Abstract
Introduction
Laser-assisted partial nephrectomy (PN) can benefit from the excellent coagulative properties of lasers to provide a bloodless tumor excision without the necessity for renal artery clamping. In this review, we aim to determine the current clinical implementation of laser assistance during laparoscopic nephron-sparing surgery.
Materials and methods
An extensive literature evaluation on laser-assisted PN was performed. Experimental work on animals and review articles were excluded.
Results
Current literature regarding laser-assisted PN is scarce. Available data consist mostly of small cohorts providing low level of evidence. Even though initial studies with currently available laser modalities demonstrated promising results, several drawbacks in each technique need to be addressed before being widely accepted as a standard care.
Conclusions
Experience with laser-assisted laparoscopic PN is steadily increasing and uniformly documenting favorable results. As urologist became more familiar with laser technology by its implementation in other clinical entities and with the increasing interest in nephron-sparing management of renal tumors, the use of laser assistance during PN should be expected to play a major role in future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic partial nephrectomy (PN) represents a challenging operation. Renal artery clamping is typically necessary to diminish hemorrhage during tumor resection with the limitation of a certain time constrain to avoid permanent functional impairment on the remaining renal tissue. Laparoscopic PN has been previously demonstrated to be associated with longer warm-ischemia times when compared to open or robotic-assisted laparoscopic PN [1, 2]. What’s more, during the limited ischemia time, besides a precise incision, the surgeon confronts with the need for a technically demanding watertight suturing that should seal the pelvicalyceal system, if opened, and ensure hemostasis before the restoration of blood flow. In addition, a potential risk for severe acute bleeding is present throughout the operation.
In an attempt to avoid the constraint of ischemia time and further improve the quality of nephron-sparing surgery, numerous techniques have been developed to avoid renal vessel occlusion [3]. Selective clamping, controlled hypotension and tumor enucleation are the most commonly applied zero ischemia approaches [4–6].
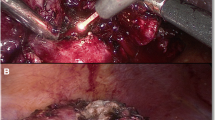
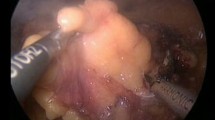
In the same context, laser-assisted PN uses the excellent coagulative properties of lasers to provide a bloodless tumor excision without the need for renal artery clamping. The concept of laser-assisted PN is old with first clinical cases being traced back in the 80s when laser energy was used in several clamped or clampless open PN cases [7–9]. Despite the promising initial outcomes, the approach was abandoned until lately when urologists became familiar with laser technology by its wide use in benign prostatic hyperplasia and stone disease. Although experience with laser renal tumor resection is still limited, the reporting literature is expanding rapidly, and based on its favorable results, laser assistance should be expected to play a significant role in nephron-sparing surgery in the future (Fig. 1).
Methods
A comprehensive PubMed review of the present literature was performed in April 2014 without a time limit. The MeSH terms used were as follows: laparoscopic and/or partial and/or nephrectomy and/or laser assisted. Additional literature was retrieved by the references of initially retrieved manuscripts (Table 1). Experimental work on animals and review articles were excluded.
Clinical experience with laser-assisted nephron-sparing surgery
Throughout the last three decades, almost every type of clinically applied laser source has been used in PN.
Carbon dioxide laser
Carbon dioxide (CO2) laser was the first laser used in clinical practice for PN. CO2 laser has a wavelength of 10,640 nm and is highly absorbed by tissue water. Thus, it has a very superficial depth of penetration (<1 mm) and can only be used as an ablative energy for small vessels rather than a cutting device. After encouraging experimental outcomes by the use of CO2 laser in dog kidneys, Barzilay et al. [7] introduced laser-assisted nephron-sparing surgery in humans with 3 cases of open lower pole partial nephrectomies. Soon after, Rosenburg published the outcomes of his small series as well [9]. Although hilar clamping was necessary, according to both studies, the addition of laser to their armamentarium reduced blood loss and time of hemostasis, and minimized parenchymal damage.
Neodymium-doped yttrium aluminum garnet and potassium titanyl-phosphate lasers
Neodymium-doped yttrium aluminum garnet (Nd:YAG) laser when operated at 1,064 nm (a 532-nm version exist for lithotripsy) has a deeper length of tissue penetration (up to 1 cm) than CO2 laser. Moreover, it demonstrates excellent cutting and coagulation properties. In 1986, Malloy et al. [8] reported their experience with Nd:YAG-assisted PN in six elderly patients with upper pole renal tumors. Three of them had transitional cell carcinoma in an upper pole calyx of a solitary kidney and where treated with a percutaneous Nd:YAG application of tumor surface prior to an open Nd:YAG-assisted PN. No renal artery occlusion was performed in any of the six cases. Similarly, Korhonen et al. [10] performed contact Nd:YAG laser PN in six patients with favorable results. Clamping of the renal artery combined with renal hypothermia ensured good intraoperative hemostasis and excellent postoperative functional results. Despite the aforementioned promising outcomes, no further adaptation of Nd:YAG laser-assisted PN has been reported ever since apart from a small series of 3 partial nephrectomies in children where both the potassium titanyl-phosphate laser (KTP; for cutting) and the Nd:YAG laser (for coagulation of large vessels) were used to allow a fast removal of kidney tissue, with minimal blood loss and minimum loss of renal parenchyma [11]. The latter article represents the only documentation of clinical application of the 532-nm KTP laser for PN with the exception of an abstract presented in the Engineering and Urology Society Congress in 2007 reporting the successful accomplishment of two KTP-assisted partial nephrectomies [12].
Holmium: yttrium aluminum garnet
Holmium: yttrium aluminum garnet (Ho:YAG) laser emits a beam with a wavelength of 2,150 nm and in water-based medium has an absorption depth of 1–2 mm. Despite the fact that Ho:YAG laser is the most widespread laser source in urology (due to its use in stone fragmentation and prostatic enucleation), currently, there is only one report documenting its efficiency in nephron-sparing surgery. Lotan et al. [13] performed three uncomplicated partial nephrectomies with a 550-μm fiber at 0.2 J/pulse and 60 Hz or 0.8 J/pulse at 40 Hz to provide a steady delivery of energy. Defocusing of the laser beam was necessary to induce hemostasis in some cases. No clamping of renal pedicles was necessary. Increased smoke during laser firing and blood splashing on the camera were the main drawbacks of the approach.
Diode laser
Diode lasers can be tuned to emit in a variety of spectral ranges depending on different elements used (e.g., aluminum, indium). A wide wavelength range within 980 and 1470 nm has been used in humans for PN. An initial report with excellent outcomes of diode laser-assisted PN was written by Khoder et al. [14] In their series with 13 patients (five open, eight laparoscopic PN), the ability of a semiconductor diode laser emitting a wavelength of 1.318 nm in a continuous-wave mode to provide a bloodless renal tissue excision without necessitating hilar clamping for the majority of cases was demonstrated. Renal artery occlusion was necessary in three cases while selective suturing of parenchymal vessels was deemed necessary in two cases where laser failed to coagulate the bleeding surface effectively. Mean blood loss for all cases (open and laparoscopic) was 240 ml. One year later, the same study group compared the same laser-assisted laparoscopic PN (LLPN) technique with laparoscopic (LPN) and open techniques (OPN). In a prospective manner, they enrolled 36 patients with small peripheral renal tumors. Patients were divided into three equal groups with 12 patients in each group. Renal tumors were excised with laser, Sonosurg or monopolar scissors during laser-assisted laparoscopic, laparoscopic and open PN, respectively. Laser-assisted laparoscopic surgery was proved to be an efficient alternative to laparoscopic or open PN with similar mean operation time and a lower estimated blood loss (170.8 ml vs. 245.2 vs. 425.8 for LLPN vs. LPN vs. OPN, respectively [15]).
In a recently published prospective study with 17 patients, Knezevic et. al. [16] performed LLPN on solitary exophytic small renal tumors (≤4 cm) with an intraparenchymal depth of ≤1.5 cm and a minimum distance of 5 mm from the urinary collecting system. For almost all cases, a diode laser 980 nm with end fire 1,000-µm laser fiber was used with the exception of a single case where a dual diode laser 980/1,470 nm was utilized. Median operative time was 170 min. In all but two cases, hilar clamping had to be performed. Median warm-ischemia time was 16 min; no intraoperative complications were observed. In only one case, perirenal hematoma was observed postoperatively.
Two-micrometer continuous thulium wave laser
In 2008, Gruschwitz et. al. [17] published one of the first reports with 2-μm continuous-wave diode-pumped solid-state laser emitting a wavelength of 2,013 nm and penetrating tissue to a depth of about 0.5 mm. They performed laser-assisted OPN on 5 patients with small exophytic kidney tumors without clamping of the renal vessels and demonstrated this technique to be a safe alternative to conventional PN. They observed no hemorrhage during any of the procedures; moreover, no sutures or other means of hemostasis were needed.
In the same year, another report with nine thulium laser-assisted PNs (8 OPN and 1 LRN) was published by Mattioli et. al. [18] In six of these PN, the pedicle was clamped and one case was performed under cold ischemia. Thulium laser presented excellent hemostasis and precise dissection of the renal cortex without any need to perform sutures of the renal parenchyma.
Sciarra et al. [19] have recently published their experience with the use of thulium laser in 10 patients subjected to open or laparoscopic laser-assisted enucleation for small peripheral renal cell carcinoma. No significant overall blood loss (<40 cc) and limited bleeding during dissection that did not interfere with the definition of surgical plane was reported.
In another series with 11 patients, Loertzer et al. [20] performed LLPN on exophytic renal tumors without clamping of the renal vessels. Mean tumor size was 32 mm. A diode-pumped solid-state laser emitting a 2-μm continuous wave with a wavelength of 2,013 nm and a tissue penetration of 0.5 mm was used. The coagulative and ablative tissue effects were gentle. Mean loss of blood was 75 ml (10–400 ml). No postoperative complications were observed. Their findings revealed LLPN without clamping of the renal vessels to be a safe alternative in exophytic renal tumors.
In their prospective analysis, Thomas et. al. [21] performed LLPN without clamping the renal vessels on a total number of 15 patients using a thulium:yttrium–aluminum–garnet (Th:YAG) laser. Mean operative time was 168 min, and mean blood loss was 341 ml. Postoperatively, neither a significant increase in serum creatinine level nor a decrease in eGFR was observed.
Robotic laser partial nephrectomy
Colli et al. [22] published case reports of two patients on whom they performed thulium laser-assisted robotic PN without clamping the hilar vessels. No intraoperative or postoperative complications were observed, and the pathological examinations revealed negative surgical margins.
Discussion
Even though PN manages to deliver lower incidence of postoperative kidney failure when compared to radical nephrectomy especially in patients with impaired kidney function prior to surgery [23], hypoxia resulting from renal vessel clamping seems to impair remaining healthy kidney tissue and leads to increased morbidity especially in previously damaged or solitary kidneys [24, 25]. Under hypoxia, oxidative radicals are produced rapidly; thus, various studies point out better clinical outcomes of tumor excision without ischemia [26, 27].
In a previous review, Klingler et al. [28] have stressed out reliability issues of existing techniques used to preserve hemostasis during LPN and demanded for newer and safer modalities. In this context, laser technology presents a promising tool, which is able to offer superior hemostasis even in the absence of hilar clamping.
Even though laser has become a part of routine urological practice in the treatment of urolithiasis and prostate hyperplasia, its utilization in nephron-sparing surgery is scarce. First, clinical experiments with laser-assisted PN almost lead back to 3 decades and over time, it has woken an increasing interest among urologists.
First, experiments with CO2 lasers revealed beneficiary results in terms of hemostasis or renal tissue damage. Nevertheless, no further clinical data on CO2 laser-assisted PN was released. This is most probably due to its inability to cut kidney efficiently and accomplish the whole operation without the need for additional instruments [7–9].
In the second decade of laser-assisted kidney surgery, urologists concentrated on Nd:YAG, KTP and Ho:YAG lasers. Reports with Nd:YAG laser revealed promising results with excellent cutting and coagulation properties but the deeper tissue penetration increased the risk of damage to healthy kidney tissue [8–12]. Even though Ho:YAG laser is widely available for urologists, there is only one publication in the literature utilizing Ho:YAG laser-assisted PN. Ho:YAG laser has a shallow tissue effect and possesses favorable hemostatic and cutting properties but due to its pulsating nature, it causes splattering and significant smoke, which can impair the vision especially during laparoscopic procedures [13, 29].
In the recent years, diode and thulium lasers were in the focus of research regarding laser-assisted PN. Promising outcomes with both laser sources were documented. Still, the major downsize of diode laser is that it can not deliver sufficient tissue ablation and cause severe carbonization [30]. On the other hand, Thulium wave laser do have excellent cutting and coagulation properties without causing blood splitting as it emits waves in a continuous manner. Unfortunately, formation of excessive smoke and tissue carbonization is also observed with this type of laser especially when it is used on dry tissues. Additional use of irrigation fluid minimizes these unwanted effects, yet concerns on possible tumor seeding due to irrigation have been raised [31].
Ideal laser
The optimal nephron-sparing surgery technique should allow precise and bloodless excision of tumors without the need to clamp hilar vessels. Even though initial studies with currently available laser modalities demonstrated promising results, several drawbacks in each technique need to be addressed before they can be widely accepted as a standard care.
The ideal laser setup should provide precise and adequate tissue cutting and ablation without causing carbonization, splattering or excessive smoke. In that case, the necessity for irrigation would be avoided and vision during resection would be improved. In addition, in such an ideal setup, hemostasis should be safely accomplished even in larger blood vessels omitting the need for suturing or additional hemostatic agents. Finally, the ideal laser should be fast and easy to use.
Adaptation of such laser in the armamentarium of urologic surgery would decrease the stiff learning curve of LPN and would increase its safety and efficacy. In the lack of renal artery occlusion, superior nephron sparing would be provided without the danger of significant blood loss.
Conclusions
The current literature regarding laser-assisted PN is scarce. Available data consist mostly of small cohorts providing low level of evidence. Nevertheless, experience with this approach is steadily increasing and uniformly documenting favorable results. As urologist become more familiar with laser technology by its implementation in other clinical entities and with the increasing interest in nephron-sparing management of renal tumors, the use of laser assistance during PN could be expected to play a major role in future.
References
Faria EF, Caputo PA, Wood CG, Karam JA, Nogueras-Gonzalez GM, Matin SF (2014) Robotic partial nephrectomy shortens warm ischemia time, reducing suturing time kinetics even for an experienced laparoscopic surgeon: a comparative analysis. World J Urol 32:265–271
Minervini A, Siena G, Antonelli A et al (2014) Open versus laparoscopic partial nephrectomy for clinical T1a renal masses: a matched-pair comparison of 280 patients with TRIFECTA outcomes (Record Project). World J Urol 32:257–263
Bessede T, Bigot P, Bernhard JC, et al. (2014 Apr 4) Are warm ischemia and ischemia time still predictive factors of poor renal function after partial nephrectomy in the setting of elective indication? World J Urol
Gill IS, Eisenberg MS, Aron M et al (2011) “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol 59:128–134
Kutikov A, Vanarsdalen KN, Gershman B et al (2008) Enucleation of renal cell carcinoma with ablation of the tumour base. BJU Int 102:688–691
Ng CK, Gill IS, Patil MB et al (2012) Anatomic renal artery branch microdissection to facilitate zero-ischemia partial nephrectomy. Eur Urol 61:67–74
Barzilay B, Lijovetzky G, Shapiro A, Caine M (1982) The clinical use of CO2 laser beam in the surgery of kidney parenchyma. Lasers Surg Med 2:81–87
Malloy TR, Schultz RE, Wein AJ, Carpiniello VL (1986) Renal preservation utilizing neodymium:YAG laser. Urology 27:99–103
Rosemberg SK (1985) Clinical experience with carbon dioxide laser in renal surgery. Urology 25:115–118
Korhonen AK, Talja M, Karlsson H, Tuhkanen K (1993) Contact Nd:YAG laser and regional renal hypothermia in partial nephrectomy. Ann Chir Gynaecol Suppl 206:59–62
Merguerian PA, Seremetis G (1994) Laser-assisted partial nephrectomy in children. J Pediatr Surg 29:934–936
Hodgson DKKR, et al. (2008) Appraisal of a novel procedure: potassium titanyl phosphate (ktp) laser laparoscopic partial nephrectomy. Abstracts of the Engineering and Urology Society, May 19, 2007, Anaheim, California. J Endourol, 22:159–212
Lotan Y, Gettman MT, Ogan K, Baker LA, Cadeddu JA (2002) Clinical use of the holmium: YAG laser in laparoscopic partial nephrectomy. J Endourol Endourol Soc 16:289–292
Khoder WY, Sroka R, Hennig G et al (2011) The 1,318-nm diode laser supported partial nephrectomy in laparoscopic and open surgery: preliminary results of a prospective feasibility study. Lasers Med Sci 26:689–697
Khoder WY, Sroka R, Siegert S, Stief CG, Becker AJ (2012) Outcome of laser-assisted laparoscopic partial nephrectomy without ischaemia for peripheral renal tumours. World J Urol 30:633–638
Knezevic N, Kulis T, Maric M, Grkovic MT, Krhen I, Kastelan Z (2014) Laparoscopic partial nephrectomy with diode laser: a promising technique. Photomed Laser Surg 32:101–105
Gruschwitz T, Stein R, Schubert J, Wunderlich H (2008) Laser-supported partial nephrectomy for renal cell carcinoma. Urology 71:334–336
Mattioli S, Munoz R, Recasens R, Berbegal C, Teichmann H (2008) What does Revolix laser contribute to partial nephrectomy? Arch Esp Urol 61:1126–1129
Sciarra A, Von Heland M, Minisola F, Salciccia S, Cattarino S, Gentile V (2013) Thulium laser supported nephron sparing surgery for renal cell carcinoma. J Urol 190:698–701
Loertzer H, Strauss A, Ringert RH, Schneider P (2013) Laser-supported partial laparoscopic nephrectomy for renal cell carcinoma without ischaemia time. BMC Urology 13:31
Thomas AZ, Smyth L, Hennessey D, O’Kelly F, Moran D, Lynch TH (2013) Zero ischemia laparoscopic partial thulium laser nephrectomy. J Endourol Endourol Soc 27:1366–1370
Colli J. MG, Lee BR (2012) Zero Ischemia Robotic Laser Partial Nephrectomy: Use of the Thulium laser in Renal Cell Carcinoma. BJU int Case Reports. BJUIw-2011-064-web
Tobert CM, Riedinger CB, Lane BR (2014) Do we know (or just believe) that partial nephrectomy leads to better survival than radical nephrectomy for renal cancer? World J Urol 32:573–579
Ghavamian R, Cheville JC, Lohse CM, Weaver AL, Zincke H, Blute ML (2002) Renal cell carcinoma in the solitary kidney: an analysis of complications and outcome after nephron sparing surgery. J Urol 168:454–459
McKiernan J, Simmons R, Katz J, Russo P (2002) Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 59:816–820
Choi JD, Park JW, Choi JY et al (2010) Renal damage caused by warm ischaemia during laparoscopic and robot-assisted partial nephrectomy: an assessment using Tc 99 m-DTPA glomerular filtration rate. Eur Urol 58:900–905
Porpiglia F, Fiori C, Bertolo R et al (2012) The effects of warm ischaemia time on renal function after laparoscopic partial nephrectomy in patients with normal contralateral kidney. World J Urol 30:257–263
Klingler CH, Remzi M, Marberger M, Janetschek G. (2006 Nov) Haemostasis in laparoscopy. European Urol, 50:948–56; discussion 56–7
Ogan K, Cadeddu JA (2002) Minimally invasive management of the small renal tumor: review of laparoscopic partial nephrectomy and ablative techniques. J Endourol Endourol Soc 16:635–643
Janda P, Sroka R, Mundweil B, Betz CS, Baumgartner R, Leunig A (2003) Comparison of thermal tissue effects induced by contact application of fiber guided laser systems. Lasers Surg Med 33:93–101
Bui MH, Breda A, Gui D, Said J, Schulam P (2007) Less smoke and minimal tissue carbonization using a thulium laser for laparoscopic partial nephrectomy without hilar clamping in a porcine model. J Endourol Endourol Soc 21:1107–1111
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kyriazis, I., Ozsoy, M., Kallidonis, P. et al. Current evidence on lasers in laparoscopy: partial nephrectomy. World J Urol 33, 589–594 (2015). https://doi.org/10.1007/s00345-014-1343-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-014-1343-0