time to reach the threshold of thermal injury increased as ODC decreased, (Fig. 3). Notably, with a 50% ODC the time to reach threshold of injury with extended trials was tenfold greater than with 60% ODC (948 vs. 94 s). Similarly, the energy that could safely be applied before reaching threshold of thermal injury increased as ODC decreased. At 30 W settings the threshold of injury was reached after delivery of only 683 J at 100% ODC compared to 1180 J at 70% ODC and 14,200 J at 50% ODC, (Table 2).
Discussion
Safe use of high-power laser settings during endoscopic LL requires proper understanding of temperature elevation resulting from a range of laser ODC. Decreasing ODC limits the rise in fluid temperature and lessens thermal dose. This allows the laser to be activated for a longer period of time and deliver greater total energy before the threshold of thermal injury is reached. Proper management of laser ODC can enhance patient safety and optimize stone treatment.
Thermal injury to biological tissues is dependent upon both temperature elevation and exposure time. Dewey and Sapareto developed the metric of thermal dose, reported in “equivalent minutes at 43 °C”, to standardize cumulative temperature exposure for varying temperature curves [14]. The threshold of thermal injury (commonly defined as 120 equivalent minutes) is reached when tissue is exposed to 43 °C for 120 min [14, 15]. Exposure to higher temperatures for shorter durations can also reach threshold. For example, laser activation at 30 W settings with 80% ODC produced a thermal dose of 1270 equivalent minutes, which exceeds the threshold of thermal injury.
While thermal dose provides a biologically relevant measure of cumulative temperature exposure, two other metrics are more intuitive. The time to threshold of thermal injury indicates how long laser energy can be applied before thermal injury will occur. Related, is the concept of maximal allowable energy which indicates how much energy can be applied before producing thermal tissue injury. As seen in this study, laser activation at 30 W and ODC of 50% allows 14,200 J of energy to be delivered before reaching threshold of injury. This is compared to only 1180 J at 70% ODC. In clinical cases with large stone burden a lower ODC may allow completion of stone treatment before threshold of injury is reached.
This study has several limitations that warrant further comment. First, the in-vitro model provides for precise measurement and control of parameters but does not fully replicate the clinical environment. However, previous research has demonstrated a close approximation of thermal effects produced from this in-vitro model and in-vivo porcine studies [3, 9]. Second, the model in this study represents a scenario with optimal drainage and minimal outflow resistance, similar to what would be seen with a 15 Fr inner diameter ureteral access sheath with tip positioned at the location of stone treatment. The effects of ODC and extent of temperature elevation are likely to be more pronounced in clinical scenarios where drainage is not as optimal. Third, energy delivery of 1800 or 2400 J, as applied in this study, is less than what is typically used in many clinical laser cases. The intent was not to replicate an entire LL case, but to provide a standardized method to compare thermal profiles from a range of ODC. These data can be used as building blocks to predict thermal dose in longer, more clinically relevant patterns of laser activation. Lastly, the entire parameter space was not sampled; 30 and 40 W power settings were selected as these are commonly used clinically.
This study quantifies the temperature elevation resulting from laser activation with increasing ODC at two power settings (30 and 40 W). These results enhance intuition regarding ODC, particularly the multiplicative benefits of using 50% compared to 60% ODC for cases requiring greater lasing time and employing 30–40 W laser power. Additionally, this work underscores the importance of ODC as a primary variable for thermal management. In conjunction with previous work on the thermal effects of five other clinically relevant variables: laser power [3], volume of fluid in which the laser is activated [16], irrigation rate [3, 4, 6,7,8, 17], temperature of irrigation fluid [18, 19], and length of pedal activation [13], these findings provide a comprehensive framework for understanding laser thermal effects. Taken together, this body of work can provide insight into the design of next generation ureteroscopy systems with better control of temperature and thermal dose within the collecting system.
Future work will further explore the effect of ODC on temperature across a broader range of laser settings and parameters. While the general trends seen in this study are expected to hold, quantifying the magnitude of the effects is important. Additional porcine studies are planned to validate the thermal response to laser ODC in the in-vivo setting.
Conclusion
Laser activation at higher ODC produced greater fluid temperature and thermal dose. The time to threshold of thermal injury and maximal allowable energy were dramatically higher for 50% compared to 60% ODC at 30 and 40 W power settings. In conjunction with recent research on laser power settings, pedal activation times, fluid volume, irrigation rate and temperature, these findings lay the foundation for a set of strategies to positively impact patient safety and efficiency, and guide future development of laser technologies. Until real-time measurement of fluid temperature in the collecting system is available, urologists should be mindful of ODC effects with high-power laser settings.
References
Dauw CA, Simeon L, Alruwaily AF, Sanguedolce F, Hollingsworth JM, Roberts WW, Faerber G, Wolf JS, Ghani KR (2015) Contemporary practice patterns of flexible ureteroscopy for treating renal stones: results of a worldwide survey. J Endourol 29:1221–1230. https://doi.org/10.1089/end.2015.0260
Molina WR, Silva IN, Donalisio Da Silva R, Gustafson D, Sehrt D, Kim FJ (2015) Influence of saline on temperature profile of laser lithotripsy activation. J Endourol 29(2):235–239. https://doi.org/10.1089/end.2014.0305
Aldoukhi AH, Ghani KR, Hall TL, Roberts WW (2017) Thermal response to high-power holmium laser lithotripsy. J Endourol 31(12):1308–1312. https://doi.org/10.1089/end.2017.0679
Wollin DA, Carlos EC, Tom WR, Simmons WN, Preminger GM, Lipkin ME (2018) Effect of laser settings and irrigation rates on ureteral temperature during holmium laser lithotripsy, an in vitro model. J Endourol 32(1):59–63. https://doi.org/10.1089/end.2017.0658
Sourial MW, Ebel J, Francois N, Box GN, Knudsen BE (2018) Holmium-YAG laser: Impact of pulse energy and frequency on local fluid temperature in an in-vitro obstructed kidney calyx model. J Biomed Opt 23(10):1. https://doi.org/10.1117/1.jbo.23.10.105002
Hein S, Petzold R, Schoenthaler M, Wetterauer U, Miernik A (2018) Thermal effects of Ho:YAG laser lithotripsy: real-time evaluation in an in vitro model. World J Urol 36(9):1469–1475. https://doi.org/10.1007/s00345-018-2303-x
Winship B, Wollin D, Carlos E, Peters C, Li J, Terry R, Boydston K, Preminger GM, Lipkin M (2019) The rise and fall of high temperatures during Ureteroscopic Holmium laser lithotripsy. J Endourol 33(10):794–799. https://doi.org/10.1089/end.2019.0084
Hein S, Petzold R, Suarez-Ibarrola R, Müller PF, Schoenthaler M, Miernik A (2020) Thermal effects of Ho:YAG laser lithotripsy during retrograde intrarenal surgery and percutaneous nephrolithotomy in an ex vivo porcine kidney model. World J Urol 38(3):753–760. https://doi.org/10.1007/s00345-019-02808-5
Aldoukhi AH, Hall TL, Ghani KR, Maxwell AD, MacConaghy B, Roberts WW (2018) Caliceal fluid temperature during high-power Holmium laser lithotripsy in an in vivo Porcine model. J Endourol 32(8):724–729. https://doi.org/10.1089/end.2018.0395
Maxwell AD, MacConaghy B, Harper JD, Aldoukhi AH, Hall TL, Roberts WW (2019) Simulation of laser lithotripsy-induced heating in the urinary tract. J Endourol 33(2):113–119. https://doi.org/10.1089/end.2018.0395
William JG, Goldsmith L, Moulton DE, Waters SL, Turney BW (2021) A temperature model for laser lithotripsy. World J Urol 39:1707–1716. https://doi.org/10.1007/s00345-020-03357-y
Class 2 Device Recall Olympus. U.S. Food and Drug Administration (FDA). Initiated June 30, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?id=188172. Accessed Aug 24, 2021.
Aldoukhi AH, Dau JJ, Majdalany SE, Hall TL, Ghani KR, Hollingsworth JM, Ambani SN, Dauw CA, Roberts WW (2021) Patterns of laser activation during ureteroscopic lithotripsy: effects on caliceal fluid temperature and thermal dose. J Endourol 35(8):1217–1222. https://doi.org/10.1089/end.2020.1067
Sapareto SA, Dewey WC (1984) Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys 10(6):787–800. https://doi.org/10.1016/0360-3016(84)90379-1
Yarmolenko PS, Moon EJ, Landon C, Manzoor A, Hochman DW, Viglianti BL, Dewhirst, (2011) Thresholds for thermal damage to normal tissues: an update. Int J Hyperth 27(4):320–343. https://doi.org/10.3109/02656736.2010.534527
Khajeh NR, Hall TL, Ghani KR, Roberts WW (2021) Pelvicalyceal volume and fluid temperature elevation during laser lithotripsy. J Endourol. https://doi.org/10.1089/end.2021.0383
Aldoukhi AH, Black K, Hall TL, Ghani KR, Maxwell AD, MacConaghy B, Roberts WW (2020) Defining thermal safe laser lithotripsy power and irrigation parameters: in vitro model. J Endourol 34(1):76–81. https://doi.org/10.1089/end.2019.0499
Dau JJ, Hall TL, Maxwell AD, Ghani KR, Roberts WW (2021) Effect of chilled irrigation on Caliceal fluid temperature and time to thermal injury threshold during laser lithotripsy in vitro model. J Endourol 35(5):700–705. https://doi.org/10.1089/end.2020.0896
Dau JJ, Khajeh NR, Hall TL, Roberts WW (2021) Chilled irrigation for control of temperature elevation during ureteroscopic laser lithotripsy: in vivo porcine model. J Endourol. https://doi.org/10.1089/end.2021.0537
Funding
Funding provided through a research grant from Boston Scientific Corporation.
Author information
Authors and Affiliations
Contributions
MM Louters: data collection and analysis, manuscript writing. WW Roberts: project development and management, data analysis, manuscript editing KR Ghani: manuscript editing. JJ Dau: manuscript editing. TL Hall: manuscript editing.
Corresponding author
Ethics declarations
Conflict of interest
WW Roberts has a consulting relationship with Boston Scientific. KR Ghani has consulting relationships with Boston Scientific, Lumenis, Olympus, Coloplast, and Karl Storz. MM Louters, JJ Dau, and TL Hall have no relevant financial or non-financial interests to disclose.
Ethical approval
This was an in-vitro study that did not require ethics approval.
Informed consent
This was an in-vitro study that did not include human participation. Informed consent was not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Louters, M.M., Dau, J.J., Hall, T.L. et al. Laser operator duty cycle effect on temperature and thermal dose: in-vitro study. World J Urol 40, 1575–1580 (2022). https://doi.org/10.1007/s00345-022-03967-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-022-03967-8